Abstract
Background: The aim of this study is to estimate the budget impact of budesonide/formoterol fixed dose combination (FDC) vs salbutamol, both used as needed, in mild asthma patients, from the perspective of the Health Insurance Organization (HIO).
Methods: A static budget impact model was developed to assess the impact of budesonide/formoterol FDC entry on HIO budget over a 3-year period in Egyptian settings. Direct medical costs, including the costs of asthma medications, exacerbations, and management of side-effects, were obtained from HIO cost data. Population data were obtained from the World Bank and supplemented with local studies, and the rates of exacerbations, adverse effects, and number of sick leave days were elicited from the SYGMA 1 trial. Scenario analyses from a societal perspective and deterministic sensitivity analyses were conducted.
Results: The total costs (drug and non-drug costs) for managing mild asthma patients from the HIO perspective were estimated to be EGP8.563 billion before budesonide/formoterol entry compared to EGP5.525 billion post-entry, leading to a total budget savings of EGP3.038 billion after 3 years. This total budget saving included an increase in drug costs (EGP104 million) and a decrease in non-drug costs (EGP3.143 billion). Drug costs were higher in the budesonide/formoterol group than in the salbutamol group, but this cost was offset by reductions in non-drug costs, resulting in a reduction in the total costs of healthcare resources. At the societal level, the total budget savings after including the indirect costs was expected to be EGP5.976 billion after 3 years of budesonide/formoterol entry.
Conclusion: Budesonide/formoterol in mild asthma instead of salbutamol produces better patient outcomes and decreases total costs, with increases in drug cost offset by reductions in non-drug costs due to fewer exacerbations. Budesonide/formoterol is a budget saving option for guideline-directed treatment, from the economic perspective of the payer and the health perspective of the patient.
Background
Asthma is a main cause of morbidity, mortality, economic burden and a major public health problem worldwideCitation1. This chronic disease is characterized by inflammation of respiratory airways, hypersensitivity of airways, and variable airflow limitation for short periods of time.
The number of people living with asthma worldwide is estimated as 334 million according to the Global Asthma NetworkCitation2. The prevalence of asthma has steadily increased alongside that of allergy, as modern lifestyles are adopted and communities become more urbanizedCitation3–6. In Egypt many studies on asthma have been performed in the pediatric population but very few addressed the adult populationCitation7. SNAPSHOT; a cross-sectional epidemiological program conducted in five Middle Eastern countries among the adult general population collected data on asthma, allergic rhinitis, benign prostatic hyperplasia and bipolar disorder; reported an asthma prevalence of 6.7% in EgyptCitation8.
Mild asthma often remains poorly controlled despite the availability of effective treatmentsCitation9. Current guidelines recommend that most patients with mild asthma be treated with regular, low-dose inhaled glucocorticoids as controller medication to reduce the risk of exacerbations, with short-acting β2-agonists (SABAs) used as needed for symptom reliefCitation9. Despite the relatively low burden of symptoms in these patients, airway inflammation, although variable in intensity, is usually presentCitation9. The under-use of inhaled glucocorticoids, even in patients with mild asthma, is associated with severe asthma exacerbations and deathCitation10. However, adherence to regular controller therapy, particularly inhaled glucocorticoids, is poorCitation11,Citation12. It has been reported that half of the treated patients do not comply correctly with their treatmentCitation13. Instead, patients rely on SABAs to relieve symptoms, and overuse is common. This behavior is also associated with a risk of severe exacerbations and deathCitation14,Citation15.
Medication adherence is a key factor for controlling progression of chronic disease. The lack of adherence is associated with increased healthcare costs due to emergency room visits and hospitalizations as well as additional diagnostic tests and stepping up therapy compared to the original less costly therapy, which also indicates higher consumption of primary care and specialist consultationsCitation16,Citation17.
The use of a combination of a budesonide and formoterol on an as-needed basis—an anti-inflammatory reliever approach—is recommended by Global Initiative for Asthma (GINA), including more than 25 trials showing the benefits of Symbicort Turbuhaler as an anti-inflammatory reliever to reduce the risk of severe asthma attacks across all disease severities, among them the SYGMA trials published in the New England Journal of MedicineCitation18–21.
In Egypt, there is no budget impact analysis or related economic information (such as cost effectiveness studies) on the effects of the budesonide/formoterol treatment combination.
The objective of this study was to conduct a budget impact analysis of budesonide/formoterol FDC (Symbicort® (Astrazeneca)−(200 μg of budesonide and 6 μg of formoterol)) vs the standard of care (salbutamol inhaler; 0.1 mg) among mild asthma patients over a 3 year period from the HIO perspective to provide decision-makers in Egypt with evidence to support resource allocation and improve patient outcomes.
Methods
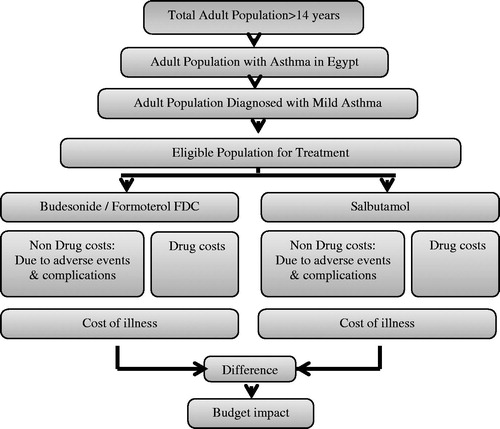
A static budget impact model was developed using Microsoft Excel to assess the impact of budesonide/formoterol FDC entry. The structure of the model () reflects the current local practices and the published studies in treating mild asthmaCitation19–22. The model structure includes disease prevalence, distribution of market shares per medication, medication use, and drug acquisition cost in Egypt. Patients aged 14 years of age and older were considered in the model. No approval was required from an Ethics Committee or Institutional Review Board as no access patient level data.
This economic study was conducted from the HIO perspective and included only direct medical costs. Scenario analyses were conducted from a societal perspective and included indirect costs such as productivity-related costs. Total healthcare costs were calculated as the quantity of each resource used multiplied by the corresponding unit cost in Egyptian Pound (EGP) derived in 2019. Indirect costs were measured as days of sick leave and were estimated using Gross Domestic Product (GDP)Citation23. A macro-costing approach was used to determine the costs.
Direct medical costs include the costs of asthma medications, exacerbations, and management of side-effects. A severe exacerbation is worsening asthma leading to the use of systemic glucocorticoids for ≥3 days, inpatient hospitalization, or an emergency department visit leading to the use of systemic glucocorticoidsCitation21. A moderate-to-severe exacerbation is worsening asthma requiring the addition of inhaled budesonide/formoterol at a dose of 320 μg twice daily to avoid progression to a severe exacerbationCitation21. These definitions were validated from an expert panel. Adverse event costs of the drugs administered for the treatment of mild asthma was estimated based on the incidence of each event and pharmacy costs associated with their management. Adverse events included are upper respiratory tract infection, pharyngitis, viral upper respiratory tract infection and bronchitis. Pharmacy costs for the management of each adverse event in the model were based on local clinical practice. Discounting was not considered in this study, consistent with recommendations of the ISPORCitation24.
Data sources
All model inputs and their ranges are shown in . Population data were obtained from the World BankCitation25, and the prevalence of asthma in Egypt was elicited from SNAPSHOT, a cross-sectional epidemiological program conducted in five Middle Eastern countries (Egypt, Turkey, Kuwait, Saudi Arabia, and the United Arab Emirates), among the adult general population, on asthma, allergic rhinitis, benign prostatic hyperplasia, and bipolar disorder from 33,486 subjectsCitation8. The prevalence of mild asthma in Egypt was obtained from a study of demographic and clinical characteristics conducted at Mahalla Chest Hospital and included 212 adult patients who had bronchial asthma with a wide range of asthma severityCitation22. The rates of exacerbations, adverse effects and number of sick leave days were elicited from SYGMA 1 trial; a 52-week, randomized double-blind trial involving 3,849 patients with mild asthmaCitation21. The number of eligible patients for “Treatment As-Needed” and the average resource use in mild and severe asthma exacerbations were obtained from an expert panel. This panel consisted of five key leading physicians who were specialists in pulmonary medicine recruited from the most representative institutions in Egypt, thus reflecting real life clinical practice. They responded to a survey on average resource use and number of eligible patients and then a consensus approach developed by using the Quasi-Delphi method consisted of an iterative series of meetings and interrogations. The costs of asthma medications, exacerbations, and management of side-effects were obtained from HIO cost data.
Table 1. Model input parameters.
Assumptions
The main assumptions used in the model were; first, since salbutamol inhaler is considered the comparator and the standard of care in mild asthma in Egypt, and terbutaline inhaler is not available in the Egyptian market, thus, it was assumed that the rate of exacerbations and side-effects of terbutaline in SYGMA 1 study is the same as salbutamol. Second, any assigned treatment remains unchanged for the study period. Third, the patients are l00% compliant and have no other comorbidities. All these assumptions were validated by national clinical experts and the following institutions; Chest Diseases Sector in Ministry of Health, Abbasya Chest Hospital, Nasrcity Health Insurance Hospital, and Cairo University Hospitals. Separate market share distributions across the treatment options were used for patients with mild asthma as shown in .
Table 2. Market penetration for treatment options.
Sensitivity analyses
In addition to the scenario analysis conducted from a societal perspective mentioned above, a one-way sensitivity analyses were conducted for certain inputs to assess the model robustness. The inputs considered in the sensitivity analyses included the exacerbation rates, all costs of drugs and laboratory tests, population data, and market share. They were varied up or down by 10% or 20% of their base case values.
Results
The target population with mild asthma treated as needed with budesonide/formoterol FDC (Symbicort) in 2019 was estimated to be 611,012 while those covered by HIO were estimated to be 359,275 in Egypt. Individuals in this group are eligible to receive budesonide/formoterol vs salbutamol in mild asthma. summarizes the budget savings of budesonide formoterol entry in terms of total annual drug and non-drug costs.
Figure 2. The total annual costs (drug and non-drug) of current and new scenarios in mild asthma from an HIO perspective. (a) The total drug costs for the two scenarios. (b) The total non-drug costs for the two scenarios. (c) The total drug costs per patient for the two scenarios. (d) The total non-drug costs per patient for the two scenarios.
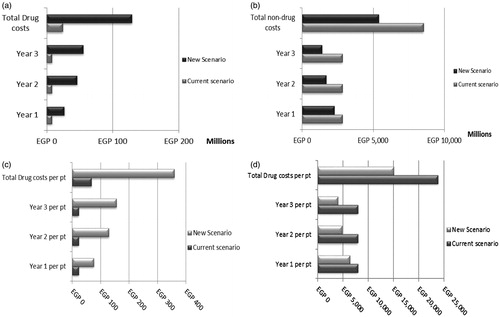
The total annual costs (drug and non-drug costs) from a HIO perspective were estimated to be EGP 8.563 billion before budesonide/formoterol entry (current scenario) compared to EGP 5.525 billion post-entry (new scenario), leading to a total budget savings of EGP 3.038 billion after 3 years. This total budget savings after 3 years included increase in drug costs (EGP 104.926 million) and a decrease in non-drug costs (EGP 3.143 billion). Drug costs were higher in the budesonide/formoterol group than in the salbutamol group, but this was offset by reductions in non-drug costs, mainly hospitalization, resulting in a reduction in the total costs of healthcare resources (; ).
Table 3. The total direct annual costs (drug and non-drug) of current and new scenario in mild asthma.
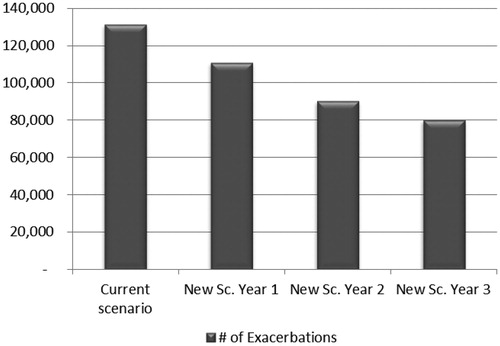
The total number of exacerbations that occurred in the salbutamol group was estimated at 131,368 compared to 110,838, 90,308, and 80,043 in budesonide/formoterol in the first, second, and third year, respectively. The total number of exacerbations avoided after 3 years of budesonide/formoterol entry was estimated at 51,325 ().
Figure 3. The total number of exacerbations of current and new scenarios in mild asthma from an HIO perspective.
The total indirect costs were estimated to be EGP 2.379 billion before budesonide/formoterol entry compared to EGP 2.232 billion post-entry, leading to a total savings/productivity gain of EGP 147 million in the first year. The total savings was estimated to be EGP 294 million and EGP 367 million in the second and third years, respectively.
Sensitivity analyses
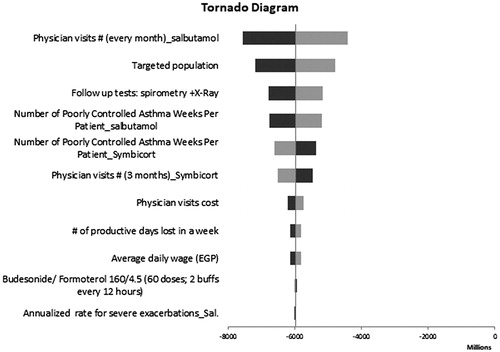
Given the uncertainty of all the parameters for the two treatments, a sensitivity analysis was conducted using plausible ranges in . One-way sensitivity analyses on the base case revealed that model results were robust to changes in key input parameters. The number of physician visits in the salbutamol group and the number of targeted patients covered by HIO were found to have the highest impact on the model results ().
Figure 4. One-way sensitivity results from an HIO perspective. Black bars indicate the higher values while the grey bars indicate the lower values.
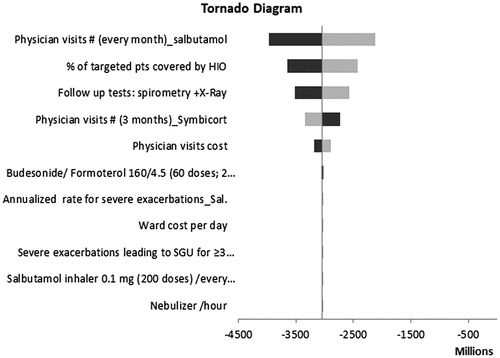
A scenario analysis was conducted by varying the perspective of the study and then the savings in productivity costs were included, there was a substantial net savings in budesonide/formoterol group. The total budget savings included an estimated pharmacy budget increase of EGP 178 million (drug costs) and a medical budget decrease of EGP 6.154 billion (non-drug costs). Overall, at the societal level the total budget savings after including the indirect costs was expected to be EGP 5.976 billion after 3 years of budesonide/formoterol entry. The results of the one-way sensitivity analyses are presented in the tornado diagram ().
Discussions
The objective of this study was to estimate the budget impact of budesonide/formoterol FDC vs salbutamol (standard care), both used as needed in mild asthmatic patients, from the perspective of the HIO. The results suggest an overall budget saving among patients who received budesonide/formoterol FDC due to reduced number of exacerbations occurred with budesonide/formoterol. The increase in upfront drug costs of budesonide/formoterol FDC in mild asthmatic patients were offset by less resource utilization due to fewer exacerbations. The SYGMA 1 study reported that as-needed budesonide/formoterol provided superior asthma-symptom control to as-needed SABA in mild asthmatic patients. They also reported that the number of patients with high use (>8 and >12 inhalations in 1 day) of as-needed medication are reported more in SABACitation21. This is evidence which supports that policy-makers prioritize the adoption of budesonide/formoterol as a better treatment intervention in mild asthma.
This study reported that the total number of exacerbations avoided after 3 years of budesonide/formoterol entry was estimated at 51,325. Avoiding exacerbations/unplanned hospital admissions is a major concern for the health insurance sector, not only because of the rising unit costs of hospital admissions, but also because of the disruption it causes to the hospitals—most notably inpatient waiting lists—and to the other patients in the society. Self-management with budesonide/formoterol seems to be effective in reducing unplanned hospital admissions. If indirect costs are included from the societal perspective, budesonide–formoterol is budget saving. The reductions in time off work varies across the working age population and also in elderly individuals, who do not usually participate directly in formal labor market activities but contribute in informal labor markets by volunteering time for various activitiesCitation26.
The results of this study are consistent with Andersson’s study that found budesonide/formoterol generates significant gain in outcomes with complete or partial offset of costs driven by a reduction in the use of other resource items in connection with mild and severe asthma exacerbations in the UK, Sweden, and SpainCitation27. Cost-effectiveness results provide an input into the decision-making process; however, they do not address the likely impact of any new treatment on healthcare budgets or their affordability to a given payer or countryCitation28. A budget impact analysis is an important part of a comprehensive economic evaluation of a new treatment and provides guidance about the acquisition and use of new treatments with budget projectionsCitation24.
The strengths of our study include that it is the first economic evaluation conducted in Egypt that specifically looks at mild asthma-related treatment costs and investigated the budget impact of budesonide/formoterol. Second, the data inputs including rates of exacerbations, adverse effects and number of sick leave days were obtained from a large double-blinded randomized clinical trial involving 3,849 patients. Third, the validity of the model estimates were tested by surveying four diverse clinical experts in Egypt to reflect on the real-life clinical practice, particularly among mild asthma patients with a history of poor compliance. Fourth, the current model is consistent with international budget impact modeling because it is transparent (i.e. assumptions are clearly stated), takes the perspective of the payer, uses a time horizon that is relevant, and captures data from reliable sourcesCitation24.
This study was limited by possible uncertainty in the proportion of targeted mild asthma patients obtained from the expert panel, however this may be acceptable when there is a difficulty in real world utilization of dataCitation29. Second, we didn’t incorporate the measurement of volunteering time for elder populations as it is hard to be locally estimated. The most neutral approach—in terms of equity and justice—would be to value all productive time at a mean wage plus fringe benefits in societyCitation26.
Conclusion
Treating mild asthma patients with budesonide/formoterol instead of salbutamol produces better patient outcomes, and decreases total costs with increases in drug cost offset by reductions in non-drug costs due to fewer exacerbations. Budesonide/formoterol in mild asthma instead of salbutamol is a budget saving option for guideline-directed treatment, from the economic perspective of the payer and the health perspective of the patient.
Transparency
Declaration of funding
This work was funded through AstraZeneca without any interference in the methodology and design of this study.
Declaration of financial/other relationships
None declared. A peer reviewer on this manuscript has disclosed participation in consulting, advisory boards, and speaker panels for, or receipt of travel reimbursement from Amphastar, Astra Zeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Mylan, Novartis, Oriel, Pearl, Sunovion, Teva, and Theravance. They have also conducted multicenter clinical research trials for ∼40 pharmaceutical companies. The remaining peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgements
We would like to thank Mohamad Taher, Market Access Manager, AstraZeneca, Cairo, Egypt for his insights and support of this work from its beginning until its completion.
References
- Wisnivesky J, Leventhal H, Halm E. Predictors of asthma-related healthcare utilization and quality of life among inner-city patients with asthma. J Allergy Clin Immunol. 2005;116:636–642.
- The Global Asthma Report 2014. In.: Global Asthma Network; 2014 [cited 2019 Apr 10]. Available from: www.globalasthmanetwork.org/publications/Global_Asthma_Report_2014.pdf.
- Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. In.: World Health Organisation; 2007 [cited 2019 Apr 1]. Available from: https://www.who.int/gard/publications/GARD_Manual/en/.
- Stanojevic TS, Moores G, Gershon AS, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204.
- Alsowaidi S, Abdulle A, Bernsen R, et al. Allergic rhinitis and asthma: a large cross-sectional study in the United Arab Emirates. Int Arch Allergy Immunol. 2010;153:274–279.
- Mahboub BH, Al-Hammadi S, Rafique M, et al. Population prevalence of asthma and its determinants based on European Community respiratory health survey in the United Arab Emirates. BMC Pulm Med. 2012;12:4.
- Adeloye D, Chan KY, Rudan I, et al. An estimate of asthma prevalence in Africa: a systematic analysis. Croat Med J. 2013;54:519–531.
- Tarraf H, Aydin O, Mungan D, et al. Prevalence of asthma among the adult general population of five Middle Eastern countries: results of the SNAPSHOT program. BMC Pulm Med. 2018;18:68.
- Global Initiative for Asthma (GINA). 2018 GINA report, global strategy for asthma management and prevention [cited 2019 April 8]. Available from: https://ginasthma.org/gina-reports/.
- Pauwels RA, Pedersen S, Busse WW, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361:1071–1076.
- Rand C, Bilderback A, Schiller K, et al. Adherence with montelukast or fluticasone in a long-term clinical trial: results from the mild asthma montelukast versus inhaled corticosteroid trial. J Allergy Clin Immunol. 2007;119:916–923.
- Jónasson G, Carlsen KH, Mowinckel P. Asthma drug adherence in a long term clinical trial. Arch Dis Child. 2000;83:330–333.
- Gonzalez F, de la Fuente-Cid R, Álvarez-Gil R, et al. [Factors associated with asthma control in primary care patients: the CHAS study]. Arch Bronconeumol. 2010;46:358–363.
- Stanford RH, Shah MB, D’Souza AO, et al. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. 2012;109:403–407.
- Suissa S, Ernst P, Boivin J-F, et al. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care Med. 1994;149:604–610.
- Dalcin Pde T, Grutcki DM, Laporte PP, et al. Impact of short-term educational intervention on adherence to asthma treatment and on asthma control. J Bras Pneumol. 2011;37:19–27.
- Darba J, Ramirez G, Sicras A, et al. The importance of inhaler devices: the choice of inhaler device may lead to suboptimal adherence in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:2335–2345.
- Papi A, Caramori G, Adcock IM, et al. Rescue treatment in asthma. More than as-needed bronchodilation. Chest. 2009;135:1628–1633.
- Global Initiative for Asthma (GINA). GINA Implementation Pocket Guide; 2019 [cited 2019 Apr 10]. Available from: https://ginasthma.org/gina-implementation-guide/gina-implementation-pocket-guide-2019/.
- O’Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J. 2017;50:1701103.
- O’Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined budesonide–formoterol as needed in mild asthma. N Engl J Med. 2018;378:1865–1876.
- Mabrouk AA, Abd El-Aziz AA, Agha MA, et al. Study of demographic and clinical characteristics of bronchial asthma patients in Mahalla Chest Hospital during the period from March 2013 to February 2014. Menoufia Med J. 2017;30:241–248.
- Kawalec P, Malinowski K. Disease activity, quality of life and indirect costs of reduced productivity at work, generated by Polish patients with ankylosing spondylitis. Reumatologia. 2015;53:301–308.
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR task force on good research practices–budget impact analysis. Value Health. 2007;10:336–347.
- The World Bank. Population data, Egypt, Arab Republic; 2017. [cited 2019 Apr 10]. Available from: https://data.worldbank.org/indicator/SP.POP.TOTL?locations=EG.
- Lakdawalla DN, Doshi JA, Garrison LP, et al. Defining elements of value in health care–a health economics approach: an ISPOR special task force report. Value Health. 2018;21:131–139.
- Andersson F, Stahl E, Barnes PJ, et al. Adding formoterol to budesonide in moderate asthma—health economic results from the FACET study. Resp Med. 2001;95:505–512.
- Truema PN, Drummond H. Developing guidance for budget impact analysis. Pharmacoeconomics. 2001;19:609–621.
- Evans C. The use of consensus methods and expert panels in pharmacoeconomic studies_practical applications and methodological shortcomings. PharmacoEconomics. 1997;12:121–129.