Abstract
Aims: The Biventricular vs Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK-HF) demonstrated that biventricular (BiV) pacing resulted in better clinical and structural outcomes compared to right ventricular (RV) pacing in patients with atrioventricular (AV) block and reduced left ventricular ejection fraction (LVEF; ≤50%). This study investigated the cost-effectiveness of BiV vs RV pacing in the patient population enrolled in the BLOCK-HF trial.
Methods: All-cause mortality, New York Heart Association (NYHA) Class distribution over time, and NYHA-specific heart failure (HF)-related healthcare utilization rates were predicted using statistical models based on BLOCK-HF patient data. A proportion-in-state model calculated cost-effectiveness from the Medicare payer perspective.
Results: The predicted patient survival was 6.78 years with RV and 7.52 years with BiV pacing, a 10.9% increase over lifetime. BiV pacing resulted in 0.41 more quality-adjusted life years (QALYs) compared to RV pacing, at an additional cost of $12,537. The “base-case” incremental cost-effectiveness ratio (ICER) was $30,860/QALY gained. Within the clinical sub-groups, the highest observed ICER was $43,687 (NYHA Class I). Patients receiving combined BiV pacing and defibrillation (BiV-D) devices were projected to benefit more (0.84 years gained) than BiV pacemaker (BiV-P) recipients (0.49 years gained), compared to dual-chamber pacemakers.
Conclusions: BiV pacing in AV block patients improves survival and attenuates HF progression compared to RV pacing. ICERs were consistently below the US acceptability threshold ($50,000/QALY). From a US Medicare perspective, the additional up-front cost associated with offering BiV pacing to the BLOCK-HF patient population appears justified.
Introduction
Heart failure (HF) is a major global health problem, and its prevalence continues to rise over time with the aging of the population, thereby imposing a significant economic burden on the healthcare systemCitation1. Cardiac resynchronization therapy (CRT) is a cardiac pacing modality, which has taken center stage in the treatment of HF patients with electromechanical dyssynchronyCitation2.
While the primary goal of right ventricular (RV) pacing is restoring ventricular systole in patients with atrioventricular (AV) block, recent studies have indicated that substantial RV pacing may result in progressive left ventricular (LV) dysfunction and HF in patients with established LV dysfunctionCitation2–4. The Biventricular vs Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK-HF) clinical trial examined the effects of biventricular pacing (BiV) using CRT compared with right ventricular (RV) pacing in patients with atrioventricular (AV) block and a reduced left ventricular ejection fraction (LVEF)Citation5,Citation6. The results of the BLOCK-HF study demonstrated that BiV pacing was superior to traditional RV apical pacing for patients with AV block, mild-to-moderate HF, and abnormal LV systolic dysfunctionCitation5.
Although the BLOCK-HF trial demonstrated that patients with AV block could benefit from BiV pacing, little is known about the cost-effectiveness in this population. Therefore, the aim of the current study was to address the potential economic impact of using BiV pacing vs traditional RV pacing for this new indication.
Methods
BLOCK HF study
BLOCK-HF was a prospective, randomized, multi-center, double-blind, controlled study which tested the hypothesis whether BiV pacing might reduce mortality, morbidity, and adverse left ventricular remodeling in patients with AV block, LV dysfunction (LVEF 50%), and mild-to-moderate HF, who meet standard indications for ventricular pacing. BiV pacing is superior to RV pacing as measured by a composite endpoint consisting of all-cause mortality, HF-related urgent care, and increase of ≥15% in LV end-systolic volume index (LVESVI)Citation6.
Economic model design
A “state-transition” model was used to generate expected lifetime costs and benefits for a range of covariate patterns and for each treatment option in each of the three comparisons (as applicable) using a lifetime time horizon and a unit of time of 1 month. Patients received pacing-only or combined pacing/defibrillation devices, resulting in essentially four groups: (a) RV pacing only (RV-P), (b) BiV pacing only (BiV-P), (c) RV pacing/defibrillation device (RV-D), and (d) BiV pacing/defibrillator device (BiV-D). All patients received BiV devices, but those randomized to RV had left ventricular (LV) pacing turned off.
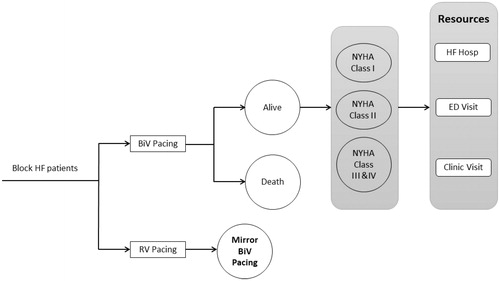
The conceptual model framework is presented in . Health states were defined by survival (“Alive” and “Dead”) in combination with five regression equationsCitation7,Citation8. The first equation was used to allocate patients between these two health states at the start of a given model cycle. A second regression equation estimated the proportion of “Alive” patients in NYHA Class I, Class II, and Class III/IV. The remaining three regression equations predicted the monthly rates of HF-related hospitalizations, emergency department, and clinic visits.
Figure 1. Conceptual model schematic. Abbreviations. BiV, biventricular pacing; RV, right ventricular pacing; HF, heart failure; ED, Emergency Department.
Treatment-specific, predicted NYHA Class distributions were used as time-varying covariates and informed/modified the HF-related event equations. Baseline variables were used as covariates in all the above analyses: age (continuous), gender (binary, i.e. male/female), LVEF (binary, i.e. >35% and ≤35%), degree of AV block (1st, 2nd, or 3rd), and NYHA Class (I, II, or III/IV).
The outcome measures of the analyses were life years (LYs) and quality-adjusted life years (QALYs) gained. The costs and benefits were discounted at 3% per yearCitation9, and the primary outcome measure was the incremental cost-effectiveness ratio (ICER) calculated as costs per LY and QALY gained. Data on battery longevity, utility weights, and payment rates/costs represented the only inputs sourced outside the trial. This approach was consistent with analytic frameworks used in the USCitation10 and elsewhereCitation7,Citation11,Citation12.
All-cause mortality
Individual patient data (IPD) from the BLOCK-HF trial were utilized to compare and extrapolate survival between BiV and RV treatment groups. Commonly used parametric functions (Exponential; Weibull; Gompertz; Log-normal, Log-logistic) were fitted to the intention-to-treat trial data for each patient group. Trial follow-up was assessed via Akaike information criteria (AIC) and Bayesian information criteria (BIC) and visual assessment of Cox-Snell residual plots. The proportional hazards assumption was assessed via examination of log-cumulative hazard plots and Schoenfeld residuals by risk group.
Disease progression
Disease progression was approximated via patient allocation to a specific NYHA Class. Multinomial logistic regression models were used to predict the proportion of patients in each class over time. NYHA Class II was considered the baseline category and results generated for NYHA Classes I and III/IV were expressed relative to Class II. Separate models were tested using permutations of time (natural or log scale), treatment, and interaction terms between time and treatment, to account for potential changes in the impact of BiV pacing over time.
HF-related hospitalizations, emergency department visits, and clinic visits
For the “base-case” modeling count-based regression models (Poisson, negative binomial and their zero-inflated versions) were applied to each of these event types, with goodness-of-fit assessed via a combination of statistical accuracy (again AIC/BIC) and predictive plausibility. The outcome from this analysis was a series of risk equations used to generate NYHA Class specific monthly event rates.
Adverse events
All adverse event (AE) rates were based on BLOCK-HF trial data. An adverse event was defined as any undesirable clinical occurrence in a subject, whether or not it was considered to be related to the investigational therapy, that is related to the subject’s cardiovascular, pulmonary, or renal system (including system and procedure-related events) or events in which the subject presented with symptoms compatible with fluid retention and/or decreased exercise tolerance. Documented pre-existing conditions were not considered adverse events unless there was a change in the nature or severity of the condition. All reported adverse events were classified for cardiovascular, heart failure, system, product, and procedure relatedness by the Adverse Event Advisory Committee (AEAC). The AEAC determined if the events reported conformed to the definitions for complications, observations, serious events, or unanticipated adverse device effect (UADE). In line with common practiceCitation8, AEs outside the therapy area of the analysis were considered unrelated and, thus, excluded.
Conservatively, implant- and device-related complications were assumed to occur concurrently with the implant, and to be reimbursed separately. The calculated rates were considered to apply to every device implant irrespective of the number of implants per patient in the trial. Because all patients received BiV devices, AEs specifically related to LV lead implantation were observed across all arms in the trial. However, for the model “base-case”, LV lead-related complications were assumed to equal zero in patients randomized to non-BiV arms. The key parameters used in the model are presented in Supplementary Table S1. For patients with RV-P and BiV-P, the RV and LV lead displacement rates were 2.1% and 3.3%, respectively; while the rates were 4.0% and 1.9% for patients with RV-D and BiV-D.
Battery longevity
Treatment choice-specific lead displacement and other device-related surgery rates were taken from the BLOCK-HF trial and applied in the first model. The time-to-battery-depletion was integrated using actual data from the cardiac clinical audit database (CCAD) that captured information from all implants across the UK. The data set used included 22,259 ICDs, 10,062 CRT-Ds, and 7,968 CRT-Ps implanted between January 1, 2000 and April 14, 2011 from all manufacturersCitation12. Any battery replacements after the first were modeled at a constant rate based on median device battery duration.
Analytic perspective and costs
Adhering to ISPOR’s Key Guideline Features of the US Academy of Managed Care Pharmacy (AMCP)Citation10 and ensuring comparability with other analysesCitation13–15, a payer perspective based on the US Centers for Medicare and Medicaid Services (CMS) Medicare program was used. Medicare severity diagnostic payment groups (MS-DRG) national average payment rates for Medicare fiscal year 2014 were employed to assess facility payments for all types of inpatient procedures. Ambulatory payment code (APC) payment rates for fiscal year 2014 were used for costs relating to outpatient procedures. The mix of inpatient and outpatient procedure implants was determined using data from the 2012 physician/supplier procedure summary (PSPS) master fileCitation16. Physician fees for device implants were also included. All payments were reduced by 2% to account for sequestration. The key cost components used in the model are presented in Supplementary Table S2.
Health-related quality-of-life (HRQoL)
The NYHA specific utility estimates were based on the study by Gohler et al.Citation17, which was published in Value Health and has been used in much research. EuroQol 5 D data from the Eplerenone Post-acute Myocardial Infarction Heart Failure Efficacy and Survival Study trial were used to estimate utilities as a function of NYHA classification. Class-specific utility decrements were applied to age- and gender-stratified utility scores from a US reference populationCitation18. Compared with NYHA Class I patients, the utility values were 0.071 and 0.161 lower for NYHA Class II and NYHA Class III/IV patients. The baseline utilities for NYHA Classes I, II, and III/IV were 0.90, 0.83, and 0.74 after adjusting for age (60 years old) and gender. The model then dynamically generated a utility value per patient per cycle based on the aforementioned variables and NYHA Class allocation. An additional utility decrement of 0.1 was associated with a HF-related hospitalization event.
Probabilistic sensitivity analysis
Probabilistic sensitivity analyses (PSA) were performed to understand the impact of parameter uncertainty on the model results. For each parameter, 95% confidence intervals (CIs) were employed to inform the uncertainty estimate. Each of the 689 patients of BLOCK-HF were run through the model a hundred times for a total of 68,900 simulations. Beta distributions were used to quantify uncertainty in utility values and for uncertainty in device longevity and cost parameters, log-normal distributions were utilized. Relationships between coefficients in all regression equations were maintained within the PSA via the use of multinomial distributions.
Results
Extrapolation of clinical endpoints
All-cause mortality
A full list of the 12 derived prediction models is presented in Supplementary Table S3. After correcting for the impact of all other covariates, the impact of BiV on all-cause mortality was consistent across all statistical models, with the relative risk reduction (RRR) values ranging from 0.78–0.80. NYHA Class was consistently the single greatest predictor of mortality, with the RRR for NYHA Class III/IV patients being between 5–8-times higher than for NYHA Class I patients. The corresponding rate for NYHA Class II was approximately twice that predicted for NYHA Class I.
The predicted proportion of patients who survived during years 0–10 post-implant are presented in , with the highest median survival (RV and BiV) being observed in patients with a defibrillator indication (RV-D and BiV-D) and the lowest in those without (RV-P and BiV-P). The predicted lifetime mean survival for all patients is reported in , with BiV patients being predicted to live 10.9% longer than RV patients (7.52 years vs 6.78 years, respectively). BiV-D offered a 10.6% improvement in survival compared with RV-D, and BiV-P an 8.8% improvement compared with RV-P.
Figure 2. Predicted overall survival values (all patients only). (a) Whole trial population; (b) Defibrillator sub-group; (c) Pacemaker sub-group.
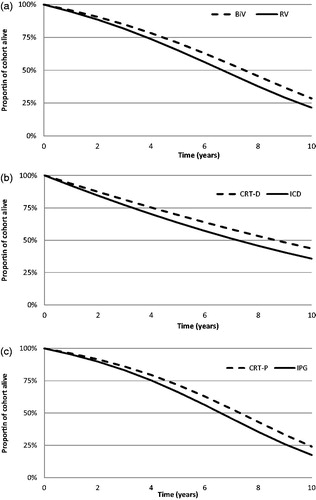
Table 1. Predicted survival estimates and lifetime event counts (per-person, “all-patients”, defibrillator, and pacing sub-groups).
Disease progression
The multinomial models used to predict NYHA Class over time in each of the three patient groups (all patients, defibrillator patients, and non-defibrillator patients) are reported in Supplementary Table S4. In the “All-Patient” analysis, BiV pacing resulted in more patients being in NYHA Class I than NYHA Classes II/III/IV, compared with RV. The NYHA Class III/IV model also achieved statistical significance (Supplementary Table S4), suggesting that BiV pacing delays disease progression. The magnitude of this effect lessened over time, leading to an overall modest effect that was consistent throughout all sub-groups.
HF-related hospitalizations, emergency department visits, and clinic visits
Poisson models were identified as the most appropriate approach to model HF hospitalizations in all three patient groups (all patients, defibrillator patients, and non-defibrillator patients), with negative binomial models selected to quantify emergency department and clinic visits. Full risk equations are reported in Supplementary Tables S5 and S6. The significant predictors were NYHA class and previous HF hospitalization. NYHA classification had a consistently significant impact on HF-related hospitalization rates. The rate observed in NYHA Class I patients was approximately half that of NYHA Class II patients, whereas NYHA Class III/IV rates were ∼ 2.5-times higher than Class II. This effect was maintained when the impact of a previous hospitalization was also taken into consideration. The HF hospitalization rate in patients who had a previous event was 3.5–4.5-times higher than in those who did not (after adjusting for other baseline covariates). Finally, BiV therapy had an additional modest impact on the rate of these hospitalizations across all patient sub-groups. Although BiV usage was not a significant predictor, the result still shows a modest impact of BiV usage on the rate of HF hospitalization (Relative Risk Ratio (RRR) = 0.93 for all patients; RRR = 0.97 for defibrillator patients; RRR = 0.91 for pacemaker patients).
BiV pacing had a positive and significant impact on the rate of emergency department visits in all patients, except for patients with a defibrillator indication. In addition, BiV pacing was also associated with a directional reduction in clinic visit rates. NYHA Class was a highly significant predictor of emergency department visits (all-patient and device sub-groups) and clinic visits in the all-patient and pacing sub-group (the impact was directional in the defibrillator sub-group).
The predicted number of lifetime HF hospitalization events, emergency department visits, and clinic visits are presented in . Taking survival into consideration (7.52 years for BiV vs 6.78 years for RV), it was predicted that patients receiving BiV therapy would, on average, experience a higher number of HF hospitalization events than those receiving RV therapy alone, even though the absolute difference in event counts between the two groups was small.
Economic results
Model “base-case” results
Treatment with BiV pacing offers patients 0.41 additional discounted QALYs at a discounted cost to the US healthcare system of $12,537 and an ICER of $30,860/QALY gained. On a LY gained basis, BiV pacing offers 0.55 additional discounted LYs, leading to an ICER of $22,700 per LY. In patients with a defibrillation indication, the magnitude of additional benefit from BiV-D compared with RV-D is 0.56 QALYs, with an additional cost of $18,067, yielding an ICER of $32,195/QALY gained. BiV therapy alone conferred a benefit of 0.35 QALYs, and, due to the incremental cost being smaller ($3,010), the lowest ICER is generated ($8,492/QALY gained). The predicted lifetime cost-effectiveness results for all patients in each of the three patient groups are presented in .
Table 2. “Base-case” cost-effectiveness analysis results (per-person, “all-patients”, defibrillator, and pacing sub-groups).
Among all sub-groups, the highest ICER observed was $41,383 (BiV-D vs RV-D in NYHA Class I patients) and the lowest $10,704 (BiV-P vs RV-P in NYHA Class II patients). Within each of the three patient groups, ICERs were similar regardless of the degree of baseline AV block, baseline LVEF categorization, baseline age (expressed as ≤65 years or >65) and sex ().
Table 3. Clinical characteristic sub-group analyses: incremental cost-effectiveness ratios (ICERs) on an additional cost ($) per quality-adjusted life year (QALY) gained.
Sensitivity analyses
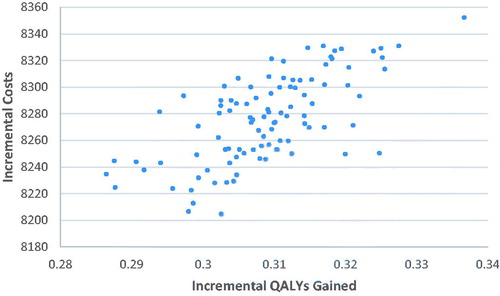
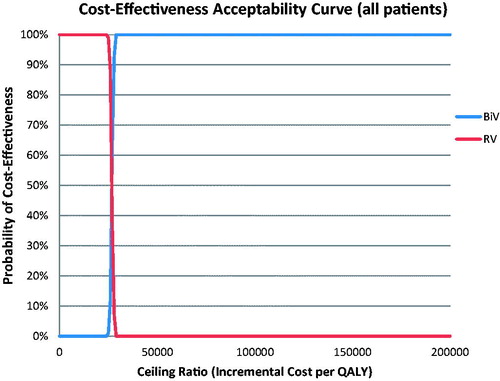
Probabilistic results, from the mean of 1,000 simulations, are detailed in . Treatment with BiV pacing offered a mean benefit of 0.31 QALYs (95% CI = 0.29–0.33) per patient vs RV pacing. Mean additional costs were $8,276 (95% CI = $8,217–$8,330), resulting in $26,816/QALY gained (95% CI = $25,487–$28,485). No simulations exceeded the US acceptability threshold of $50,000/QALY gained. The cost-effectiveness plane with the PSA results plotted is provided in . The cost-effectiveness acceptability curve (CEAC) for BiV/RV pacing is provided in .
Table 4. Probabilistic cost-effectiveness results (per-person, “all-patients”).
Discussion
Analysis of the BLOCK-HF trial data demonstrated BiV pacing to be cost-effective compared to RV pacing, across all patient sub-groups and device types, with ICERs well below the cost-effective threshold of $50,000 per QALY gainedCitation19.
The cost-effectiveness of BiV using CRT in “traditional” indications has been examined previously, and our current findings indicate a similar level of cost-effectiveness. Specifically, a data analysis of the COMPANION study generated ICERs of $19,600/QALY [CRT-P vs optimal medical therapy (OMT)] and $43,000/QALY (CRT-D vs OMT)Citation13. In addition, the MADIT-CRT and RAFT trials reported ICERs for CRT-D vs ICD of $58,330/QALY and $33,593/QALY, respectivelyCitation14,Citation15. However, the MADIT-CRT results were generated using a truncated time horizon of 4 years and assumed a cost perspective different to ours, making comparisons more difficult.
In addition to the higher inherent cost of BiV devices, there are other potential costs associated with BiV pacing, such as AEs related to the LV lead and shorter battery lifespan. However, the sensitivity analyses indicated that only battery duration altered the results, without a decision-altering impact on cost-effectiveness. The cornerstone of the analysis was the NYHA Class allocation across time, since all other outputs (apart from survival) depended on them. This was appropriate because: (a) BLOCK-HF had a minimal amount of missing NYHA Class allocation data (only 16 data-points out of 2,130 in 12 out of 691 subjects), (b) NYHA Class assessments were performed by blinded personnel, and (c) NYHA Class was associated with statistically significant differences in all healthcare utilization rates.
The current analysis projected an ∼10% increase in survival across the patient population studied. Notably, these increases were more pronounced in the lower baseline risk sub-groups (e.g. NYHA Class I, 1st degree AV block), which benefited the most from a prevention strategy. These additional LYs raised overall patient healthcare utilization (and thus payer-incurred costs), an effect highlighted when truncating the time. The approximate 62% cost reduction (from $30,860/QALY gained to $11,869/QALY gained) is explainable by the very few patient deaths by the end of the fifth year (thus no additional survival), and the infrequent need for battery replacements.
It is noticed that the projected lifetime HF hospitalization was slightly higher for BiV after taking longer survival into consideration (7.52 years for BiV vs 6.78 years for RV), although our statistical model based on the trial data shows BiV usage marginally reduced HF hospitalization (RRR = 0.93 for all patients) compared to RV only. This is because the longer a patient survives, the higher chance that the patient could experience HF events. In fact, we didn’t see much difference in terms of HF-related hospitalization between the two arms across the lifetime horizon (0.95 for BiV vs 0.94 for RV).
Despite this, we have likely under-estimated the cost-effectiveness of BiV pacing in patients with AV block. First, our analyses were based on HF-related event rates observed in the BLOCK-HF trial. However, randomized controlled trials in this space have consistently reported lower HF-hospitalization rates than in the “real-world”. A recent registry-based study reported a 3-year cumulative incidence of 59.4% in cardiovascular-related hospitalizations among the 61.1% surviving CRT-D recipients who received a device at an age similar to that of the BLOCK-HF patientsCitation20. BLOCK-HF observed a 47.2% cumulative incidence of broadly equivalent events, over a mean of 37 months. Similarly, battery longevity is likely to continue to increase. Applying current expected longevities to our model will result in lower ICERs. Second, patient crossover was substantial in BLOCK-HF. In the control arm (RV pacing), 86 out of 342 (24.56%) patients crossed over to BiV pacing during the trial. BLOCK-HF as well as our analyses were performed under an intention-to-treat design, likely under-estimating the effect of BiV pacing. Third, a number of patients had new onset atrial fibrillation (AF) in the trial; all of these patients were kept in the analysis in their respective arms, regardless of the occurrence of AF. AF may have impact on the efficacy of BiV pacing in these patients, and thus affects the magnitude of our results. Excluding those patients would likely allow us to report better results than those reported here.
Our cost-accounting assumptions tended to be conservative. AEs that occurred during a replacement hospitalization would normally receive minimal additional payment from Medicare. We have accounted for them as fully paid additional HF-related hospitalizations. When calculating AE rates, we assumed that the risk of an AE occurring during the full trial follow-up duration applied to a single replacement event. This constituted an over-estimation of AE-related costs and led to higher ICERs.
Limitations
This study is characterized by several limitations. Pertaining to HF-related event rates, self-containment in a trial may lead to lower reported AE rates. We used an infection risk per generator replacement of 0.9%, as observed in the BLOCK-HF study. A recent systematic review and meta-analysisCitation21 reported this rate to be in the range of 2–3% for CRT devices. In addition, LV lead-related complications were assumed to be zero in patients randomized to the non-BiV arm since all patients received BiV devices. This may introduce a bias by under-estimating the complication rate in the RV arm. However, this assumption would have skewed the data in favor of the RV arm and weakened the overall findings of the study. Furthermore, we believe that our sensitivity analyses scenarios effectively managed the risk of cost-effectiveness thresholds violations, since an increase in AE rates to nearly implausible levels had little impact on ICERs.
The battery longevity calculations were based on data sourced outside BLOCK-HF. The CCAD represents the largest single source of device battery data among all the manufacturers. Since similar devices are sold in the UK and the US, we believe that these data are applicable to a US-based analysis. Time-to-event curves were only used for the first implant, whereas subsequent implants were assessed on a fixed expiration rate per month based on median longevities. Despite this apparent simplification, we believe that the additional effort to create a model with device memory (discrete event simulation) was not justified by the relatively few replacements expected.
Health-related quality-of-life (HRQoL) was assessed in BLOCK-HF via the commonly used Minnesota Living with Heart Failure (MLWHF) questionnaire and the patient global assessmentCitation22. Neither instrument has been weighted to become a translatable healthcare utility that can be used in QALY calculations. Other analysesCitation23 were able to link MLWHF data with QALY because they also included EQ-5D. BLOCK-HF only measured the MLWHF scores for QoL, and thus used external literature to source the required inputs. The underlying data informing the publication usedCitation17 are sourced from the EPHESUS studyCitation24, a population distinct from the current study. Nevertheless, because sensitivity analyses showed little impact of the utility scores themselves, we felt this was a valid methodology.
Finally, our analytic approach was based—to the maximum extent possible—on trial-collected data that were used to generate risk equations informing long-term predictions. External data were limited to the necessary costs, healthcare utility, and battery longevity. This approach maximized internal validity of the analyses, but potentially limited transferability of our findings to healthcare settings different from the US.
Conclusions
Analysis of individual patient data in the BLOCK-HF trial revealed that BiV pacing, combined with a defibrillator in indicated patients, provides a cost-effective use of resources and value-for-money to the US Medicare program.
Transparency
Declaration of funding
The BLOCK-HF trial was funded in its entirety by Medtronic plc. ICON/Oxford Outcomes was commissioned by Medtronic to conduct this analysis. Medtronic played no role in the actual analyses apart from providing directional and explanatory input when needed.
Declaration of financial/other relationships
ESC has received consulting fees from Medtronic plc and Boston Scientific Corporation. MGSJS has no financial or other relationships to declare. SM, MKS, and AP are employees of ICON/Oxford Outcomes. SIT is an employee of, and owns shares in, Medtronic plc. XL, KLPV, and AAL are employees of Medtronic plc. ABC has received consulting fees from Medtronic plc, honoraria from Medtronic plc and St. Jude Medical Inc., and participates in a St. Jude Medical Inc. Advisory Board. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
No assistance in the preparation of this article is to be declared.
Supplemental data for this article is available online at https://doi.org/10.1080/13696998.2019.1652184.
Download MS Word (60.6 KB)References
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492.
- Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281–2329.
- Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) trial. JAMA. 2002;288:3115–3123.
- Lamas GA, Lee KL, Sweeney MO, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002;346:1854–1862.
- Curtis AB. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;369:579.
- Curtis AB, Adamson PB, Chung E, et al. Biventricular versus right ventricular pacing in patients with AV block (BLOCK HF): clinical study design and rationale. J Cardiovasc Electrophysiol. 2007;18:965–971.
- Linde C, Mealing S, Hawkins N, et al. Cost-effectiveness of cardiac resynchronization therapy in patients with asymptomatic to mild heart failure: insights from the European cohort of the REVERSE (Resynchronization Reverses remodeling in Systolic Left Ventricular Dysfunction). Eur Heart J. 2011;32:1631–1639.
- National Institute for Health and Care Excellence (NICE): guide to the methods of technology appraisal; 2013 [cited 2015 May 11]. Available from: https://www.nice.org.uk/guidance/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.
- Khazanie P, Liang L, Qualls LG, et al. Outcomes of medicare beneficiaries with heart failure and atrial fibrillation. JACC Heart Fail. 2014;2:41–48.
- Academy of Managed Care Pharmacy (AMCP): a format for submission of clinical and economic evidence in supprt of formulary consideration. The AMCP format for formulary Submissions: Version 4.0; 2016 April [cited 2019 March 8]. Available from: https://www.amcp.org/FormatV4.
- Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices–overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Med Decis Making. 2012;32:667–677.
- National Institute for Health and Care Excellence (NICE): technology appraisal number TA314. Final appraisal determination (FAD); 2014 [cited 2015 May 11]. Available from: https://www.nice.org.uk/guidance/ta314.
- Feldman AM, de Lissovoy G, Bristow MR, et al. Cost effectiveness of cardiac resynchronization therapy in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial. J Am Coll Cardiol. 2005;46:2311–2321.
- Noyes K, Veazie P, Hall WJ, et al. Cost-effectiveness of cardiac resynchronization therapy in the MADIT-CRT trial. J Cardiovasc Electrophysiol. 2013;24:66–74.
- Wells GA, Douglas C, Nichol G, et al. Cost-effectiveness of cardiac resynchronization therapy (CRT-D) for mild to moderate heart failure. Heart Rhythm Society Congress; 2012 [cited 2015 May 11]. Available from: http://ondemand.hrsonline.org/media/HRS2012AM_4/AB32/7766/7766.pdf.
- van Gelder BM, Meijer A, Bracke FA. The optimized V-V interval determined by interventricular conduction times versus invasive measurement by LVdP/dtMAX. J Cardiovasc Electrophysiol. 2008;19:939–944.
- Gohler A, Geisler BP, Manne JM, et al. Utility estimates for decision-analytic modeling in chronic heart failure–health states based on New York Heart Association classes and number of rehospitalizations. Value Health. 2009;12:185–187.
- Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45:1162–1170.
- Russell LB, Gold MR, Siegel JE, et al. The role of cost-effectiveness analysis in health and medicine. Panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1172–1177.
- Khazanie P, Hammill BG, Qualls LG, et al. Clinical effectiveness of cardiac resynchronization therapy vs medical therapy alone among patients with heart failure: an analysis of the ICD and ADHERE national registries. Circ Heart Fail. 2014;7:926–934.
- Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace. 2015;17:767–777.
- Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail. 2001;7:176–182.
- Calvert MJ, Freemantle N, Yao G, investigators C-H, et al. Cost-effectiveness of cardiac resynchronization therapy: results from the CARE-HF trial. Eur Heart J. 2005;26:2681–2688.
- Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321.