Abstract
Background: Guidelines recommend febrile neutropenia (FN) prophylaxis following myelotoxic chemotherapy with either daily injections of filgrastim (Neupogen®) or biosimilar filgrastim-sndz (Zarzio/Zarxio®), single-injection pegfilgrastim (Neulasta®), or pegfilgrastim administered through an on-body injector (PEG-OBI; Neulasta® Onpro®). PEG-OBI failure rates up to 6.9% have been reported, putting patients at incremental risk for FN and FN-related hospitalization. Our objective was to estimate, from a US payer perspective, the incremental costs of FN hospitalizations and the total incremental costs associated with PEG-OBI prophylaxis at varying device failure rates over assured FN prophylaxis with daily injections of filgrastim or filgrastim-sndz or a single injection of pegfilgrastim.
Methods: Cost simulations comparing prophylaxis with PEG-OBI at failure rates of 1–10% versus assured prophylaxis in cycle 1 of chemotherapy were performed for panels of 10,000 patients with lung cancer treated with cyclophosphamide, doxorubicin, and etoposide (1 analysis) or non-Hodgkin lymphoma (NHL) treated with CHOP or CNOP (2 analyses). Daily injection scenarios were 4.3, 5, and 11 injections for lung cancer and 5, 6.5, and 11 for NHL. The analyses are from the US payer perspective.
Results: For lung cancer, the total incremental cost of PEG-OBI prophylaxis at varying failure rates and durations ranged from $6,691,969‒$31,765,299 over filgrastim and $18,901,969‒$36,538,299 over filgrastim-sndz. For NHL, in scenario 1, the total incremental costs ranged from $6,794,984‒$30,361,345 over filgrastim and $19,004,984‒$35,911,345 over filgrastim-sndz; in scenario 2, the incremental costs ranged from $7,003,657‒$32,448,067 over filgrastim and $19,213,657‒$37,998,067 over filgrastim-sndz.
Conclusions: In this simulation, the incremental costs of FN-related hospitalization due to PEG-OBI failure in cycle 1 compared to assured prophylaxis with reference pegfilgrastim, reference filgrastim, and biosimilar filgrastim-sndz varied depending upon the PEG-OBI failure rate and the alternative G-CSF prophylaxis option. Biosimilar filgrastim-sndz offers the greatest cost-efficiency.
Introduction
Febrile neutropenia (FN) is a potentially life-threatening complication in cancer patients undergoing myelosuppressive chemotherapyCitation1–6. FN often results in hospitalization, can jeopardize anti-neoplastic treatment caused by dose delays, dose reductions, or cycle cancellations, is associated with increased morbidity and mortality, impairs quality of life, and increases health care costsCitation7–9. In the US in 2012, 91,560 adult patients were hospitalized for cancer-related neutropenia, with a mean length of stay of 9.6 days and mean hospital cost of $24,770Citation10.
Daily-injection filgrastim (Neupogen®, Amgen, Thousand Oaks, CA, USA.) and single-injection pegfilgrastim (Neulasta®, Amgen, Thousand Oaks, CA, USA.) are granulocyte colony-stimulating factors (G-CSF) that stimulate neutrophil production in the bone marrowCitation11. Following the patent expirations for reference filgrastim, the biosimilar agent filgrastim-sndz was approved in Europe in 2008 and in the US in 2015. Evidence-based guidelines, including those by the ASCOCitation7, the NCCNCitation8, and the EORTCCitation9 recommend that, generally, prophylaxis with G-CSFs be initiated no sooner than 24 hours and up to 72-96 hours following chemotherapy.
There may be circumstances, however, in which the 24-hour time lag cannot be observed or is inconvenient for patients. In 2015, an on-body injector device (Neulasta®, Onpro®, Onpro, Amgen, Thousand Oaks, CA, USA.) was approved, which is applied the same day of chemotherapy and administers pegfilgrastim approximately 27 hours later. In December 2017, the US FDA label for NeulastaCitation12 was amended in response to accounts of the device not performing as intended. Studies have reported failure rates between 1.7–6.9%Citation13–16. Being a single-administration formulation, a missed or partial dose puts patients undergoing myelotoxic chemotherapy at risk of levels of FN and FN-related hospitalizations that exceed the rates associated with assured prophylaxis with pegfilgrastim or filgrastim or available G-CSF biosimilars.
We report here on cost simulation analyses, from the perspective of a US payer, of FN prophylaxis in cycle 1 of chemotherapy with the pegfilgrastim on-body injector (PEG-OBI) at varying device failure rates compared to assured prophylaxis with single-injection pegfilgrastim and different scenarios of daily-injection reference or biosimilar filgrastim. Specifically, for two US panels of 10,000 US patients in cycle 1 of chemotherapy, we estimated (1) the 2018 incremental costs of FN-related hospitalizations associated with varying failure rates of the PEG-OBI device, and (2) the 2018 total incremental costs of PEG-OBI prophylaxis at varying device failure rates.
We chose to apply these cost simulation analyses to lung cancer and non-Hodgkin lymphoma (NHL), two highly incident and prevalent cancers that are commonly treated with myelotoxic chemotherapy and are therefore associated with significant FN risk. In 2018, there were an estimated 234,030 new cases of lung and bronchus cancer in the US, or 13.5% of all new cancer cases; and an estimated 154,050 deaths attributable to this cancer, or 25.3% of all cancer deaths. The estimated 5-year survival rate is 18.6%Citation17. In that same year, there were an estimated 74,680 new cases of NHL, or 4.3% of all new cancer cases; and an estimated 19,910 deaths, or 4.3% of all cancer deaths. More positively, the estimated 5-year survival rate is 71.4%Citation18.
Methods
Model
We specified a general simulation model for 2018 that considered PEG-OBI failure rates of 1% to 10% versus assured prophylaxis (failure rate of 0%) in cycle 1 of chemotherapy. Applying cost inputs and published data as detailed below, the model estimated, for a panel of 10,000 patients and from a US payer perspective, the number of patients at risk for FN-related hospitalization due to PEG-OBI failure and the incremental costs associated with such device failure. These costs were compared subsequently to the costs of assured prophylaxis with pegfilgrastim and various published daily regimens of reference filgrastim and biosimilar filgrastim-sndz to estimate the total incremental costs associated with varying rates of PEG-OBI failure. The 1% to 10% device failure rates reflect the published 1.7 to 6.9% failure ratesCitation13–16 with an additional margin to accommodate any upper-bound imprecision in these point estimates.
Assumptions
The following assumptions governed the cost simulation analyses:
All G-CSF treatments were considered to have equal efficacy and safety. This assumption was based on published evidence including that reference filgrastim is superior to placebo for chemotherapy-induced neutropeniaCitation19. Phase III clinical trials also show that pegfilgrastim is non-inferior to reference filgrastimCitation20,Citation21. Biosimilar filgrastim-sndz is equivalent to reference filgrastimCitation22–24. Therefore, by extension, biosimilar filgrastim-sndz is considered equivalent to pegfilgrastim. Despite meta-analysis attemptsCitation25–27 to imply superiority of pegfilgrastim over standard filgrastim, we have argued elsewhereCitation28 that the primary trial evidence is from non-inferiority trials, that meta-analyses of non-inferiority trials do not establish superiority (even when phase II trials are included), and that there is no phase III evidence of the clinically relevant superiority of pegfilgrastim. This is also consistent with Klastersky and Awad’s conclusionCitation29 that pegfilgrastim ‘is definitely not inferior to classical’ filgrastim, but ‘whether it might be more superior […] cannot be derived from the presently available data, but it seems unlikely that major differences do exist’.
The application of the PEG-OBI device and all G-CSF injections were administered by a clinician, and therefore it was assumed that Current Procedural Terminology (CPT) reimbursement is the true cost of drug administration.
Daily filgrastim regimens (reference and biosimilar) were modeled under 3 scenarios. In a first scenario, we applied the real-world median duration data from the observational MONITOR-GCSF study: 5 days for all patientsCitation30, for patients with non-small-cell lung cancer [data on file], and for patients with NHL [data on file]. In a second scenario, we used the mean daily duration reported from a US healthcare claims database: 4.3 days for lung cancer patients and 6.5 days for NHL patientsCitation31. A third scenario was based on the common 11 days derived from the initial filgrastim trials and corresponding to the principle of treating through the absolute neutrophil count nadir.
The incremental risk of FN-related hospitalization in lung cancer patients was extrapolated from a phase III randomized trial in small cell lung cancer patients receiving chemotherapy with cyclophosphamide, doxorubicin, and etoposideCitation32. In this study, the rate of FN-related hospitalization with G-CSF support was 10.0% and the rate of FN-related hospitalization without G-CSF support was 23.5%. Hence, we assumed that the incremental risk of FN-related hospitalization associated with PEG-OBI failure was the difference of 13.5%.
The incremental risk of FN-related hospitalization in NHL patients was extrapolated using FN-related hospitalization data from two published sources. The first was a historical case series study conducted in 12 diverse practice settings in the US among NHL patients treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)Citation33. This study reported an FN-related hospitalization rate with G-CSF support of 13.25% compared to 23.28% without G-CSF support. Thus, the incremental risk of FN-related hospitalization associated with PEG-OBI failure was assumed to be the difference of 10.03%. The second source was a randomized trial with bifactorial design including either CHOP or cyclophosphamide, mitoxantrone, vincristine, and prednisone (CNOP) with or without G-CSF supportCitation34. In this study, the FN-related hospitalization rate was 33% with versus 50% without G-CSF support. The incremental risk of FN-related hospitalization associated with PEG-OBI failure was assumed to be 17%.
Cost inputs
Cost estimates and sources are detailed in . We used the average selling price (ASP) for the first quarter of 2018 from Medicare Part B drug payment limitsCitation35. Drug administration costs were per the 2018 CPT reimbursementCitation36. For FN-related hospitalization costs in lung cancer and NHLCitation10, 2012 costs were adjusted to 2018 pricing based upon the US Bureau of Labor Statistics Consumer Price Index for Medical CareCitation37.
Table 1. Cost inputs.
Simulation analyses
We specified bespoke simulation models that included modules (1) to estimate prophylaxis costs; (2) to estimate the additional patients at risk for FN-related hospitalization under varying PEG-OBI device failure rates and the associated incremental costs of hospitalization; (3) to calculate the differential total costs of failure-rate adjusted prophylaxis with PEG-OBI relative to assured prophylaxis with single-injection pegfilgrastim or daily injection regimens of varying duration with reference or biosimilar filgrastim-sndz; and (4) to apply these estimates to (a) the lung cancer case, and (b) the two NHL cases.
Results
Prophylaxis costs
The costs of prophylaxis with the PEG-OBI device, single-injection pegfilgrastim, and various durations of reference and biosimilar filgrastim are summarized in . Being single administrations, the costs of PEG-OBI and pegfilgrastim are constant, the difference between both being minor variations in reimbursement for administration. The variations in costs of prophylaxis for filgrastim and filgrastim-sndz are a function of drug costs and number of injections administered.
Table 2. Cycle 1 prophylaxis costs for panels of 10,000 lung cancer and 10,000 NHL patients.
Cost-simulation: lung cancer
and present the additional patients at risk for FN-related hospitalization under varying failure rates of the PEG-OBI device and the associated incremental costs of FN-related hospitalization for a panel of 10,000 lung cancer patients. and further review the total incremental costs of prophylaxis with PEG-OBI compared to pegfilgrastim and various durations of reference and biosimilar filgrastim-sndz under varying failure rates. Patients at incremental risk of FN-related hospitalization ranged from 13.5 at failure rate of 1% to 135 at failure rate of 10%, with corresponding incremental costs ranging from $197,269 to $1,972,689. The total incremental costs of PEG-OBI prophylaxis relative to single-injection pegfilgrastim under varying failure rates ranged from $(175,931) to $1,599,489 (the negative being an artifact of the lower reimbursement rate for administration of PEG-OBI). Compared to prophylaxis with reference filgrastim, the incremental total cost of prophylaxis with PEG-OBI under varying failure rates and across three different duration scenarios ranged from $6,691,969 (11 days at 1% failure rate) to $31,765,299 (4.3 days at 10% failure rate). The incremental total cost of prophylaxis with PEG-OBI under varying failure rates and across duration scenarios over prophylaxis with biosimilar filgrastim-sndz ranged from $18,901,969 (11 days at 1.0% failure rate) to $36,538,299 (4.3 days at 10% failure rate).
Figure 1. Incremental FN-related hospitalization related to PEG-OBI failure: number of patients at risk and hospitalization costs. Abbreviations. FN, febrile neutropenia; FN-HOSP risk, risk hospitalization due to febrile neutropenia; NHL, non-Hodgkin lymphoma; PEG-OBI, pegfilgrastim on-body injector.
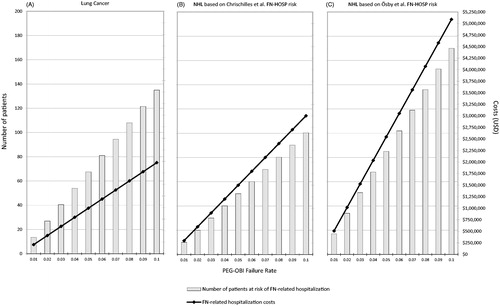
Figure 2. Incremental costs of PEG-OBI vs PEG, reference filgrastim and biosimilar filgrastim-sndz (includes cost of prophylaxis + FN-related hospitalization related to PEG-OBI failure) in lung cancer patients (A, B, and C, respectively) and in NHL patients (D, E, F, respectively). Abbreviations. NHL, non-Hodgkin lymphoma; PEG, pegfilgrastim; PEG-OBI, pegfilgrastim on-body injector; USD, US dollars.
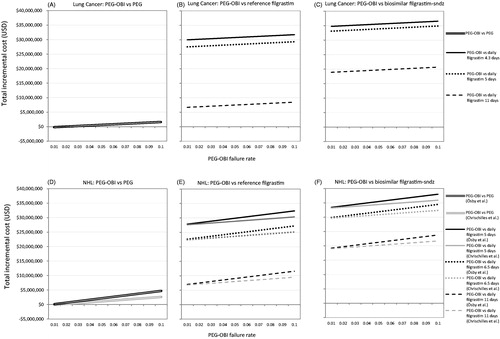
Table 3. Lung cancer: incremental cycle 1 costs of FN-related hospitalization due to PEG-OBI failure and total incremental cycle 1 costs of PEG-OBI prophylaxis compared to other G-CSFs.
Cost-simulation: non-Hodgkin lymphoma
Per Chrischilles et al. FN hospitalization risk
Applying the incremental hospitalization-risk as estimated from Chrischilles et al.Citation33, and present the additional patients at risk for FN-related hospitalization under varying failure rates of the PEG-OBI device and the associated incremental costs of FN-related hospitalization for a panel of 10,000 NHL patients. and ) further detail the total incremental costs of prophylaxis with PEG-OBI compared to pegfilgrastim and various durations of reference and biosimilar filgrastim under varying failure rates. Patients at incremental risk of FN-related hospitalization ranged from 10.03 at failure rate of 1% to 100.30 at failure rate of 10%, with corresponding incremental costs ranging from $300,284 to $3,002,845. The total incremental costs of PEG-OBI prophylaxis relative to single-injection pegfilgrastim under varying failure rates ranged from $(72,916) to $2,629,645 (this negative also being an artifact of the lower reimbursement rate for administration of PEG-OBI). Compared to prophylaxis with reference filgrastim, the incremental total cost of prophylaxis with PEG-OBI under varying failure rates and across three different duration scenarios ranged from $6,794,984 (11 days at 1.0% failure rate) to $30,361,345 (5 days at 10% failure rate). The incremental total cost of prophylaxis with PEG-OBI under varying failure rates and across duration scenarios over prophylaxis with biosimilar filgrastim-sndz ranged from $19,004,984 (11 days at 1% failure rate) to $35,911,345 (5 days at 10% failure rate).
Table 4. Non-Hodgkin lymphoma: incremental cycle 1 costs of FN-related hospitalization due to PEG-OBI failure and total incremental cycle 1 costs of PEG-OBI prophylaxis compared to other G-CSFs.
Per Ösby et al. FN hospitalization risk
Similarly, using the incremental hospitalization-risk as estimated from Ösby et al.Citation34, and present the additional patients at risk for FN-related hospitalization under varying PEG-OBI failure rates and the associated incremental costs of FN-related hospitalization for a panel of 10,000 NHL patients. and ) exhibit the total incremental costs of prophylaxis with PEG-OBI compared to pegfilgrastim and various durations of reference and biosimilar filgrastim under varying failure rates. Patients at incremental risk of FN-related hospitalization ranged from 17 at failure rate of 0.1 to 170 at failure rate of 10%, with corresponding incremental costs ranging from $508,957 to $5,089,567. The total incremental costs of PEG-OBI prophylaxis relative to single-injection pegfilgrastim under varying failure rates ranged from $135,757 to $4,716,367. Compared to prophylaxis with reference filgrastim, the incremental total cost of prophylaxis with PEG-OBI under varying failure rates and across three different duration scenarios ranged from $7,003,657 (11 days at 1% failure rate) to $32,448,067 (5 days at 10% failure rate). The incremental total cost of prophylaxis with PEG-OBI under varying failure rates and across duration scenarios over prophylaxis with biosimilar filgrastim-sndz ranged from $19,213,657 (11 days at 1% failure rate) to $37,998,067 (5 days at 10% failure rate).
Discussion
The PEG-OBI has the clinical benefit of providing prophylaxis with the convenience for the patients of not requiring a return visit to the clinic the day after chemotherapy. However, with point estimates of device failure rates as high as 6.9%, and 10% not being an unlikely correction for relative imprecision, a significant number of patients are at risk for receiving no or insufficient G-CSF prophylaxis with a corresponding rise in the likelihood of experiencing an FN episode and requiring hospitalization. In addition, with almost two decades of clinical experience with daily filgrastim injections, clinical practice has evolved from treating through the absolute neutrophil nadir for about 11 days to shorter regimens of 4 to 7 days. Weycker et al.Citation31 related a mean of 4.3 filgrastim injections for patients with lung cancer and 6.5 injections for patients with NHL, though with a slight increase in FN risk. The European MONITOR-GCSF studyCitation30 of biosimilar filgrastim-sndz reported a median of 5 daily injections for lung cancer and NHL with no increase in FN rateCitation30,Citation38. Increasingly, US payers may decline to reimburse for prophylaxis with pegfilgrastim and authorize up to 7 days of daily filgrastim injections. Against this background of changing FN prophylaxis patterns and the approval of biosimilar versions of filgrastim and pegfilgrastim in the US, the principal findings of the cost simulation analyses reported here are two-fold.
First, in a panel of 10,000 lung cancer patients receiving PEG-OBI, and considering failure rates as high as 10%, up to 135 patients may be at risk of FN and FN-related hospitalization at an additional cost of almost $2 million. Capping the failure rate at the highest reported point estimate of 6.9% would put 93 patients at risk at an additional cost of almost $1.4 million. Depending on the FN hospitalization risk estimate used, in the setting of NHL, between 100 and 170 out of 10,000 patients fitted with the PEG-OBI device would be at risk of experiencing FN at a 10% failure rate, at incremental costs of $3 million and $5.1 million, respectively. At the 6.9% rate, between 69 and 117 PEG-OBI patients would be at risk with incremental costs of $2.1 million and exceeding $3.5 million, respectively.
Second, while one option is to treat instead with single-injection pegfilgrastim, assure prophylaxis, and avoid additional FN episodes, similar effectiveness can be achieved with daily filgrastim injection (though, perhaps, at the expense of patient convenience and comfort). In the setting of lung cancer, using pegfilgrastim instead of the PEG-OBI device yields savings of $1.6 million per 10,000 lung cancer patients. However, treating with an average 4.3 daily filgrastim injections instead of the device, generates savings of almost $32 million if reference filgrastim is used, but $36.5 million if biosimilar filgrastim-sndz is administered. In the setting of NHL and applying the incremental 17% FN risk, assured prophylaxis with pegfilgrastim is associated with savings per 10,000 patients of $4.7 million, over $32 million with reference filgrastim, and about $38 million with filgrastim-sndz.
Reconciling these two analyses, the economic dynamics of the known problem of failure of the PEG-OBI device, the loss of prophylaxis, and the increase in FN risk versus prophylaxis with injected pegylated or standard filgrastim result in a triple benefit: with injection, prophylaxis is assured, FN outcomes are better, and costs are lower. To this the extra savings should be added if reference filgrastim is used; and, especially, the additional 15% to 18% savings generated by prophylaxis with biosimilar filgrastim-sndz.
Weycker et al.Citation31 provided evidence linking real-world evidence of shorter courses of prophylaxis with reference filgrastim to an increased risk of FN-related hospitalization. However, using data from the MONITOR-GCSF study Aapro et al.Citation38 found no such association with biosimilar filgrastim-sndz in real-world practice. As both filgrastim and filgrastim-sndz are known to be equivalent, this difference between these two studies is unlikely to be pharmacological. Instead it may be a function of the additional 10 years of clinical experience with daily filgrastim between the Weycker et al.Citation31 and Aapro et al.Citation38 reports. In addition, it may also be related to the MONITOR-GCSF finding that prophylaxis patterns deviated from what is recommended by the EORTC guidelinesCitation9. About a quarter of patients received prophylaxis that exceeded these guidelinesCitation39, and this over-prophylaxis was associated with a lower likelihood of FN episodes and FN-related hospitalizationsCitation38.
Our study has limitations. We used publicly available cost inputs. Few trials reported the data needed to extrapolate incremental FN and FN-related hospitalization risk, and we were obliged to use older trials. Available PEG-OBI failure rates did not include confidence intervals, hence we adopted an arbitrary 10% as the upper limit. The ASP reflects Medicare reimbursement; however, the equivalent metric for private payers is not public. Our analyses did not include indirect costs. There is the possibility that prophylaxis with filgrastim for short durations holds unknown risks. Clinical experience and prophylaxis effectiveness need to be better understood. Our analyses were limited to cycle 1 of chemotherapy.
Our study suggests areas for future research. The US payer perspective adopted in our simulations may merit from replication from a health system perspective to support internal decision-making by, for instance, Pharmacy and Therapeutics Committees. In addition to focusing on FN-related hospitalization events, simulations are also needed on the differential impact of PEG-OBI device failure on the frequency of chemotherapy-induced neutropenia (especially CIN grades 3 and 4) and FN episodes. As both CIN and FN may result in disruptions to the chemotherapy regimen and impact cancer control, the cost implications of these merit investigation as well.
Conclusion
In this simulation of panels of 10,000 lung cancer and 10,000 NHL patients in the US, the incremental and total costs of FN-related hospitalization due to PEG-OBI failure in cycle 1 compared to assured prophylaxis with reference pegfilgrastim, reference filgrastim, and biosimilar filgrastim-sndz varied depending upon the PEG-OBI failure rate and the alternative G-CSF prophylaxis option. Biosimilar filgrastim-sndz offers the greatest cost-efficiency.
Transparency
Declaration of financial/other interests
AM serves on a Speakers Bureau and Steering Committee for Sandoz, Inc. He was subcontracted by Matrix45 for work on this project. AK is a former employee, and NM and MN are current employees of Hexal AG. KC and SB are employees of Sandoz, Inc. IA and KM are employees of Matrix45, which was contracted by Hexal AG to conduct the simulations. By company policy, employees are prohibited from owning equity in client organizations (except through mutual funds or other independently administered collective investment instruments) or contracting independently with client organizations. Matrix45 provides similar services to those described in this article to other biopharmaceutical companies on a non-exclusivity basis. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors met ICMJE and COPE criteria and contributed substantively to the study as follows: study concept: IA, SB, KC, AK, KM, NM, AM; study design: IA, AK, KM, NM, AM; model development and simulations: IA, KM, AM; review and interpretation of results: IA, SB, KC, AK, KM, NM, MN, AM; development of manuscript: IA, KM, AM; review of the manuscript for intellectual content: SB, KC, AK, NM, MN.
Acknowledgements
Editorial and manuscript management support was provided by Spirit Medical Communications Group Ltd. under funding from Hexal AG. SB is now at Novartis Pharmaceuticals Corporation.
This work was previously presented as poster presentations at: 2018 American Society of Hematology Annual Meeting: AM, AK, NM, KC, SB, KM, IA. Cost simulation for febrile neutropenia hospitalization in the US due to pegfilgrastim on-body injector failure compared to single-injection pegfilgrastim and daily injections with reference and biosimilar in non-Hodgkin lymphoma. Blood 2018;132(Suppl 1):2251. European Society for Medical Oncology (ESMO) Asia 2018 Congress: AM, AK, NM, KC, SB, KM, IA. Cost simulation for the US of febrile neutropenia hospitalization due to pegfilgrastim on-body injector failure compared to single-injection pegfilgrastim and daily injections with reference and biosimilar filgrastim in lung cancer. Annals of Oncology 2018;29(Suppl 9):ix133.
Additional information
Funding
References
- Lalami Y, Paesmans M, Aoun M, et al. A prospective randomised evaluation of G-CSF or G-CSF plus oral antibiotics in chemotherapy-treated patients at high risk of developing febrile neutropenia. Support Care Cancer. 2004;12:725–730.
- Lyman GH, Lyman CH, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005;10:427–437.
- Kuderer N, Dale D, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor or febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158–3167.
- Paessens B, von Schilling C, Berger K, et al. Health resource consumption and costs attributable to chemotherapy-induced toxicity in German routine hospital care in lymphoproliferative disorder and NSCLC patients. Ann Oncol. 2011;22:2310–2319.
- Weycker D, Barron R, Kartashov A, et al. Incidence, treatment, and consequences of chemotherapy-induced febrile neutropenia in the inpatient and outpatient settings. J Oncol Pharm Pract. 2014;20:190–198.
- Weycker D, Li X, Edelsberg J, et al. Risk and consequences of chemotherapy-induced febrile neutropenia in patients with metastatic solid tumors. J Oncol Pract. 2015;11:47–54.
- Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015;33:3199–3212.
- Crawford J, Becker PS, Armitage JO, et al. Myeloid growth factors, Version 2.2017. Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1520–1541.
- Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eu J Cancer. 2011;47:8–32.
- Tai E, Guy GP, Dunbar A, et al. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13:e552–e561.
- Raposo CG, Marin AP, Barón MG. Colony-stimulating factors: clinical evidence for treatment and prophylaxis of chemotherapy-induced febrile neutropenia. Clin Transl Oncol.. 2006;8:729–734.
- Amgen. Neulasta® highlights of prescribing information; 2018 Jun [updated 2019 Apr; cited 2019 May 14]. Available from: https://www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/neulasta/neulasta_pi_hcp_english.ashx.
- Joshi RS, Egbuna OI, Cairns AS, et al. Performance of the pegfilgrastim on-body injector as studied with placebo buffer in healthy volunteers. Curr Med Res Opin. 2017;33:379–384.
- Stuessy P, Sanchez FA, Schober M. Retrospective review of pegfilgrastim on-body injector delivery rates in a large health system [abstract e18273]. J Clin Oncol. 2017;35:e18273.
- Mahler LJ, DiBlasi R, Perez A, et al. On-body injector: an administration device for pegfilgrastim. Clin J Oncol Nurs. 2017;21:121–122.
- Townley C, Porter C, McMullen N. Comparing grade 4 neutropenia associated with pegfilgrastim administered via the Onpro device versus manual injection with a prefilled syringe. J Hematol Oncol Pharm. 2018;8:3397.
- National Cancer Institute. SEER Cancer Stat Facts: Lung and bronchus cancer; [cited 2019 May 14]. Available from: https://seer.cancer.gov/statfacts/html/lungb.html.
- National Cancer Institute. SEER Cancer Stat Facts: Non-Hodgkin lymphoma; [cited 2019 May 14]; Available from: https://seer.cancer.gov/statfacts/html/nhl.html.
- Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–170.
- Holmes FA, O’Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20:727–731.
- Green MD, Koebl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14:29–35.
- Gascón P, Fuhr U, Sorgel F, et al. Development of a new G-CSF product based on biosimilarity assessment. Ann Oncol. 2010;21:1419–1429.
- Blackwell K, Semiglazov V, Krasnozhon D, et al. Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26:1948–1953.
- European Medicines Agency. CHMP Assessment report for Zarzio. EMEA/CHMP/651339/2008; 2008. [cited 2019 May 14]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000917/WC500046528.pdf.
- Pinto L, Liu Z, Doan Q, et al. Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade IV neutropenia and bone pain: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 2007;23:2283–2295.
- Cooper KL, Madan J, Whyte S, et al. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404.
- Wang L, Baser O, Kutikova L, et al. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2015;23:3131–3140.
- McBride A, Campbell K, Bikkina M, et al. Reply: cost-efficiency analyses for the US of biosimilar filgrastim-sndz, reference filgrastim, pegfilgrastim, and pegfilgrastim with on-body injector in the prophylaxis of chemotherapy-induced (febrile) neutropenia. J Med Econ. 2018;21:606–609.
- Klastersky J, Awada A. Prevention of febrile neutropenia in chemotherapy-treated cancer patients: pegylated versus standard myeloid colony stimulating factors. Do we have a choice? Crit Rev Oncol Hematol. 2011;78:17–23.
- Gascón P, Aapro M, Ludwig H, et al. Treatment patterns and outcomes in the prophylaxis of chemotherapy-induced (febrile) neutropenia with biosimilar filgrastim (the MONITOR-GCSF study). Support Care Cancer. 2016;24:911–925.
- Weycker D, Hackett J, Edelsberg JS, et al. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40:402–407.
- Timmer-Bonte JN, deBoo TM, Smit HJ, et al. Prevention of chemotherapy-induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony-stimulating factor in small-cell lung cancer: a Dutch randomized phase III study. J Clin Oncol. 2005;23:7974–7984.
- Chrischilles E, Delgado DJ, Stolshek BS, et al. Impact of age and colony-stimulating factor use on hospital length of stay for febrile neutropenia in CHOP-treated non-Hodgkin’s lymphoma. Cancer Control. 2002;9:203–211.
- Ösby E, Hagberg H, Kvaløy S, et al. CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: results of a Nordic Lymphoma Group randomized trial. Blood. 2003;101:3840–3848.
- Centers for Medicare and Medicaid Services. Medicare Part B drug average sales price. 2018 ASP drug pricing files: April 2018 ASP pricing file; 2018. [cited 2019 May 15]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2018ASPFiles.html.
- Centers for Medicare and Medicaid Services. Details for title: CMS-1676-F; 2018. [cited 2019 May 15]. Available from: www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1676-F.html.
- Official Data Foundation. Medical care priced at $1,000 in 2000 -> $1,882.09 in 2019; 2018. [cited 2019 May 15]. Available from: www.officialdata.org/Medical-care/price-inflation.
- Aapro M, Ludwig H, Bokemeyer C, et al. Predictive modeling of the outcomes of chemotherapy-induced (febrile) neutropenia prophylaxis with biosimilar filgrastim (MONITOR-GCSF study). Ann Oncol. 2016;27:2039–2045.
- Bokemeyer C, Gascón P, Aapro M, et al. Over- and under-prophylaxis for chemotherapy-induced (febrile) neutropenia relative to evidence-based guidelines is associated with differences in outcomes: findings from the MONITOR-GCSF study. Support Care Cancer. 2017;25:1819–1828.
