Abstract
Aims: To examine healthcare resource utilization (HRU) and costs within 12 months after hospitalization for respiratory syncytial virus (RSVH) or unspecified bronchiolitis (UBH) in infants.
Materials and methods: Infants born July 1, 2009–June 30, 2015 were identified in the MarketScan Medicaid and Commercial databases and were assigned to one of three cohorts: RSVH (with/without UBH), UBH, or comparator (no RSVH or UBH). Each infant was identified as pre-term (5 groups) or term (2 groups) based on weeks gestational age (wGA). Index dates were the first admission dates for RSVH or UBH infants and were randomly assigned to comparator infants based on time from birth to index in the RSVH cohort. HRU, all-cause costs, and incremental cost differences between hospitalized and comparator infants were assessed over 12 months post-index with and without the index hospitalization. Results were propensity score weighted to balance pre-index characteristics across hospitalization cohorts.
Results: This study identified 15,872 RSVH infants, 6,081 UBH infants, and 986,087 comparator infants in the Medicaid population and 5,755 RSVH infants, 1,888 UBH infants, and 696,302 comparator infants in the commercial population. HRU in follow-up was greater for RSVH and UBH infants relative to comparator infants in both populations, including hospitalizations (commercial: 7.4%, 11.0%, 1.7%; Medicaid: 12.3%, 15.3%, 3.2%) and emergency department visits (commercial: 33.0%, 33.3%, 17.2%; Medicaid: 65.8%, 68.5%, 51.4%). HRU was highest among RSVH and UBH infants born at <29 wGA. Hospitalized infants had numerically higher follow-up costs than comparator infants, with incremental differences reaching $19,896 among Medicaid UBH infants and $37,417 among commercial RSVH infants.
Limitations: RSV/UB may be miscoded in claims data.
Conclusions: Infants hospitalized for RSV or UB largely had greater subsequent HRU and costs in the first year after index hospitalization than comparator infants. Absolute and incremental follow-up costs relative to comparator infants were highest among infants <29 wGA.
Introduction
Respiratory syncytial virus (RSV) is a common seasonal infection that typically presents as a cold-like upper respiratory infection. In at-risk populations, including infants and the elderly, it may progress to severe lower respiratory complications including bronchiolitis, pnemonia, bronchitis, respiratory failure, and even deathCitation1,Citation2. RSV is responsible for an estimated 50–90% of bronchiolitis hospitalizations among children aged <2 years in the US, with infection rates peaking between December and February depending on regionCitation1,Citation3. Despite decreases in rates of hospitalization and inpatient death between 1997 and 2012, bronchiolitis remains a leading cause of inpatient admissions among infants and children in the US, with roughly 2–3% of infants aged ≤12 months requiring hospitalization each yearCitation4–6.
Certain characteristics, such as pre-term birth, congenital heart disease, and bronchopulmonary dysplasia put infants at increased risk of an RSV-related hospitalizationCitation7. This risk may be mitigated by the prophylactic use of palivizumab, an intramuscular injection administered monthly during the RSV seasonCitation8.
Since 1998, palivizumab has been indicated for the prevention of serious RSV lower respiratory tract disease in pediatric patients that are born prematurely at 35 weeks gestational age (wGA) or less and who are 6 months of age or younger at the beginning of an RSV season, with bronchopulmonary dysplasia (BPD) that required medical treatment within the previous 6 months and who are 24 months of age or younger at the beginning of the RSV season, and with hemodynamically significant congenital heart disease (CHD) and who are 24 months of age or younger at the beginning of RSV seasonCitation9. In 2014, the American Academy of Pediatrics (AAP) stopped recommending palivizumab for otherwise healthy 29–34 wGA pre-term infants, stating they should no longer be considered high risk because “infants born at or after 29 weeks, 0 days' gestation have an RSV hospitalization (RSVH) rate similar to the rate of full-term infants”Citation10. Goldstein et al.Citation11 showed that infants born at 29–34 wGA had ∼2.5–5.5-times higher risk of RSVH compared with term infants. Also, the risk of RSVH was ∼1.5–2-times higher after 2014 (2014–2016 season vs 2012–2014 season) among 29–34 wGA infants.
Although the infant mortality rate associated with RSV lower respiratory tract infections (LRTIs) is low – 3–4 deaths per 10,000 inpatient admissionsCitation12 – pre-term infants hospitalized with RSV have more severe and costly hospitalizations than infants born at termCitation13. In addition, RSVH has been associated with increased odds of subsequent wheezing, respiratory-related hospitalizations, and higher downstream healthcare resource utilization (HRU) and costsCitation14–16. This increased healthcare burden may continue for several years and is not just restricted to severely premature (<29 wGA) infantsCitation17. Using US data from the MarketScan Multi-State Medicaid database covering births in 2003–2005, Shi et al.Citation16 demonstrated that the burden following RSV-LRTI or unspecified bronchiolitis (UB) was highest for infants born at ≤33 wGA and that infants born at 34–36 wGA had higher HRU and costs in the year following these infections than term (≥37 wGA) infants. The objective of the current study is to provide updated estimates of the impact of hospitalization for RSV-LRTI in the first year of life on subsequent HRU and direct costs in Medicaid- and commercially insured infants across different gestational ages.
Methods
Study design and data source
This was a retrospective, observational cohort study of Medicaid and commercially insured infants born in US hospitals between July 2009 and June 2015. The study was conducted using administrative healthcare claims data from Truven Health MarketScan Medicaid Multi-State (Medicaid) and Commercial Claims and Encounters (Commercial) databases for the study period July 2009 to September 2016. Each database includes inpatient and outpatient medical claims, outpatient pharmacy claims, and enrollment information for their covered populationCitation18. The Medicaid database contains data for ∼44.2 million Medicaid enrollees across multiple geographically dispersed states. The Commercial database includes data for 145.5 million employees and their dependents enrolled in a variety of fee-for-service and managed care health plans.
Data management protocols were compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996 regulations and fully de-identified. Therefore, we did not seek Institutional Review Board approval.
Patient selection and cohort assignment
All infants in the Medicaid and Commercial databases born between July 1, 2009 and June 30, 2015 (study period) who had a linked birth hospitalization record and were discharged alive from the birth hospitalization were included in the analysis. Eligible infants were then classified into one of three mutually-exclusive diagnosis-based cohorts: (1) infants with an RSV-LRTI hospitalization in the first year of life; (2) infants with a UB hospitalization in the first year of life and no evidence of a RSV-LRTI hospitalization during the study period; or (3) a comparator cohort, infants with no evidence of either a RSV-LRTI or UB hospitalization in the first year of life. Infants with hospitalizations for both RSV-LRTI and UB were classified into cohort 1. For infants in cohorts 1 and 2, the index date was set to the date of the first inpatient claim with a diagnosis of RSV-LRTI or UB, respectively. For infants in the comparator cohort 3, the index date was randomly assigned based on the distribution of times between birth and index dates for infants in the RSV-LRTI cohort, making age at index date similar between cohorts 1 and 3. The time from birth to index date was defined as the baseline period, and the 12 months following the index date was defined as the post-index period. To ensure complete data capture, continuous enrollment with medical and pharmacy benefits was required during the baseline and post-index periods for all infants. All study infants were also required to have an identifiable gestational age (based on ICD diagnosis codes and diagnostic-related groupings [DRGs]) and were segmented into seven gestational age risk cohorts: <29 wGA, 29–30 wGA, 31–32 wGA, 33–34 wGA, 35–36 wGA, term with major health problems (based on DRG code 793), term without major health problems (i.e. normal newborn, DRG code 795). Infants with selected complex, rare medical conditions (e.g. cystic fibrosis, immunodeficiency, congenital anomalies of the respiratory system, neuromuscular, immunological or genetic conditions, or organ transplants), chronic lung disease of prematurity, or CHD were excluded because of potential palivizumab use.
Baseline characteristics
Baseline demographic characteristics included age at the index date, age at the start of the first RSV season (defined as November 1), sex, and birth weight. Some clinical characteristics, including length of stay (LOS) of birth hospitalization, presence of a neonatal intensive care unit (NICU) admission, and presence of mechanical ventilation were measured during the birth hospitalization. Outpatient use of palivizumab prophylaxis was recorded for the baseline and follow-up periods (inpatient prophylaxis data were not available). The presence of diagnosis codes for any congenital disease, respiratory problems, or failure to thrive was measured during the baseline period only (Supplementary Appendix 1).
Study outcomes
The primary outcomes of interest were all-cause HRU and costs in the post-index period. HRU was measured by the percentages of infants using particular services during this follow-up period (inpatient admission, emergency department [ED] visit, outpatient physician office visits, pharmacy fills, other services [e.g. diagnostics]) and the mean number of encounters per patient. All-cause costs for inpatient services, outpatient services (ED visits, physician office visits, laboratory services, other outpatient services), and outpatient pharmacy services were calculated for the full year post-index (including the index hospitalization) and from the end of the index hospitalization until 12-months post-index (follow-up). This allowed reporting total costs including (i.e. full year) and excluding (i.e. follow-up) the cost of the index hospitalization. Costs were based on fully adjudicated paid healthcare claims and were adjusted to 2016 dollars using the medical care component of the Consumer Price IndexCitation19.
Statistical analysis
For all outcomes, data for continuous variables were summarized with means and SDs, whereas data for categorical variables were summarized with counts and proportions. Median values are also reported for cost outcomes. Data for Medicaid- and commercially insured infants were analyzed separately.
For HRU and cost outcomes, propensity score weighting was used to adjust for baseline differences in key demographic and clinical characteristics among cohorts. Multinomial logistic regression modeling was performed to calculate a generalized propensity score which indicated the conditional probability of having an inpatient admission for RSV-LRTI, UB, or neither. Age at first RSV season (defined as November 1 through March 31), birth year, sex, gestational age group, geographic region (Commercial only), birth hospitalization LOS, NICU admission and NICU LOS during the birth hospitalization, and any use of palivizumab prophylaxis were used in these models and propensity score weights derived from the results.
For HRU, weighted means and weighted proportions were calculated. To provide for the possibility that some infants might have no costs in follow-up, two-part models for the cost analysis were constructed for each gestational age group to estimate propensity score weighted costs for the full year and follow-up intervals with the above covariates. In addition, birth weight and the presence of respiratory problems in the baseline period were included as covariates in these models to reduce residual confounding.
Incremental costs were calculated to show the difference in full-year or follow-up costs between the RSV-LRI/UB infants and the comparator infants (infants without an inpatient admission for RSV-LRTI or UB) within the same risk cohort.
Weighted proportions were compared using chi-squared tests and weighted means were compared using t-tests with unequal variances. p-values < 0.05 were considered statistically significant.
Results
A total of 1,008,040 infants covered by Medicaid and 703,945 infants covered by commercial plans met all selection criteria (). Of these, over 77% of infants from each payer population were coded as term without major health problems in the birth hospitalization records, whereas 11% of Medicaid infants and 9% of commercial infants were identified as pre-term (36 wGA or less).
Table 1. Patient selection.
Within each payer population, the proportions of infants with an RSV-LRTI and UB hospitalization in the first year of life were lowest among term infants and increased with decreasing gestational age (Supplementary Appendix 2). Within the Medicaid-insured population, the proportions with a hospitalization in the first year of life ranged from 1.3% in healthy term infants to 3.9% among infants <29 wGA for RSV-LRTI and from 0.5% in healthy term infants to 3.6% among infants <29 wGA for UB. Within the commercially insured population, the proportions with a hospitalization in the first year of life ranged from 0.7% in healthy term infants to 1.7% among infants <29 wGA for RSV-LRTI and from 0.2% in healthy term infants to 1.8% among infants <29 wGA for UB. A small number of infants (1–2%) had multiple RSV-LRTI hospitalizations during the first 12 months.
Consistent with previous reportsCitation6,Citation7, males made up 50.1% and 50.9% of the comparator cohorts for the Medicaid and commercial populations, respectively, but comprised a greater proportion of the RSV-LRTI (55.3% and 58.5%) and UB (60.5% and 60.0%) cohorts.
Among infants with Medicaid coverage, 45.6% (RSV-LRTI), 62.4% (UB), and 46.8% (comparator) were over 90 days old at the time of hospitalization (). The distribution was similar for infants with commercial insurance, of whom 42.8% (RSV-LRTI), 61.6% (UB), and 48.9% (comparator) were over 90 days old at the time of hospitalization. In the RSV-LRTI cohort, 52.9% of Medicaid-insured infants and 56.7% of commercially insured infants were born during the RSV season (November 1–March 31) compared with 41.1% and 38.5% of infants in their respective comparator cohorts. Infants born during the RSV season comprised 42.6% of the UB-hospitalized cohort in the Medicaid population and 45.5% of this cohort in the commercial population. NICU admission among Medicaid and commercially insured infants during the birth hospitalization was most common among infants in the UB cohorts (20.2% and 21.3%), followed by the RSV-LRTI cohorts (16.3% and 15.6%), and the comparator cohorts (9.4% and 9.7%).
Table 2. Patient baseline characteristics.
Propensity score weighting was used to adjust for baseline differences among cohorts (Supplementary Appendix 3). The results presented here are weighted unless otherwise specified. The proportion of infants with an admission in the follow-up period (after discharge from the index hospitalization) for infants in the RSV-LRTI/UB cohorts ranged from 31–37% for infants <29 wGA at birth and from 7–14% for healthy term infants (p < 0.01, RSV-LRTI vs comparator and UB vs comparator) (). The percentages of term infants with at least one inpatient admission in the first year after index date within the Medicaid- and commercially insured comparator cohorts were 4.1% and 2.6%, respectively (Supplementary Appendix 4). Among infants <29 wGA at birth, the percentages of comparator infants with at least one inpatient admission rose to 18.5% and 13.3%.
Figure 1. Propensity score weighted healthcare utilization of (A) Medicaid- and (B) Commercially insured infants in the follow-up period (excludes the index hospitalization). All p-values comparing RSV-LRTI to comparator and UB to comparator are <0.01. Abbreviations. LRTI, lower respiratory tract infection; MHP, major health problems; RSV, respiratory syncytial virus; UB, unspecified bronchiolitis; wGA, weeks gestational age.
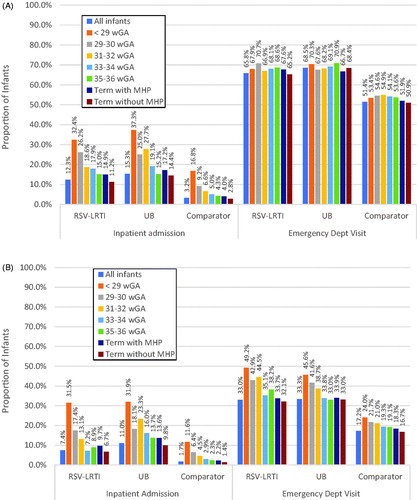
ED utilization was higher among Medicaid-insured infants with an RSV-LRTI or UB hospitalization, regardless of gestational age, and lower among commercially-insured comparator infants in both the full year and follow-up period (p < 0.05 except for commercially insured 29–30 wGA infants with UB) ( and ). In all gestational age groups and regardless of payer, more than 90% of RSV-LTRI and UB hospitalized infants had at least one outpatient office visit in addition to pediatric wellness visits in the first year after index date.
Table 3. Propensity score weighted differences of healthcare resource utilization vs the comparator cohort in 12 months after the index date.
In general, pre-term infants in both payer populations had higher all-cause post-index healthcare utilization in the first year after index date than term infants, including more inpatient admissions, more ER visits, and more outpatient physician office visits (). HRU in the hospitalized and comparator cohorts was lowest among term infants and tended to increase with the degree of prematurity.
The highest propensity score adjusted mean total all-cause healthcare costs (including index hospitalization costs) were incurred by infants with an RSV-LRTI hospitalization who were <29 wGA at birth (Medicaid: $89,202 [standard error, SE = $11,031]; commercial: $263,509 [SE $91,219]), whereas the lowest costs were accrued by healthy term infants in the comparator cohorts (Medicaid: $1,286 [SE $84]; commercial: $2,804 [SE $34]) (Supplementary Appendix 5). Among Medicaid infants, propensity score adjusted mean total costs excluding the index hospitalization ranged from $2,018 [SE $174] to $31,987 [SE $3,683] in the RSV-LRTI cohort, $2,130 [SE $230] to $43,317 [SE $5,235] in the UB cohort, and $1,214 [SE $84] to $23,421 [SE $1,549] in the comparator cohort. Term infants defined the lower cost bounds and infants <29 wGA at birth defined the upper cost bounds. The same gestational age pattern was observed in commercial infants, in which propensity score adjusted mean follow-up costs excluding the index hospitalization ranged from $4,040 [SE $338] to $68,838 [SE $14,812] in the RSV-LRTI cohort, $4,327 [SE $445] to $51,125 [SE $16,422] in the UB cohort, and $2,637 [SE $34] to $31,421 [SE $1,604] in the comparator cohort (Supplementary Appendix 5).
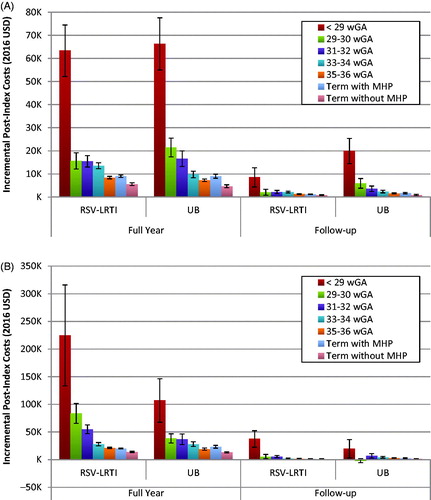
Overall, across both payer populations, there was a trend towards increasing incremental costs with decreasing gestational age (). In the Medicaid population, the incremental follow-up costs incurred in the period after infants were discharged from an RSV-LRTI hospitalization ranged from $804 [SE $193] for healthy term infants up to $8,566 [SE $4,162] for infants born at <29 wGA (Supplementary Appendix 5). Incremental follow-up costs were even higher for infants after discharge from an UB hospitalization, ranging from $916 [SE $245] for healthy term infants up to $19,896 [SE $5,459] for infants born at <29 wGA. In the commercial population, incremental costs in the follow-up period for infants discharged from an RSV-LRTI hospitalization were $1,403 [SE $340] for healthy term infants and $37,417 [SE $14,899] for infants born at <29 wGA; whereas, for infants discharged from a UB hospitalization the incremental costs were $1,690 [SE $446] for healthy term infants and $19,704 [SE $16,500] for infants born at <29 wGA. Among infants born at 29–30 wGA who were hospitalized with UB, the incremental costs in the follow-up period were negative; however, this may have been biased by the small number of infants in this cohort (n = 45).
Figure 2. Adjusted incremental difference in mean total all-cause healthcare costs after RSV-LRTI or UB inpatient admission for (A) Medicaid- and (B) Commercially insured infants. p < 0.05 for all full year costs. p < 0.05 for all follow-up costs, except: Medicaid 29–30 wGA RSV vs no hospitalization (p = 0.111), commercial <29 wGA UB vs no hospitalization (p = 0.2368), commercial 29–30 wGA RSV vs no hospitalization (p = 0.156), commercial 29–30 wGA UB vs no hospitalization (p = 0.603), commercial 31–32 wGA UB vs no hospitalization (p = 0.089). Abbreviations. LRTI, lower respiratory tract infection; MHP, major health problems; RSV, respiratory syncytial virus; UB, unspecified bronchiolitis; USD, United States dollar; wGA, weeks gestational age.
Discussion
This study confirmed that hospitalization among infants insured by Medicaid or commercially for RSV-LRTI/UB in the first year of life is associated with substantially higher subsequent HRU and costs across all gestational ages when compared with those of infants who were not hospitalized for these conditions. Hospitalized infants had more inpatient admissions, ED visits, outpatient office visits, and numerically higher mean healthcare costs in the follow-up period than comparator infants across all gestational ages. Infants born before 29 wGA had the highest overall post-hospitalization HRU and costs.
Our results support the gestational age pattern reported in an earlier Medicaid studyCitation16. Using the MarketScan Medicaid database covering 2003–2005, Shi et al.Citation16 reported that incremental follow-up healthcare costs after an inpatient admission for RSV were $7,419 for infants born at ≤33 wGA, $3,455 for infants born at 33–36 wGA, and $1,902 for term infants. A similar trend of numerically higher costs among infants born at younger gestational ages was also reported among those hospitalized for UB ($3,978 for infants born at ≤33 wGA, $1,852 for infants born at 33–36 wGA, and $1,005 for term infants).
The current study expands beyond the previous Medicaid-based study by also reporting outcomes for a commerically insured population and by providing results for more detailed wGA categories within each payer population. In addition, we focused exclusively on infants with RSV-LRTI or UB hospitalizations (rather than evaluating RSV-LRTI/UB outpatient episodes) in the first year of life, as Shi et al.Citation16 noted that this group of hospitalized infants had the greatest downstream cost impacts. Our results provide increased detail and substantiate the Shi et al.Citation16 finding that both early- and late-pre-term RSV-LRTI/UB infants have higher costs than term infants, subsequent to an RSV or UB hospitalization.
Our study also corroborates findings from smaller studies that investigated the impact of an RSV hospitalization on the long-term health of premature infants. A study of moderately premature (32–35 wGA) infants in two British hospitals examined healthcare utilization and costs among infants with an inpatient admission for RSV (n = 20) and found that those infants had significantly more hospital admissions, longer hospital stays, more ED visits, and higher healthcare costs in their first 2 years of life than similar infants without a respiratory-related hospitalization (n = 108)Citation15. A study of 434 moderately pre-term infants in Spain found that wheezing was more common through the first 6 years of life among children with an infant inpatient admission for RSV, and that these children also had more outpatient visits, ED visits, and increased use of asthma medications than moderately pre-term infants without an inpatient RSV admissionCitation14. More recently, an Italian matched cohort study reported that pre-term (<37 wGA) infants with an inpatient admission for RSV-LRTI/UB (n = 22) had 83% higher healthcare costs over their first 4 years of life, and that 18.2% of the RSV-LRTI/UB infants were re-hospitalized for related respiratory conditions between ages 2–4 compared with 1.6% of comparator infants (n = 62)Citation17.
Compared to term infants, pre-term infants have a higher risk of being hospitalized due to RSV and have more severe and costly admissions. As shown in this study, pre-term infants hospitalized due to RSV have higher resource utilization and costs in the year following hospitalization compared to pre-term infants not hospitalized for RSV disease.
Limitations
The limitations of this study are typical of those for retrospective analyses of administrative claims data. First, administrative data is used for billing purposes and not collected with the same rigor as clinical trial data; therefore, it is subject to miscoding and under-coding. The databases include adjudicated claims. Any healthcare services for which a claim was not processed would not be included in the databases or this analysis. Additionally, we cannot account for infants who were included in both databases. Laboratory tests are not available in claims, and the American Academy of Pediatrics does not recommend routine virologic testing; therefore, we relied on diagnostic coding to identify RSV hospitalizations. To address the potential under-coding of RSV, we included a cohort of infants with UB, because it is estimated that 50–90% of UB cases are caused by infection with RSVCitation1,Citation3. Although we used propensity score weighting and multivariable modeling to control for differences in baseline characteristics, residual confounding may still exist.
Although the standardized differences suggest that the propensity score weights adequately adjusted for baseline differences between cohorts, we also examined the feasibility of including healthcare utilization for non-respiratory conditions (digestive and genitourinary) as potential negative control outcomes. Initial analysis indicated that infants who had experienced an RSV or UB hospitalization were also likely to be at increased risk for a wide-range of other outcomes including non-respiratory outcomes. This makes it exceedingly challenging to identify outcomes that are truly independent of the index hospitalizations, and it was beyond the scope of the current study to explore this question further.
We also acknowledge that there are several known factors that influence infant health, such as breastfeeding, the presence of siblings, day-care attendance, and exposure to second-hand smoke, that are not captured in administrative claims and, therefore, could not be addressed. In addition, some of the RSV-LRTI and UB risk cohorts, particularly among commercially insured infants <32 wGA, had fewer than 100 patients, which makes results obtained for those cohorts more sensitive to outliers.
Conclusions
This descriptive, retrospective analysis demonstrates that infants who were hospitalized for RSV-LRTI/UB have increased healthcare utilization and costs at least through the remainder of the year following that inpatient admission than comparator infants. In addition, these results add weight to the hypothesis that there is an inverse relationship between gestational age and the healthcare utilization and cost burden incurred after an RSV-LRTI/UB hospitalization in the first year of life.
Transparency
Declaration of funding
This study was funded by AstraZeneca. Employees of AstraZeneca were involved in the design and execution of this study.
Declaration of financial/other interests
LB is employed by AstraZeneca. TG was employed by AstraZeneca at the time this study was completed and is a current employee of Sobi, Inc. AMK is employed by IBM Watson Health, which received funding from AstraZeneca to conduct this study. SWW is a paid consultant of IBM Watson Health. JL was in the AstraZeneca speaker program for Synagis (palivizumab) at the time this study was completed. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Complying with ethics of experimentation statement
All database records are statistically de-identified and certified to be fully compliant with US patient confidentiality requirements set forth in the Health Insurance Portability and Accountability Act of 1996. Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, Institutional Review Board approval to conduct this study was not necessary.
Supplemental Material
Download MS Word (65.3 KB)Acknowledgements
Medical writing support was provided by Jessamine P. Winer-Jones, of IBM Watson Health, and these services were paid for by AstraZeneca. Editorial support was provided by ETHOS Health Communications and was funded by Sobi, Inc.
Data availability
The data that support the findings of this study are available from IBM Watson Health. Restrictions apply to the availability of these data, which were used under license for this study. Derived data are available from the corresponding author with the permission of IBM Watson Health.
References
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928.
- Falsey AR, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759.
- Rose EB, Wheatley A, Langley G, et al. Respiratory syncytial virus seasonality — United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2018;67:71–76.
- Doucette A, Jiang X, Fryzek J, et al. Trends in respiratory syncytial virus and bronchiolitis hospitalization rates in high-risk infants in a United States nationally representative database, 1997–2012. PLoS One. 2016;11:e0152208.
- Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598.
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143:127–132.
- Boyce TG, Mellen BG, Mitchel EF, et al. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137:865–870.
- IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537.
- Synagis (palivizumab) [prescribing information]. Gaithersburg (MD): AstraZeneca; 2014.
- Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502.
- Goldstein M, Krilov LR, Fergie J, et al. Respiratory syncytial virus hospitalizations among U.S. preterm infants compared with term infants before and after the 2014 American Academy of Pediatrics guidance on immunoprophylaxis: 2012–2016. Am J Perinatol. 2018;35:1433–1442.
- Byington CL, Wilkes J, Korgenski K, et al. Respiratory syncytial virus–Associated mortality in hospitalized infants and young children. Pediatrics. 2015;135:e24.
- McLaurin KK, Farr AM, Wade SW, et al. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol. 2016;36:990.
- Carbonell-Estrany X, Pérez-Yarza EG, García LS, et al. Long-term burden and respiratory effects of respiratory syncytial virus hospitalization in preterm infants—The SPRING study. PLoS One. 2015;10:e0125422.
- Shefali-Patel D, Paris MA, Watson F, et al. RSV hospitalisation and healthcare utilisation in moderately prematurely born infants. Eur J Pediatr. 2012;171:1055–1061.
- Shi N, Palmer L, Chu B-C, et al. Association of RSV lower respiratory tract infection and subsequent healthcare use and costs: a Medicaid claims analysis in early-preterm, late-preterm, and full-term infants. J Med Econ. 2011;14:335–340.
- Roggeri DP, Roggeri A, Rossi E, et al. Impact of hospitalizations for bronchiolitis in preterm infants on long-term health care costs in Italy: a retrospective case-control study. CEOR. 2016;8:407–412.
- IBM MarketScan Research Databases for health services researchers. In: IBM, editor. 2018. Available from: https://www.ibm.com/downloads/cas/6KNYVVQ2
- Consumer Price Index details report tables annual average 2016: United States Department of Labor; 2017 [cited 2018 22 Jun 2018]. Available from: https://www.bls.gov/cpi/tables.htm
