?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives: Atrial fibrillation (AF) is the most common arrhythmia and a major marker of ischemic stroke risk. Early detection is crucial and, once diagnosed, anticoagulation therapy can be initiated to reduce stroke risk. The aim of this study was to assess the cost-effectiveness of employing an insertable cardiac monitor (ICM), BIOMONITOR, for the detection of AF compared to standard of care (SoC) ECG and Holter monitoring in patients with cryptogenic stroke, that is, stroke of unknown origin and where paroxysmal, silent AF is suspected.
Materials and methods: A Markov model was developed which consisted of five main health states reflecting the potential lifetime evolution of the AF disease: post cryptogenic stroke (index event), subsequent mild, moderate and severe stroke, and death. Sub-states were included to track a patient’s AF diagnostic status and the use of antiplatelet or anticoagulant therapy. AF detection was assumed to result in a treatment switch from aspirin to anticoagulants, except among those with a history of major bleeding. Detection yield and accuracy, clinical actions and treatment effects were derived from the literature and validated by an expert clinician. All relevant costs from a US Medicare perspective were included.
Results and conclusions: An ICM-based strategy was associated with a reduction of 37 secondary ischemic strokes per 1000 patients monitored compared with SoC. Total per-patient costs with an ICM were higher (US$90,052 vs. US$85,157) although stroke-related costs were reduced. The use of an ICM was associated with a base-case incremental cost-effectiveness ratio of US$18,487 per life year gained compared with SoC and US$25,098 per quality-adjusted life year gained, below established willingness-to-pay thresholds. The conclusions were found to be robust over a range of input values. From a US Medicare perspective the use of a BIOMONITOR ICM represents a cost-effective diagnostic strategy for patients with cryptogenic stroke and suspected AF.
1. Introduction
One of the most frequent cardiac arrhythmias in any given population is atrial fibrillation (AF)Citation1. The presence of AF is associated with an increased risk of ischemic strokeCitation2, although this risk can be mitigated with the use of anticoagulantsCitation3–5. Approximately 25–30% of ischemic strokes are classified as cryptogenic, that is, they are of obscure or unknown originCitation6,Citation7 and in these cases AF involvement is often suspectedCitation8–10. Clinical guidelines require diagnostic confirmation of AF before anticoagulant therapy can be initiatedCitation11–13 thus the need for effective diagnostic tools.
As a diagnostic procedure, the standard of care (SoC) for many years has included the use of an external device known as a Holter monitor, which records the electrical activity of the heart via electrocardiography (ECG). A Holter monitor is typically employed over a period of at least 24 hours and may be used on a repeated basisCitation14. However, as AF is often silent (asymptomatic) and intermittent (paroxysmal) it frequently goes undetected with the use of a Holter monitorCitation15.
An alternative for patients with a history of cryptogenic stroke and suspected AF is the use of an insertable cardiac monitor (ICM), such as BIOMONITORCitation16,Citation17. All ICMs are inserted under the skin in the left pectoral area. They continuously monitor heart rhythm for abnormal events, store ECG and other data from these episodes and communicate the information to the treating physician for further assessmentCitation18. In the case of a BIOMONITOR and when used in combination with BIOTRONIK Home Monitoring®, this information is transmitted on a daily basis. Alternatives to BIOMONITOR include the Reveal LINQ (Medtronic) and Confirm Rx (Abbott). There are small differences among the technologies (for example in terms of size, battery life and information storage and transmission) although no studies directly comparing their sensitivity and specificity for AF detection.
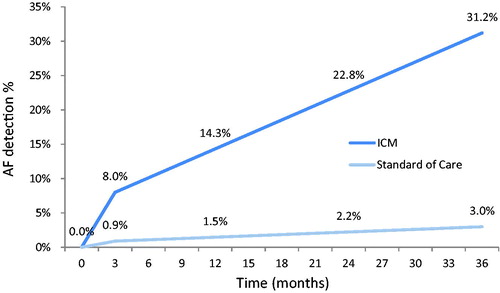
In the only multi-national study of its type to date, known as CRYSTAL-AF, 441 patients with cryptogenic stroke were randomized in a 1:1 ratio to receive either monitoring with an ICM (Reveal XT, an earlier version of the Reveal LINQ) or standard of care (SoC) involving a combination of physician assessment, ECG and/or Holter monitoringCitation1. After 12 months of follow up, AF was detected in 12.4% of patients in the ICM group versus 2.0% of patients in the control (SoC) group. After 3 years, among patients followed long-term, the rates were 30% and 3%, respectively. These findings were subsequently confirmed in a real-world cryptogenic stroke cohort with suspected AF, with a reported detection rate of 21.5% at 2 yearsCitation19.
ICMs are currently underutilized in patients with cryptogenic stroke and suspected AF despite their potential value in improving patient outcomesCitation11,Citation13,Citation18,Citation20. There are different groups involved in the care and decision making of patients who are candidates for ICMsCitation21 which may in part explain current ICM use. Given the large volume of data that is stored and transmitted some clinicians may also have concerns about managing and reviewing the informationCitation18. Furthermore, the initial cost to public health care systems associated with the purchase, insertion and later removal of ICM devices is significant. ICM technology is itself evolving and smaller devices are now available, which, with a simplified insertion procedure are expected to improve acceptance and ultimately device useCitation22,Citation23.
Patients with a history of ischemic stroke are typically prescribed antiplatelet therapy, with the aim of reducing the risk of subsequent stroke. Upon AF detection most will switch to anticoagulants, depending on their individual stroke risk (using the CHADS2 Score, an acronym for congestive heart failure, hypertension, age > 75 years, diabetes mellitus, and prior stroke or transient ischemic attack) and the potential for bleeding as a side effect of anticoagulant therapy. The ultimate decision on the initiation of anticoagulants rests with the clinician, who must weigh the expected benefits and risks associated. Novel oral anticoagulants (NOACs) appear to confer at least a similar clinical benefit to warfarin in reducing the risk of ischemic stroke and systemic embolism and are associated with a lower risk of hemorrhagic stroke compared with warfarin, although have higher acquisition costsCitation24–26.
The objective of this study was to assess the economic value of BIOMONITOR use for the detection of AF compared with SoC monitoring in patients with a history of cryptogenic stroke. The relevant patient population is those who would be eligible for anticoagulants if AF was diagnosed. The main outputs of the analysis are the number of secondary strokes avoided, life years gained, quality-adjusted life years (QALYs) gained and costs, further summarized as cost per life year gained and cost per quality-adjusted life year (QALY) gained.
2. Methods
A Markov cost-effectiveness model was developed in Microsoft Excel considering a hypothetical cohort of patients diagnosed with a recent cryptogenic stroke, allocated to receive either an ICM or SoC monitoring as observed during the CRYSTAL-AF trialCitation1. The core model structure was inspired by existing studies and models developed for evaluating the diagnosis and management of patients with AF and/or cryptogenic stroke and the use of anticoagulants. These included a cost-effectiveness analysis by Diamantopolous et al. based on CRYSTAL-AF for the UK settingCitation27, a US analysis by Miller et al.Citation28 on the benefits of anticoagulation in patients with nonvalvular AF and a CADTH therapeutic review on anticoagulant use in patients with AFCitation29. Existing US clinical guidelines were also used to support model developmentCitation11 and details were subsequently validated with input from a local clinical expert.
To ensure all relevant costs and outcomes were captured, a lifetime horizon was deemed appropriate. A cycle length of three months was applied, with half-cycle correction. This three-month interval is consistent with the scheduled follow-up intervals in the CRYSTAL-AF and other relevant NOAC trialsCitation1,Citation3,Citation4. Given the mean age of the cohort at baseline (65 years) Medicare was determined to be the most relevant payer. An overview of the key features of the model is given in .
Table 1. Overview of the key features of the model.
3. Model structure
The core model structure is illustrated in . The model is composed of five mutually exclusive health states and each of these (except the absorbing health state of death) is further divided into sub-states depending on the patient’s AF diagnostic status (i.e. true negative [TN], false positive [FP], false negative [FN] or true positive [TP]).
Figure 1. Markov model structure for cost-effectiveness of ICM use in patients with a history of cryptogenic stroke and suspected AF. *These additional adverse events can cause treatment switches, but no transitions between shown health states. AF: atrial fibrillation; CRNM: clinically relevant non-major; ECH: extracranial hemorrhage; FN: false negative; FP: false positive; GI: gastrointestinal; HS: hemorrhagic stroke; ICH: intracranial hemorrhage; TIA: transient ischemic attack; SE: systemic embolism; TN: true negative; TP: true positive.
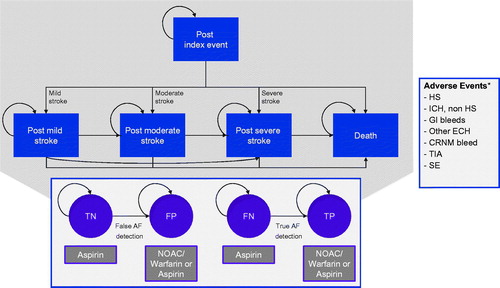
Following their index event (cryptogenic stroke), patients enter the model in either the TN or FN sub-states of the “post-index event” health state, reflecting their initial lack of AF diagnosis. Over time, patients without further stroke events will remain in this health state and will transition to the TP or FP subgroups upon AF detection.
Those who experience a subsequent stroke (ischemic or hemorrhagic) will transition to one of several other post-stroke health states reflecting the severity of this secondary stroke (i.e. post-mild stroke, post-moderate stroke, post-severe stroke or death). In each of these health states, during the AF detection period, there is a possibility that AF is diagnosed, which may lead to a change in management. Patients can progress to a worse health state if they suffer another, more severe stroke but it was assumed that transitions would not occur in the opposite direction.
At any time there is a risk that patients experience an adverse event, such as bleeding as a side-effect of anticoagulant therapy. Bleeding events incorporated into the model include hemorrhagic stroke (HS) (via the post-index stroke health states), intracranial hemorrhage excluding HS (ICH, non-HS), gastrointestinal bleed (GI), other extracranial hemorrhages (ECH) and clinically relevant non-major (CRNM) bleeds. Clinically, there are four main types of ICH: epidural hematoma, subdural hematoma, subarachnoid hemorrhage and intracerebral hemorrhage. In this model, hemorrhagic stroke (HS) was considered as a subcategory of ICH and includes subarachnoid hemorrhage and intracerebral hemorrhage. The non-HS ICH category includes epidural and subdural bleeds, frequently associated with head trauma and thus less relevant to this analysis. Other potential adverse events considered were transient ischemic attack (TIA) and systemic embolism (SE). With the exception of HS, it was assumed that these additional events may result in the need for a change in drug treatment but would not lead to a transition in primary health states as defined in the model. It was also assumed that both AF could be detected and an adverse event could occur in a single cycle.
Patients with ischemic or hemorrhagic stroke were assumed to experience long-lasting and severity-related consequences in terms of health-related quality of life (HRQL), resource utilization and costs (i.e. patients cannot transition from a more severe to a less severe post-stroke state). Non-fatal ECH, other ICH and CRNM bleeds were assumed to carry temporary consequences (i.e. one-off treatment costs and a utility decrement) lasting only the cycle in which the bleed occurred at maximum. The same was assumed for TIAs and SEs.
4. Model input parameters
Event probabilities employed in the model were derived from published sources. The most important studies used included the pivotal CRYSTAL-AF trialCitation1, as well as the subgroup analyses of patients with a history of ischemic stroke or TIA from the key AVERROES (apixaban vs. aspirin)Citation3 ARISTOTLE (apixaban vs. warfarin)Citation4, RE-LY (dabigatran vs. warfarin)Citation30 and ROCKET-AF (rivaroxaban vs. warfarin)Citation31 studies. CRYSTAL-AF is considered the most robust evidence available on which to base an ICM treatment comparison although the population enrolled was younger than would be expected to receive ICMs in routine clinical practice and included patients with a history of TIAs. Given these limitations our model incorporated relevant observational data, including the study by Ziegler et al.Citation32 on real-world use of ICMs and the analysis by Luengo-Fernandez et al.Citation33 on stroke burden and outcomes as part of the UK Oxford Vascular Outcomes Study. Evidence from the pivotal edoxaban trialCitation34 was not formally incorporated, as the product was not well established at the time the model was developed.
4.1. Population and AF detection
Based on the observational study by Ziegler et al. it was assumed that 53% of the cohort would be male and that the mean age of the patient population was 65 years. The distribution of CHADS2 scores for patients entering the model, which influences the baseline risk of ischemic stroke, was derived from the CRYSTAL-AF population. The vast majority of patients had CHADS2 scores of 2, 3 or 4 (34.01%, 41.50% and 19.05%, respectively).
Based on the findings of CRYSTAL-AF, with a 30% AF detection rate after 3 years and an assumed 96.1% diagnostic sensitivity of the ICM involvedCitation1,Citation35, it was estimated that the “true” AF prevalence among this patient population was 31.2%. This estimate potentially reflects an upper rangeCitation36 and we explored the impact of lower assumptions via our sensitivity analysis. More extensive use of ICMs will highlight cases of subclinical AF which may have gone undetected with the use of external devicesCitation37. These are nonetheless important as subclinical AF is also associated with an increased risk of cryptogenic strokeCitation9,Citation10,Citation14.
4.1.1. AF detection
The probability of AF detection was calculated based on sensitivity and specificity estimates for the monitoring methods. BIOMONITOR estimates were derived from the BioMonitor 2 Master StudyCitation16 and we assumed that all ICMs were successfully planted. SoC was taken to consist of the same mix of monitoring methods as reported for CRYSTAL-AF.
4.1.1.1. Sensitivity
The patient-based sensitivity parameter for BIOMONITOR in the base-case analysis was set at 100% as reported in the BioMonitor 2 Master Study and was reduced to 90% and then to 75% in our sensitivity analysis. The sensitivity of the SoC comparator was set at 9.6%, as derived from CRYSTAL-AF (9.6% sensitivity × 31.2% AF will lead to 3% true positives after three years) with an estimated range of 7.8–11.6% based on the assumption that the standard error would be 10% of the mean in the sensitivity analysis. A further variation of 25% was also explored.
4.1.1.2. Specificity
In CRYSTAL-AF, episodes of AF that qualified for analysis were adjudicated by an independent clinical committee and in routine practice every ICM-recorded ECG is ultimately judged by a clinician. Hence, assuming that a physician will validate and rectify any false positive AF findings our base-case scenario assumes 100% specificity for both interventions. In our sensitivity analysis, this value was reduced to 75% for both monitoring methods.
4.1.1.3. Estimated AF detection rates
As the rate of detection recorded in CRYSTAL-AF was higher in the first three months post-intervention, a separate per-cycle probability of AF detection was calculated for the very first cycle (months 0–3: 8.0 vs. 0.9%). Thereafter, the model assumes a constant detection rate until the end of the AF monitoring period (months 3–36: 2.11 vs. 0.19%). Based on these detection rates, three-month cycle transition probabilities were derived using the formulas proposed by Briggs et al.Citation38. These base-case settings result in AF detection rates of 3.0% in the SoC group (as observed in the CRYSTAL-AF control arm) and 31.2% in the ICM group after 36 months of monitoring ().
4.2. Drug treatment
In our model, all patients begin on treatment with aspirin (in the TN and FN health states). NOACs or warfarin are initiated upon AF detection (false and true positives) unless the patient is ineligible due to prior bleedings, in which case they are assumed to continue with aspirin. Of those receiving anticoagulant therapy, based on an earlier claims data analysis, it was assumed that 37.8% would be taking NOACs and others warfarinCitation39. The influence of higher levels of NOAC prescription and the use of dual antiplatelet therapy with aspirin plus a platelet P2Y12 receptor inhibitor was explored in our sensitivity analysis.
In each model cycle, there is a probability of treatment discontinuation due to the occurrence of an adverse event or for other reasons. Of the subgroups on anticoagulant therapy (as in the model developed by Diamantopoulos), it was assumed that throughout the observation period all patients suffering a non-fatal HS, 56% of patients having another ICHCitation40, and 25% of patients suffering a non-fatal ECHCitation41 would permanently switch to antiplatelet therapy. The same percentages were precluded from subsequently receiving anticoagulant therapy if the bleeding event occurred while being treated with aspirin.
For the remainder of the patients (44% of ICH and 75% of ECH events) it was assumed that anticoagulant therapy would be temporarily halted (for a period of six weeks in the base case). HS patients on aspirin did not discontinue treatment following major bleeding events and were precluded from changing to anticoagulant therapy, even if they were subsequently diagnosed with AF. Patients experiencing CRNM bleeds, TIA or SE were assumed to remain on anticoagulation treatment. The annual probability of treatment discontinuation for reasons other than adverse events was assumed to be 13.2 and 14.4% for NOACs and warfarin, respectively in the base-case analysisCitation42 although discontinuation rates of up to 40% with NOACs and up to 50% with warfarin after 12 months were explored in the sensitivity analysis.
4.3. Ischemic stroke risk and severity
In each cycle and health state, patients have a risk of suffering a recurrent ischemic stroke. This risk was assumed to be dependent on their treatment, AF status, CHADS2 score and ageCitation43,Citation44. Evidence available from various studies was synthesized to calculate a risk of ischemic stroke for each post-index health sub-state based on these four factors, according to the methods described below.
4.3.1. Ischemic stroke risk in patients with AF
A clinical registry study from the UK published by Mohan et al.Citation45 reported that the Hazard Ratio (HR) for secondary ischemic stroke in patients with AF compared to patients without AF was 1.51 (95% CI: 1.09–2.09). Although this value appears conservative in comparison to the results of the Framingham StudyCitation2 (suggesting that non-valvular AF was independently associated with a threefold to fivefold increased risk for stroke) we assumed this estimate. Thus a key assumption of our model is that the ischemic stroke risk for patients without AF is around two-thirds of the risk of patients with AF, regardless of the treatment they are receiving.
4.3.2. Ischemic stroke risk in patients receiving aspirin
Gage et al.Citation46 reported the annual risk of ischemic stroke among patients with diagnosed AF receiving aspirin broken down by CHADS2 score. Similar to Diamantopoulos et al., these risks were applied to the cohort’s distribution of CHADS2-scores to derive a baseline risk for AF patients on aspirin. It was assumed that the risk of ischemic stroke for TP and FN patients on aspirin is identical, as both groups actually have AF and receive the same treatment (.). This baseline ischemic stroke risk for patients on aspirin was subsequently synthesized with treatment effects for warfarin and NOACs to derive the ischemic stroke risk for patients in the other sub-states.
Table 2. Estimated annual ischemic stroke risk by CHADS2 score.
4.3.3. Ischemic stroke risk for patients in other sub-states
For patients diagnosed with AF, the treatment effect for warfarin versus aspirin was estimated through an indirect comparison with apixabanCitation3,Citation4,Citation28. The model outputs were found to be very sensitive to this particular parameter and it has a significant impact on the ischemic stroke risk because it directly determines the efficacy of warfarin and indirectly the efficacy assumed for NOACs. A published network meta-analysis of trials comparing any NOAC versus warfarin specifically reporting results for patients with prior stroke or TIA was used to inform the treatment effect of NOAC vs. warfarin (Peto odds ratio: 1.03, 95% CI: 0.87–1.21)Citation47. In our model, all odds ratios (ORs) were transformed into relative risks (RR) according to the method described by Edlin et al.Citation48. The above-mentioned methodology is identical to the approach taken by Diamantopoulos et al.
4.3.4. Ischemic stroke risk with age
The ischemic stroke risks of all groups were increased by a factor of 1.46 (95% CI: 0.80–2.16) per decade from 70 years to account for the higher risk of stroke as patients become olderCitation47. All annual risks were converted into three-month cycle probabilities using the formulas described by Briggs et al.Citation38.
4.3.5. Ischemic stroke severity
Subsequent ischemic strokes were divided into mild (42.17%), moderate (26.17%), severe (9.50%) and fatal (22.17%) cases. The proportions of each were sourced from two published cost-effectiveness analyses, the first comparing apixaban with warfarin and aspirinCitation49 and the second comparing warfarin with other NOACsCitation50 and are based on average values. Since there is no specific evidence available on ischemic stroke severity proportions for the subgroup of patients with a history of stroke, evidence from the overall stroke population of the relevant clinical trials was included. For simplicity and data availability reasons it was assumed that the severity distribution of subsequent stroke episodes was not treatment dependent.
4.4. Risk of adverse events including bleeding
The risk of adverse events among patients receiving NOACs, warfarin or aspirin was calculated in a manner as for the risk of ischemic stroke. The major difference was that, in general, the risk for patients on warfarin therapy was used as the reference rather than the risk on aspirin. Event risk was assumed to be a function of both treatment and age. Model inputs on adverse event risks are documented in .
Table 3. Annual risks of adverse events in patients with prior stroke or TIA.
4.4.1. Bleeding risk on warfarin
For patients on warfarin, the baseline risk of an adverse event was obtained by averaging the annual risks reported in patients with a history of stroke or TIA in the key ARISTOTLECitation4, RE-LYCitation30, and ROCKET-AFCitation31 studies. For CRNM bleeds, only overall population data were available from ARISTOTLECitation26 and RE-LYCitation24 (i.e. no subgroup data was presented). As values for TIAs were not reported in these studies, an annual risk for TIA in patients with AF was obtained from a study published by CADTH in 2013Citation30.
Diamantopoulos et al. derived the annual risk for HS by using data suggesting that 59.7% of ICHs are HSCitation27. The resulting annual risk (0.71%) is very close to the average of values reported in the three key NOAC studies (0.74%). In a similar manner, however, Diamantopoulos used data suggesting that 41.8% of ECHs are GI bleeds. With a reported annual risk of GI bleeds of 1.11%, the calculated annual risk for other ECH bleeds (1.54%) is considerably higher than the risk reported in ROCKET-AF (0.35%)Citation31. In this model, we used the conservative value (1.54%).
4.4.2. Bleeding risk on aspirin
The treatment effect for aspirin versus warfarin was again estimated through an indirect comparison with apixabanCitation3,Citation4. Somewhat unexpectedly, the HR for aspirin vs. warfarin obtained in this manner for HS (0.40/0.25 = 1.60) seems to suggest a higher risk of HS whilst being treated with aspirin as compared to warfarin. It should be noted here that the number of certain AEs were limited in the subgroup of patients with prior stroke/TIA in the NOAC studies (in particular AVERROESCitation3) and thus estimates for the HRs obtained in the indirect comparison are uncertain as indicated by their wide 95% confidence intervals. To our knowledge, however, this is the only data available for patients with prior stroke/TIA and no randomized studies comparing the effects of aspirin and warfarin have been conducted in this subset of patients.
4.4.3. Bleeding risk on NOACs
To derive the probabilities of bleeding events on NOAC, Peto odds ratios for NOAC vs. warfarin from a meta-analysis undertaken by Ntaios et al.Citation47 were applied (after conversion of the ORs to RRs) to the warfarin baseline risks. For CRNM bleeds we used overall population data from the RE-LY trialCitation24.
4.4.4. Adverse event risk and age
A risk adjustment factor per decade was included in our model to account for the increased probability of having an adverse event with ageCitation27. In line with the approach by Diamantopoulos et al., the risk adjustment factor (HR) for bleedings was set at 1.97 in the base case (95% CI: 1.79–2.16)Citation42. For the other adverse events (TIA and SE), a placeholder for such a risk adjustment factor was included and its impact assessed in the one-way sensitivity analysis (HR = 1.00 in the base case; varied between 0.50 and 2.50). Again all annual risks were converted into three-month cycle probabilities according to the methods described by Briggs et al.Citation38.
4.4.5. Hemorrhagic stroke severity
As for secondary ischemic stroke, HS episodes were divided into mild (28.17%), moderate (22.50%), severe (12.17%) and fatal (37.17%) strokes. Again the proportions of these categories were sourced from the two key cost-effectiveness studies of apixaban for stroke prevention in patients with AFCitation49,Citation50. In line with Diamantopoulos et al., it was assumed that the severity distribution was not treatment dependentCitation27.
4.5. Mortality
All-cause mortality rates were derived from age and gender-specific mortality rates for the general population as reported in US life tables for 2011Citation51. As described by Diamantopoulos et al. mortality rates were adjusted for cerebrovascular-related deaths to avoid double counting of deaths caused by strokes and bleedings. Background mortality rates were further adjusted for treatment effects and elevated risk in the post-stroke health states.
Aspirin was assumed to reduce the risk of background mortality by 9% (HR = 0.91), based on data reported by the European Atrial Fibrillation Trial Study GroupCitation52. The treatment effect of warfarin on background mortality was calculated using indirect evidence from the subgroup analyses of the AVERROES and ARISTOTLE trials (HR = 1.09, SE = 0.359)Citation3,Citation4. The effect of NOAC compared to warfarin (HR = 0.98, SE 0.329), was derived from the network meta-analysis by Ntaios et al.Citation47.
Based on data used by Diamantopoulos et al.Citation27 and Miller et al.Citation28, background mortality risk was increased for the post-stroke health states (mild stroke HR = 2.56, moderate stroke HR = 4.63, severe stroke HR = 13.18). Furthermore, during the acute phase patients may die as a direct result of adverse events. To account for this risk, a case fatality rate was also included for each adverse event ().
Table 4. Case fatality rates.
Table 5. Baseline health state utility estimates used in the model by diagnosis and health state.
4.6. Health-related quality of life
HRQL was incorporated in the model through the use of health state utility values and disutilities associated with the acute stage of adverse events. Baseline utility values (i.e. values for the model’s first year) for the post-index and post-stroke health states were obtained from CRYSTAL-AFCitation1 and the OXVASC studyCitation33, respectively. In order to reflect the fact that the HRQL of people tends to decrease over time due to aging and comorbidities, the following formulaCitation53 was used to adjust the health state utility values over time:
(1)
(1)
UBase represents the utility of the general population as a function of age and gender and can be used as a multiplier to the baseline utilities of our health states. For a population similar in age and gender as the CRYSTAL-AF cohort (61.5 years and 63.5% males), UBase is 0.823. For a population similar to the one entering our model (65 years and 53% male), UBase is 0.805.
The baseline utility for patients in CRYSTAL-AF was reported to be 0.774. An annual utility decrement of 0.056 was applied for patients with AF, resulting in a baseline utility value of 0.718 for patients with AF. When these values were adjusted for age and gender, we obtained baseline utility values of 0.757 (0.774 × 0.805/0.823) and 0.702 (0.718 × 0.805/0.823) for our model for patients without and with AF, respectively ().
Utility decrements associated with adverse events were derived from various cost-effectiveness studiesCitation28,Citation49,Citation50,Citation54. Each time an adverse event occurred, a one-off utility decrement was taken into account to reflect the negative HRQL impact of these events in their acute phase. For strokes (ischemic or hemorrhagic) and other ICHs, the acute disutility was assumed to last for the duration of one cycle (i.e. 3 months). For ECHs, the acute disutility was assumed to last for 2 weeks and for CRNM bleeds for 2 days. Additional acute event disutilities for TIA and SE were collected from prior economic analyses, as reported in .
Table 6. Health state disutilities from adverse events.
4.7. Resource utilization and costs
Only direct medical costs were included in the analysis ().
Table 7. Costs US$ included in the analysis.
4.7.1. Costs related to AF detection
Costs related to the ICM specifically included the acquisition cost of the device, one-off costs for its insertion and later removal and a per-cycle cost related to routine monitoring and patient follow-up. It was assumed that AF detection efforts would end after the three-year monitoring period and that there would be no additional long-term follow-up as a direct result of ICM use once the device had been removed.
Costs for SoC were calculated on the basis of utilization patterns observed in CRYSTAL-AFCitation1,Citation27. Unit costs for individual tests were based on procedural terminology (CPT) codes 93000 (ECG) and 93224 (Holter) as published in policy guidelines by the American managed health care company AETNACitation40. The model separated first, second and third-year tests performed.
4.7.2. Costs of anticoagulation therapy
The costs per cycle of NOAC treatment were based on the average cost of apixaban, rivaroxaban, dabigatran, and edoxaban as reported by Shah et al.Citation41 For warfarin, additional per-cycle costs for INR monitoring were taken into accountCitation49,Citation50.
4.7.3. Costs of managing adverse events
A one-off cost was included for the management of the acute phase of adverse events, obtained from a US cost-effectiveness study by Miller et al.Citation28 comparing two types of NOAC for stroke prevention in patients with nonvalvular AF. For the base-case scenario, we assigned zero cost to fatal strokes although in a sensitivity analysis we assumed the same acute costs for fatal stroke as for a severe ischemic stroke event i.e. $28,192 for a fatal ischemic stroke and $37,725 for a fatal hemorrhagic stroke. This change reduces the ICER slightly, to $23,105 ($97,115–$92,609/6.575–6.380). Case fatalities due to other adverse events were assumed to incur zero costs.
4.7.4. Health states costs
To reflect the longer-term cost of managing patients who have experienced stroke recurrence, health state costs were also included in the model. These longer-term expenditures for the US setting were derived from the study by Miller et al.Citation28
5. Results
5.1. Base-case analysis
All results presented in the base-case (primary) analysis use the inputs described and are considered to be representative of the cryptogenic stroke patient population in the US. Model outputs are presented in , including the absolute number of adverse events expected per patient for each of the treatment strategies. Incremental findings are summarized in .
Table 8. Key model outputs based on discounted values for a cohort of one patient.
Table 9. Events, total costs, life-years and QALYs per patient.
5.1.1. Clinical outcomes
The results suggest that AF detection using a BIOMONITOR device would result in fewer ischemic strokes, TIAs, systemic embolisms and hemorrhagic strokes, but would lead to more other bleedings as a consequence of the use of anticoagulants. The number-needed-to-treat (NNT) in order to prevent one secondary ischemic stroke was estimated at 27.
The model estimates that for every 1,000 patients using an ICM, 37 secondary ischemic strokes and an additional 9 hemorrhagic strokes could be avoided as a consequence of increased AF diagnosis and subsequent treatment. The reduction in the number of strokes causes a larger proportion of ICM patients to remain in the “post-index event” health state and thus, on average, this population retains a better HRQL. The HRQL impacts of adverse events in their acute phase appear to be relatively minor by comparison. Overall, our model suggests that the ICM strategy would lead to an average of 0.265 life years gained and 0.195 QALYs gained per patient over a lifetime.
5.1.2. Cost outcomes
The total lifetime costs associated with patients receiving an ICM are higher than those managed with SoC, by $4,895 on average per patient treated. This difference is largely due to the acquisition, insertion and removal costs associated with the ICM device. The additional costs associated with ICM use are partly offset by a reduction in long-term care costs for patients with recurrent stroke.
5.1.3. Cost-effectiveness
The ICER was estimated to be US$18,487 per life-year gained and US$25,098 per QALY gained (). The cost per stroke avoided (ischemic and hemorrhagic) with the use of an ICM was estimated at $104,941.
6. Sensitivity analysis
6.1. One-way sensitivity analysis
To test the uncertainty around the model’s input parameters, one-way (univariate) sensitivity analyses were performed. Variations in the sensitivity and specificity parameters for both the ICM and SoC strategy comparisons and the prevalence of AF have been discussed previously. For other input values, point estimates included in the base-case analysis were varied within their 95% confidence intervals (CI) where available, otherwise, a standard error of up to 25% around the mean was assumed to generate a low and high estimate. The time horizon was reduced to a low of 10 years.
The main results are shown in the tornado diagram (), which presents the variation in output of the univariate sensitivity analysis of the fifteen parameters (in order of influence) having the largest single impact on the base-case incremental cost-effectiveness ratio. Additional details of these parameters are given in . Key drivers of the ICER were treatment-specific HRs for ischemic and hemorrhagic stroke, the specificity and sensitivity of AF monitoring, in particular in the SoC arm (as this influences the proportion of patients placed on anticoagulation therapy) and the assumed true prevalence of AF. The time horizon over which costs and outcomes are considered was also important given that there are additional costs incurred with ICM use initially, which are somewhat compensated for when the longer-term benefits of monitoring and AF detection are taken into consideration.
Figure 3. Tornado graph of parameters shown in the one-way sensitivity analysis to have the greatest impact on the cost-effectiveness of ICM use (cost in US$per QALY gained). The vertical line represents the base-case ICER for ICMs versus SoC. The horizontal bars indicate the ICER range between their low and high values.
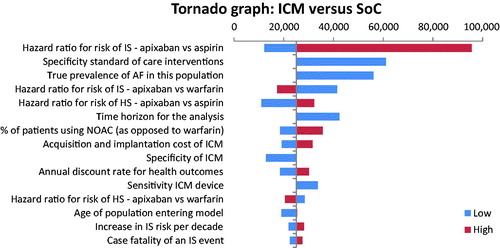
Figure 4. Cost-effectiveness plane illustrating the lifetime differences in costs and outcomes (QALYs) between the two management strategies: ICM versus SoC.
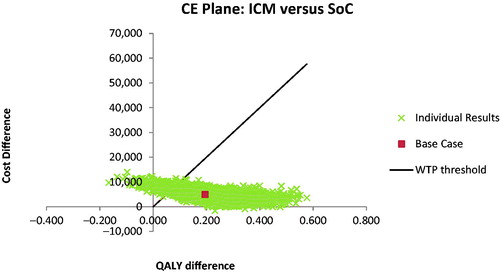
Figure 5. Cost-acceptability curve summarizing the impact of uncertainty on the cost-effectiveness or willingness-to-pay threshold.
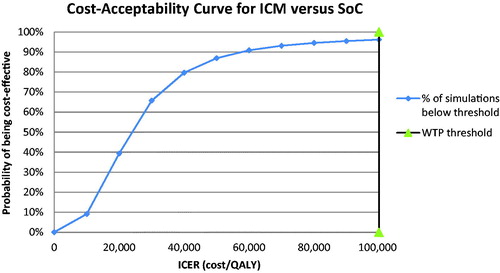
Table 10. Parameters having the greatest impact in terms of cost-effectiveness outcomes on the one-way sensitivity analysis.
As an additional analysis, we assumed a further discontinuation rate of up to 40% with NOACs and up to 50% with warfarin after 12 months. In this extreme case then the ICER increases to $75,320/QALY gained. An increase in discontinuation rates would mainly impact the total QALYs in the ICM group. Higher NOAC/warfarin discontinuation rates lead to a higher number of strokes, especially in the ICM group where more patients have been diagnosed with AF and are taking these therapies. Our model output suggests that the long-term consequences of these strokes (in terms of reduced HRQL due to disability) are more significant than the acute HRQL impact.
6.2. Probabilistic sensitivity analysis
Probabilistic sensitivity analysis (PSA), during which random draws from the distributions of all relevant input parameters are simultaneously taken, was also carried out. We assumed a beta distribution for input parameters that were associated with a probability, proportion, utility and duration (as for adverse events). In the case of proportions for which the sum needed to add to 100% a Dirichlet distribution was assumed. For hazard ratios or relative risks we assumed a log-normal distribution, for costs a gamma distribution and for disutility values a normal distribution.
With a mean incremental cost of $4,897 ($92,082–$87,185) and mean incremental benefit of 0.205 QALYs (6.333–6.128), the PSA based on 10,000 iterations returned a probabilistic ICER of $23,980 per QALY gained for the ICM vs. SoC strategy. Furthermore, it showed that the use of an ICM would be cost-effective in 96% of cases (iterations) considering a willingness-to-pay threshold of $100,000 per QALY.
6.3. Scenario analysis
Treatment-specific HRs for various adverse events in patients with prior ischemic stroke or TIA were found to have a significant impact on the model’s outputs. Given that the 95% confidence intervals around multiple of these parameters did not indicate a statistically significant difference from 1.00, we conducted a scenarios analysis where the specific HRs was set to 1.00, to investigate the potential impact on the model outputs. The differences in outcome were found to be minor, suggesting that the uncertain HRs have only a limited impact on the ICER and that the overall conclusions of the analysis are robust. Thus, although the observation that with the use of an ICM monitoring and subsequent treatment switches from aspirin to anticoagulation therapy would decrease the number of hemorrhagic strokes remains counterintuitive, its impact on the overall model outcomes seems minimal.
7. Discussion
The aim of this study was to evaluate the cost-effectiveness of an ICM, and specifically the BIOMONITOR, versus SoC monitoring for the detection of AF in patients with cryptogenic stroke in the US setting. Our results suggest that, due to the higher proportion of AF patients being diagnosed with the disease and subsequently placed on anticoagulation therapy, use of the ICM rather than SoC would result in fewer ischemic strokes, TIAs, systemic embolisms and hemorrhagic strokes, but would lead to more other bleedings. Approximately 27 patients would need to be “treated” in order to prevent one ischemic stroke over their remaining lifetime. With an incremental cost of $4,895 and incremental health benefit of 0.195 QALYs in the base-case scenario, the ICM versus SoC was found to be associated with an ICER of $25,098 per QALY gained, well below the commonly accepted threshold of $100,000. The increased and earlier access of patients to anticoagulation therapy as a result of a clear AF diagnosis reduces the burden and cost of secondary strokes for both patients and payers.
To our knowledge this is only the second study evaluating the cost-effectiveness of continuous long-term monitoring with an ICM in patients with cryptogenic stroke and the first for the US setting. The analysis published by Diamantopoulos et al. was conducted from a UK National Health Service perspective and was based mainly on the interventions and outcomes observed in the randomized, multinational CRYSTAL-AF trial. Although there are some differences between our models, the overall conclusions are similar. For example, we used gender ratios and age rates from an ICM observational study rather than the CRYSTAL-AF trial population, to better reflect the real-world setting. Another difference was that we assumed a percentage of patients would be taking warfarin, rather than exploring the impact of warfarin use via the sensitivity analysis. Furthermore, in our model patients experiencing subsequent strokes could transition from one post-stroke health state to a more severe health-state whereas in the study by Diamantopoulos et al. patients reaching a post-stroke health state were assumed to remain in that state until the time of death.
We conducted sensitivity analyses to explore the impact of parameter-related uncertainty on our base-case ICER results. Of particular interest was the probability of AF detection assumed for the SoC arm given there is no gold standard for AF detection and that Holter monitoring may be used more intensively than was studied with CRYSTAL-AFCitation11,Citation36,Citation55,Citation56. Our one-way sensitivity analyses revealed that AF detection rates were indeed a key area of sensitivity in the model. Although Holter monitoring may be used more intensively than was observed in CRYSTAL-AF there is a clear limit to the time period over which such a device can reasonably be usedCitation14. There are newer alternatives such as an ECG monitoring patch which may be used for up to 30 daysCitation57,Citation58. However, given that most cases of AF are paroxysmal, longer-term monitoring may be needed in those with suspected AF where that remains undetected with conventional monitoringCitation59.
Another key area of sensitivity in our model was the impact of the HRs assumed for each of the treatments evaluated on the risk of ischemic and hemorrhagic stroke and in particular the HR for aspirin versus apixaban on the risk of ischemic stroke. It should be noted that the effects of NOACs in this patient population, although found to be consistent in patients with and without previous stroke or TIA (apart from vascular death), were based on predefined subgroup analyses of much larger pivotal trials. On the other hand, in the case of apixaban versus aspirin, the AVERROES trial was stopped early because of a clear benefit in favor of apixabanCitation60 suggesting that the overall effect differences observed in the trial have important implications for decision-making in everyday clinical practice. More recently, two large RCTs, NAVIGATE ESUSCitation61 and RE-SPECT ESUSCitation61,Citation62, explored the value of NOACs versus aspirin in patients with cryptogenic stroke without knowledge of AF status. The results revealed no advantage in treating all post-cryptogenic stroke patients thus confirming the need for an accurate AF diagnosis before treatment with anticoagulants is initiated.
Most HRs employed in our analysis of treatment outcomes were derived from large, pivotal RCTs and it is important to understand if similar outcomes could be expected in routine clinical practice and beyond the follow-up periods observed in these studies. We believe there is evidence to suggest at least similar trends in benefits could be expected although again most of the available data is not limited to patients with a specific history of cryptogenic stroke. For example, Lip et al.Citation63 recently published the largest retrospective observational study of its kind to date of nonvalvular AF patients initiating treatment with apixaban, dabigatran, rivaroxaban or warfarin. Data for the analysis was derived from Centers for Medicare and Medicaid Services and four US commercial claims databases. The risk of stroke/systemic embolism and major bleeding was compared across matched cohorts. All three NOACs were found to be associated with at least equivalent and potentially lower rates of stroke or systemic embolism compared with warfarin and lower rates of intracranial hemorrhage. Apixaban and dabigatran also had lower rates of major bleeding overall, although rivaroxaban had a higher rate of gastrointestinal bleeding compared with warfarin.
In order to simplify our model, we assumed that the severity distribution of subsequent stroke episodes was treatment independent. This means that the proportions of subsequent mild, moderate, severe and fatal strokes (also for intracerebral hemorrhage) were applied consistently regardless of whether the patient was taking aspirin or anticoagulants. In reality, clinical evidence indicates that strokes due to large vessel thrombotic occlusion, in particular, are much rarer in anticoagulated patients, even amongst those suffering recurrent ischemic events. It would require additional clinical data plus a rework of the model to introduce treatment-dependent severities. It should be noted that Diamantopoulos et al. used the same data and assumption, referring to treatment-dependent data used in earlier CE models developed by Dorian et al. and Lip et al. Furthermore, we have consistently used averages to model NOAC effects as our analysis does not aim to distinguish between NOACs but rather to assess the likely value of an accurate AF diagnosis. We have also varied the input parameters in our sensitivity analyses and the conclusions were found to remain robust.
Cost-Effectiveness analysis compares two treatment strategies and the incremental results, therefore, represent the primary outcome. The outputs of our model are similar to those of Diamantopolous et al. in terms of recurrent ischemic strokes prevented and lifetime QALY benefit per person. Both studies suggest that, regardless of setting (US or UK), the difference in total cost between the two treatment strategies is actually small. However the absolute costs are higher for the US as compared with the UK. Although it is somewhat difficult to compare intervention costs across countries, it is generally known that healthcare costs in the US are relatively high. Furthermore, when comparing the two studies, it appears that the short-term costs for managing adverse events (minor impact) and particularly the long-term costs for providing care for patients with recurrent stroke (major impact) are the main drivers of cost differences.
The number needed to treat (NNT) is an epidemiological measure used to communicate the effectiveness of a health-care intervention. The NNT with ICMs, estimated at 27 in our study, is high nonetheless ICMs can still be considered a worthwhile investment. This is because the risk to the patient associated with the use of an ICM is very low and in contrast, the clinical endpoint of secondary stroke can be devastating, both in terms of functional impairment and cost to the health care system. We believe that further research is needed to identify patients with cryptogenic stroke who may derive the greatest benefit from ICMs. For example, should patients who have had more severe strokes be the priorities for ICMs or those with concomitant diseases known to increase stroke risk such as diabeties or sleep apnoea? In the future biomarkers may also help to identify patients who can potentially benefit most from ICMs.
Although independent of the ICM technology, persistence with anticoagulant therapy is an important variable impacting the value of an accurate AF diagnosis and thus the real-world cost-effectiveness of ICM use in patients with cryptogenic stroke. Discontinuation rates for anticoagulants vary widely in studiesCitation64–66 and are likely to be higher for warfarin than for NOACs with the additional monitoring, dietary and other restrictions associated. Given the overwhelming evidence in favor of the benefits of anticoagulation in patients with confirmed AF it would appear that, once AF has been diagnosed, specific effort is needed to encourage patients to persist with anticoagulant therapy over the longer term.
8. Conclusions
In conclusion, our results suggest that the use of ICMs in patients with a history of cryptogenic stroke and with suspected AF that is undiagnosed with the short term use of external devices is a cost-effective use of scarce Medicare resources. Facilitating an accurate AF diagnosis and subsequent access to anticoagulation therapy will reduce the burden of secondary stroke on both patients and payers.
Transparency
Declaration of funding
This study was supported by BIOTRONIK SE & Co. KG, Berlin, Germany.
Declaration of financial/other relationships
Richard P. Borge Jr., is Medical Director of the Heart Rhythm Center, Abington - Jefferson Health. Dr Borge is a consultant to Biotronik, St Jude Medical and Cardiofocus corporations. He has received honoraria for speaking, participating in clinical trials, contributing to advisory boards and for clinical training. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author Contributions
The authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported and that no important aspects of the study have been omitted. J. Maervoet designed and developed the model and performed the computations, N. Bossers identified the key data inputs, R. Borge provided expert clinical input, S. Thompson Hilpert took the lead in writing the manuscript, A. van Engen verified the analytical methods and helped supervise the project, A. Smala developed the model concept, provided information and helped supervise the project. All authors contributed to the interpretation of the results and reviewed the final manuscript.
Previous presentations
A poster of this work was presented at ISPOR 20th Annual European Congress, November 2017 in Glasgow, Scotland.
Acknowledgements
None reported.
References
- Sanna T, Diener H-C, Passman RS. Cryptogenic stroke and atrial fibrillation. N Engl J Med. 2014;371(13):1261.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke. 1991;22(8):983–988.
- Diener H-C, Eikelboom J, Connolly SJ, et al. Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol. 2012;11(3):225–231.
- Easton JD, Lopes RD, Bahit MC, et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012;11(6):503–511.
- Coleman CI, Peacock WF, Bunz TJ, et al. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and previous stroke or transient ischemic attack. Stroke. 2017;48(8):2142–2149.
- Li L, Yiin GS, Geraghty OC, et al. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. The Lancet Neurology. 2015;14(9):903–913.
- Hart RG, Diener H-C, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. The Lancet Neurology. 2014;13(4):429–438.
- Verma N, Ziegler PD, Liu S, et al. Incidence of atrial fibrillation among patients with an embolic stroke of undetermined source: insights from insertable cardiac monitors. Int J Stroke. 2019;14(2):146–153.
- Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120–129.
- Kishore A, Vail A, Majid A, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke. 2014;45(2):520–526.
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962.
- Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467–2477.
- Albers GW, Bernstein RA, Brachmann J, et al. Heart rhythm monitoring strategies for cryptogenic stroke: 2015 diagnostics and monitoring stroke focus group report. J Am Heart Assoc. 2016;5(3):e002944.
- Piorkowski C, Busch M, Nölker G, et al. Clinical evaluation of a small implantable cardiac monitor with a long sensing vector. Pacing Clin Electrophysiol. 2019;42(7):1038.
- Reinsch N, Ruprecht U, Buchholz J, et al. The BioMonitor 2 insertable cardiac monitor: clinical experience with a novel implantable cardiac monitor. J Electrocardiol. 2018;51(5):751–755.
- Giancaterino S, Lupercio F, Nishimura M, et al. Current and future use of insertable cardiac monitors. JACC Clin Electrophysiol. 2018;4(11):1383–1396.
- Ziegler PD, Rogers JD, Ferreira SW, et al. Long-term detection of atrial fibrillation with insertable cardiac monitors in a real-world cryptogenic stroke population. Int J Cardiol. 2017;244:175–179.
- Angelis G. d, Cimon K, Sinclair A, et al. Monitoring for atrial fibrillation in discharged stroke and transient ischemic attack patients: recommendations. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016. (CADTH Optimal Use Report; No. 5.2c)
- Kirchhof P. Integrated care of patients with atrial fibrillation: the 2016 ESC atrial fibrillation guidelines. Heart. 2017;103(10):729–731.
- Conti S, Reiffel JA, Gersh BJ, et al. Baseline demographics, safety, and patient acceptance of an insertable cardiac monitor for atrial fibrillation screening: the REVEAL-AF Study. J Atr Fibrillation. 2017;9(5):1551.
- Ciconte G, Giacopelli D, Pappone C. The role of implantable cardiac monitors in atrial fibrillation management. J Atr Fibrillation. 2017;10(2):1590.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992.
- Diamantopoulos A, Sawyer LM, Lip GYH, et al. Cost-effectiveness of an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke. Int J Stroke. 2016;11(3):302–312.
- Miller JD, Ye X, Lenhart GM, et al. Cost-effectiveness of edoxaban versus rivaroxaban for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF) in the US. Clinicoecon Outcomes Res. 2016;8:215–226.
- Canadian Agency for Drugs and Technologies in Health. Antithrombotic agents for the prevention of stroke and systemic embolism in patients with atrial fibrillation. Ottawa (ON): CADTH; 2013.
- Diener H-C, Connolly SJ, Ezekowitz MD, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol. 2010;9(12):1157–1163.
- Hankey GJ, Patel MR, Stevens SR, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11(4):315–322.
- Ziegler PD, Rogers JD, Ferreira SW, et al. Real-world experience with insertable cardiac monitors to find atrial fibrillation in cryptogenic stroke. Cerebrovasc Dis. 2015;40(3-4):175–181.
- Luengo-Fernandez R, Paul NLM, Gray AM, et al. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke. 2013;44(10):2854–2861.
- Rost NS, Giugliano RP, Ruff CT, et al. Outcomes with edoxaban versus warfarin in patients with previous cerebrovascular events: findings from ENGAGE AF-TIMI 48 (effective anticoagulation with factor Xa next generation in atrial fibrillation-thrombolysis in myocardial infarction 48). Stroke. 2016;47(8):2075–2082.
- Sanna T, Ziegler PD, Crea F. Detection and management of atrial fibrillation after cryptogenic stroke or embolic stroke of undetermined source. Clin Cardiol. 2018;41(3):426–432.
- Sposato LA, Cipriano LE, Saposnik G, et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. The Lancet Neurol. 2015;14(4):377–387.
- Glotzer TV, Ziegler PD. Silent atrial fibrillation as a stroke risk factor and anticoagulation indication. Can J Cardiol. 2013;29(7):S14–S23.
- Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford (UK): Oxford University Press; 2006.
- Lauffenburger JC, Farley JF, Gehi AK, et al. Factors driving anticoagulant selection in patients with atrial fibrillation in the United States. Am J Cardiol. 2015;115(8):1095–1101.
- AETNA. Holter Monitors [Internet]. Hartford (CT): AETNA; 2016 [cited 2016 Dec 15]. Available from: http://www.aetna.com/cpb/medical/data/1_99/0019.html.
- Shah A, Shewale A, Hayes CJ, et al. Cost-effectiveness of oral anticoagulants for ischemic stroke prophylaxis among nonvalvular atrial fibrillation patients. Stroke. 2016;47(6):1555–1561.
- Ariesen MJ, Claus SP, Rinkel GJE, et al. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34(8):2060–2065.
- Paciaroni M, Agnelli G, Caso V, et al. Causes and risk factors of cerebral ischemic events in patients with atrial fibrillation treated with non-vitamin K antagonist oral anticoagulants for stroke prevention. Stroke. 2019;50(8):2168.
- Moerch-Rasmussen A, Nacu A, Waje-Andreassen U, et al. Recurrent ischemic stroke is associated with the burden of risk factors. Acta Neurol Scand. 2016;133(4):289–294.
- Mohan KM, Crichton SL, Grieve AP, et al. Frequency and predictors for the risk of stroke recurrence up to 10 years after stroke: the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2009;80(9):1012–1018.
- Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110(16):2287–2292.
- Ntaios G, Papavasileiou V, Diener H-C, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43(12):3298–3304.
- Edlin R, McCabe C, Hulme C, et al. Cost effectiveness modelling for health technology assessment: a practical course. Cham (Switzerland): ADIS; 2015.
- Dorian P, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J. 2014;35(28):1897–1906.
- Lip GYH, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther. 2014;36(2):192.e20–210.e20.
- Arias E. United States Life Tables, 2011. Natl Vital Stat Rep. 2015;64(11):1–63.
- Group EEATFTS. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. The Lancet. 1993;342(8882):1255–1262.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–518.
- Miller PSJ, Andersson FL, Kalra L. Are cost benefits of anticoagulation for stroke prevention in atrial fibrillation underestimated? Stroke. 2005;36(2):360–366.
- Cipriano LE, Sposato LA. Estimating the sensitivity of holter to detect atrial fibrillation after stroke or transient ischemic attack without a gold standard is challenging. Am J Cardiol. 2016;117(2):314–316.
- Choe WC, Passman RS, Brachmann J, et al. A comparison of atrial fibrillation monitoring strategies after cryptogenic stroke (from the cryptogenic stroke and underlying AF trial). Am J Cardiol. 2015;116(6):889–893.
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ecg monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA. 2018;320(2):146–155.
- Reinke F, Bettin M, Ross LS, et al. Refinement of detecting atrial fibrillation in stroke patients: results from the TRACK-AF study. Eur J Neurol. 2018;25(4):631–636.
- Mairesse GH, Moran P, van Gelder IC, et al. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). Europace. 2017;19(10):1589–1623.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–817.
- Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378(23):2191–2201.
- Diener H-C, Easton JD, Granger CB, et al. Design of randomized, double-blind, evaluation in secondary stroke prevention comparing the efficacy and safety of the oral thrombin inhibitor dabigatran etexilate vs. acetylsalicylic acid in patients with embolic stroke of undetermined source (RE-SPECT ESUS). Int J Stroke. 2015;10(8):1309–1312.
- Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49(12):2933–2944.
- Beyer-Westendorf J, Förster K, Ebertz F, et al. Drug persistence with rivaroxaban therapy in atrial fibrillation patients-results from the Dresden non-interventional oral anticoagulation registry. Europace. 2015;17(4):530–538.
- Beyer-Westendorf J, Ehlken B, Evers T. Real-world persistence and adherence to oral anticoagulation for stroke risk reduction in patients with atrial fibrillation. Europace. 2016;18(8):1150–1157.
- Gallagher AM, Rietbrock S, Plumb J, et al. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis?. J Thromb Haemost. 2008;6(9):1500–1506.
- Nölker G, Mayer J, Boldt L-H, et al. Performance of an implantable cardiac monitor to detect atrial fibrillation: results of the DETECT AF study. J Cardiovasc Electrophysiol. 2016;27(12):1403–1410.