Abstract
Background: Few studies have examined the safety and efficacy of transcatheter aortic valve implantation (TAVI) for severe aortic stenosis (AS) in Australian patients. SOLACE-AU was a single-arm, open-label clinical trial conducted in Australian hospitals to determine the safety, performance, and cost implications of TAVI in patients with severe, symptomatic AS at intermediate surgical risk.
Methods: This was a prospective, pragmatic, single-arm, multi-center, observational trial of 199 patients with severe, symptomatic AS treated with transfemoral TAVI using the SAPIEN XT transcatheter heart valve (THV) at 11 hospitals in Australia. The primary outcome was Valve Academic Research Consortium-2 (VARC-2) criteria – a composite of seven safety endpoints. Kaplan–Meier (KM) estimates and descriptive analyses were used to evaluate the impact of transfemoral TAVI on safety and valve performance. We also evaluated patient health-related quality of life (QoL) and healthcare resources used throughout the trial.
Results: The valve was successfully implanted in 88% of patients. The VARC-2 outcome at 30 days was 12.1% (95% CI: 8.3–17.5%), and almost 90% of patients had improved heart failure symptoms at 1 year based on New York Heart Association functional class criteria. Patient QoL remained stable over time, with mean EQ-5D-3L scores being 0.71 ± 0.20 at baseline and 0.71 ± 0.21 at 2 years. Duration of post-procedure hospitalization (mean: 6.9 ± 4.7 days) decreased as procedural familiarity increased. The median total cost of TAVI decreased 10.1% at 3 years after introduction of the procedure at the sites.
Conclusions: The SOLACE-AU trial demonstrated favorable safety and performance of the SAPIEN XT valve in patients with AS at intermediate risk of surgical complications.
Introduction
Aortic stenosis (AS) is the progressive narrowing of the aortic valveCitation1,Citation2. The ensuing hemodynamic changes further contribute to valvular calcification, stenosis and left-ventricular hypertrophy, worsening cardiac output, and ultimately leading to patient deathCitation2. Patients with AS are often asymptomatic, despite significant stenosis. However, upon disease progression, aortic stenosis is associated with high morbidity and mortality; the expected mortality rate is 50% for untreated, symptomatic patientsCitation3. In Australia, the prevalence of AS in the general population is estimated to range between 2.9% and 13.6%Citation4. Of this prevalent population, 21.6% were estimated to have severe AS, with the majority (71.1%) of the severe AS population being symptomaticCitation4.
Current treatment options for patients with severe, symptomatic AS involve aortic valve replacement, either through surgical or transcatheter methods. For decades, surgical aortic valve replacement (AVR), in which the diseased valve is replaced by opening the sternum to access the heart, excise the diseased valve and suture a new valve in place, was the only treatment option [Citation4,Citation5]. In AVR, the diseased valve may be replaced with biological or mechanical valve prostheses [Citation5,Citation6]. However, advancing age and comorbidities may preclude patients from AVR, with at least one-third of patients being ineligible for surgeryCitation7. Patients are considered inoperable or at ultra-high risk of surgical complications upon assessment of key patient co-morbidities by a heart team; this assessment is facilitated through the determination of the Society of Thoracic Surgeons (STS) score for predicting patient mortality. Patients with STS scores predicting 30-day mortality >8% or risk of major morbidity >50% may be considered as high riskCitation7. Other considerations in the assessment of patient risk include the degree of aortic calcification, patient frailty and cirrhosis of the liverCitation5,Citation7. Patients determined to be at significant risk of complications from AVR may be eligible for transcatheter aortic valve implantation (TAVI) in AustraliaCitation8. Transcatheter aortic valve implantation is a minimally invasive intervention involving the catheter-based implantation of a prosthetic valve through either the transfemoral or the transapical approach, within the diseased native aortic valveCitation8. Transcatheter bioprosthetic valves may be balloon-expandable or self-expanding. Previous trials have shown that TAVI is non-inferior to AVR in patients at high risk for surgical complications, including operative death, and superior to medical managementCitation3,Citation9–11.
The SAPIEN XT transcatheter heart valve (THV; Edwards Lifesciences; Irvine, CA) is a second-generation TAVI system. It was developed to reduce the incidence of complications associated with TAVI, including stroke and vascular access-site bleedingCitation9,Citation12. Globally, improved transcatheter valve technologies and increasing operator experience have led to the selection of patients of intermediate and low risk for surgical complications for TAVICitation7,Citation12. Currently, there is a Class IIa recommendation for TAVI for patients at intermediate surgical risk, based on the American College of Cardiology/American Heart Association (ACC/AHA) guidelinesCitation13. Furthermore, the European Society of Cardiology/European Association for Cardio-Thoracic Surgery (EACTS) guidelines favor TAVI over AVR for elderly patients with suitable transfemoral access and elevated surgical riskCitation5. These recommendations were based on the increasing evidence for the safety and efficacy of TAVI amongst a population at an intermediate level of risk of AVR complicationsCitation7,Citation12. Importantly, recent studies comparing TAVI with AVR in a low-risk population setting (STS <4%) have demonstrated that TAVI is at least non-inferior to AVRCitation14,Citation15. Hence, it is likely that guidelines for the recommendation of TAVI will be extended to include the low-risk AS population.
The Safety, Performance, Quality of Life and Cost Effectiveness Outcomes of the Edwards SAPIEN XT Transcatheter Heart Valve in an Australian Population (SOLACE-AU) study was conducted to determine the safety and performance of the SAPIEN XT THV in intermediate-risk patients with severe, symptomatic AS. This study also explored changes in quality of life (QoL) post-TAVI, and health care resources usage compared with matched AVR patients in an Australian healthcare environment.
Methods
Study design and oversight
SOLACE-AU was a prospective, pragmatic, single-arm, multi-center, observational trial (NCT01675596) of SAPIEN XT delivered via transfemoral artery access. The trial was designed and monitored by the sponsor (Edwards Lifesciences) in conjunction with a steering committee composed of the principal investigator, selected co-investigators, and other clinical trial experts. The steering committee met regularly to monitor patient enrollment and site compliance. An independent clinical events committee (CEC) was also appointed for the adjudication of select outcomes. The CEC was composed of three interventional cardiologists who were not investigators in the trial and had no financial relationship with the sponsor or the clinical sites. A human research ethics committee approved the study protocol before study activities commenced. The manuscript was prepared in accord with the Strengthening of Reporting of Observational Studies in Epidemiology (STROBE) guidelinesCitation16.
Patients
From April 2012 to February 2016, patients with severe, symptomatic AS were enrolled across 11 sites in Australia. Eligible patients had senile, degenerative AS with echocardiographically derived criteria. Symptomatic AS was demonstrated by the New York Heart Association (NYHA) functional class II or greater. Patients with severe, symptomatic AS were assessed by a multidisciplinary heart team and considered eligible for the study if they had an intermediate risk of mortality within 30 days of AVR. An intermediate risk of surgical complications was defined based on patient age ≥70 years and an STS-predicted risk of mortality (PROM) score ≥4. Patients with an STS <4 were eligible for the study if other clinical characteristics, such as advanced liver disease or complications with prior cardiac surgery, indicated an intermediate risk of complications from AVR. If concomitant coronary disease was present, the heart team had to agree on the treatment strategy.
Patients were excluded if they were <70 years of age, or had a native aortic annulus <18 mm or >25 mm. Additional exclusion criteria included the presence of congenital aortic stenosis, unicuspid or bicuspid aortic valves, non-calcific acquired AS, mixed aortic valve disease, and myocardial infarction (MI) within 1 month of treatment, and inability to tolerate anticoagulation therapy. (Full inclusion and exclusion criteria are presented in Supplementary Appendix A).
Patients who received transfemoral SAPIEN XT were followed for a minimum of 2 years and a maximum of 5 years.
Device and procedure
The balloon-expandable SAPIEN XT system and the TAVI procedure have been previously describedCitation12,Citation17. Valve placement was through the transfemoral artery, using a 23, 26, or 29 mm THV. All patients received ≥100 mg aspirin, and low molecular weight or unfractionated heparin, pre-procedure. Post-procedure, patients received ≥100 mg aspirin daily, and clopidogrel, warfarin, or dabigatran for a minimum of 1 month.
Study outcomes
Primary outcome
The primary outcome for SOLACE-AU was a composite of 7 safety variables collected 30 days post-index procedure, based on Valve Academic Research Consortium-2 (VARC-2) criteriaCitation18. The safety variables were all-cause mortality, all stroke, life-threatening bleeding, acute kidney injury (stage 3), coronary artery obstruction requiring intervention, major vascular complications, and valve-related dysfunction requiring a repeat procedure.
Secondary outcomes
Key secondary safety and performance outcomes were evaluated throughout the SOLACE-AU trial at 30 days, 6 months, and annually up to 2 years post-index procedure. The outcomes were the rate of all-cause mortality, cardiovascular mortality, stroke, vascular complications, acute kidney injury, device success, and NYHA functional class changes.
Patient quality of life and resource use
Health-related QoL was assessed using both the short form-36 version 2 (SF-36v2) and EuroQol-5-dimension-3-level (EQ-5D-3L) questionnairesCitation19,Citation20 at 30 days, and 1 and 2 years post-procedure, at the minimum. The SF-36v2 is a self-reported, 36-item questionnaire that assesses patient QoL across 35 items for eight scales of physical and mental health. The aggregated physical health component score (PCS) and the mental health component score (MCS) may be estimated when scores for all of the eight domains are available.
The EQ-5D-3L is a multi-attribute utility instrument for assessing the overall health state of an individualCitation20. The questionnaire encompasses five dimensions of health (mobility, self-care, usual activities, pain or discomfort, and anxiety or depression). Three levels of responses, ranging from no problems to severe problems, are possible for each dimension in the EQ-5D-3L. The scores were converted into patient utility scores using Australian population-specific weightsCitation20. Utility values range in scale from 0–1, with 1 representing full health and 0 representing deathCitation21. In economic evaluation studies, utility values are used to weight the clinical benefits of interventions (survival) by the QoL associated with post-procedure healthCitation21.
Additionally, data pertaining to the number of admissions and total days of stay in hospital at 12 months post-index procedure were collected as secondary outcomes, as were data on patient emergency department (ED) attendances and outpatient visits. The rate of permanent pacemaker (PPM) implantation was collected as part of the key safety outcomes. The incidence of new PPM implantation was included in the health care resource use analysis, to determine its impact on health resources useCitation2,Citation22–25.
All data were site-reported and monitored with independent adjudication of serious adverse events. All adverse events were reclassified according to VARC-2 criteria after these definitions became available. There were no core laboratories used.
Statistical analyses
Data on patient QoL, duration of hospitalization, and resources usage were presented through descriptive and summary statistics unless otherwise specified. For continuous variables, mean (±standard deviation [SD]) or median (interquartile range [IQR]) were presented as descriptive statistics. For binary variables, proportions or rates were presented as descriptive statistics.
Two populations were analyzed. The as-treated population (AT) comprised all enrolled patients who started the procedure, whether it was completed or not. This population was analyzed for the safety of SAPIEN XT. The valve implant (VI) population was a subset of the AT population analyzed for SAPIEN XT performance. Patients in the VI population completed the SAPIEN XT implant procedure.
Patient safety and performance analyses
The primary outcome was analyzed using Kaplan Meier (KM) event rates and the Greenwood standard error; SAS defaults were used for confidence bands and transformations on survival time for the confidence limits. Secondary outcomes, except for NYHA functional class, device success, patient length of stay (LOS) in the hospital, health care resources usage and QoL, were also analyzed using KM estimates.
Patient quality of life and resource use analyses
The impacts of the site and procedural familiarity with TAVI on patient length of stay (LOS) in hospital were also explored. Median, IQR, lower and upper quartiles, and outlier values for patient LOS were estimated over each year from site introduction of TAVI to describe changes in patient LOS across 11 sites over time. Furthermore, patient LOS was stratified based on the number of procedures performed during the trial to explore the impact of operator familiarity with TAVI on LOS and hospital resources use. Sites performing ≤20 procedures were considered to have performed a low volume of TAVI cases, based on Australian guidance for operator and institutional requirements for a TAVI programCitation8.
All statistical analyses were performed with the use of STATA software, version 14 (StataCorp, College Station, TX) and SAS version 9.4 (SAS Institute, Cary, NC).
Cost analyses
At the time of this report, there was no Australian Refined Diagnosis Related Group (AR-DRG) code listed for TAVI. As AR-DRGs are used in the estimation of health care resources usage and health care costs, an exploratory analysis was performed using hospital ‘cost-bucket’ data and data from the National Hospital Cost Data Collection (NHCDC) reports. As this represents an exploratory analysis, the methodology used in the estimation of the cost impacts of TAVI for patients with severe, symptomatic AS at intermediate surgical risk is presented in Supplementary Appendix C.
Results
Patient characteristics and procedural outcomes
A total of 199 patients were enrolled in the SOLACE-AU trial. Of these patients, 15 withdrew consent, one patient was lost-to-follow-up and eight patients exited the study for other reasons. A total of 88.7% of patients had completed the 2-year follow-up visit, and the mean follow-up time was 2.7 ± 1.34 years. The AT population comprised of 199 patients, and 198 patients completed the implantation procedure (VI population). summarises the baseline patient characteristics and procedural characteristics for the AT population.
Table 1. Patient characteristics at baseline (all treated population).
The mean patient age was 86 years, and there were slightly more females (54%) in the cohort. Common patient comorbidities included hypertension (76.9%), coronary artery disease (64.3%), congestive heart failure (46.7%), atrial fibrillation (36.2%), previous coronary artery bypass grafting (22.1%) and prior percutaneous coronary intervention (20.5%). The mean procedure time was 76 min, and there were no device malfunctions. No patients required conversion from TAVI to surgery.
Patient safety outcomes
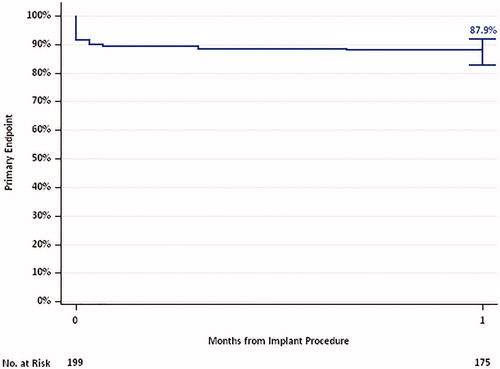
The primary and key secondary safety outcomes of the SOLACE-AU trial are summarised below (see and ). Based on the KM analyses of the VARC-2 composite outcome, the primary outcome safety variables (all-cause mortality, stroke and other key safety criteria) occurred among 12.1% (95% CI: 8.3–17.5%) of patients in the AT population within 30 days (see ). Of the VARC-2 outcomes, patients had experienced major vascular complications (6.0%), bleeding (3.5%), and stroke (3.5%) within the first 30 days (see Supplementary Appendix B). The occurrence of key secondary safety events throughout the study was low, with the most commonly observed safety outcome at 30 days being vascular complications (KM estimate: 13.1%; 95% CI: 9.1–18.6%) (see ).
Table 2. Key primary and secondary outcomes (AT population).
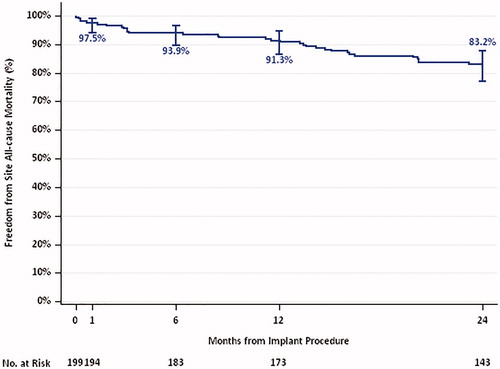
The KM curve for all-cause mortality through 2 years for the AT population is presented in . Over a period of 2 years, 32 deaths (16.8%) occurred. Of these deaths, 16 patients (8.8%) died due to cardiac causes, 14 (7.8%) due to non-cardiac causes, and two (1.1%) died due to unknown causes.
Patient performance outcomes
summarises the trends in patient NYHA functional class and valve hemodynamics over time observed for the VI population. A significant proportion of patients experienced improvement in NYHA functional class at 30 days, which was maintained to 1 year post-implant. Furthermore, the proportion of individuals with NYHA Class III declined markedly from 71.7% at baseline, to 6.7% at 1 year post-implantation. Only a small proportion (6.3%) of patients had moderate-to-severe paravalvular leakage (PVL) at baseline, and the majority of patients had mild or less PVL throughout the study. Declines in mean (mean decline: 39.5 mmHg) and peak (mean decline: 61.7 mmHg) aortic valve gradients, along with increases to valve effective orifice area (EOA) (mean increase: 1.0 cm2, SD: 0.43 cm2) were observed between baseline and 30 days, and were maintained over 2 years of follow-up (Supplementary Appendix B).
Table 3. NYHA functional class and valve haemodynamics over time (VI population).
Quality of life
summarises patient QoL over time. Standardized population norms for both the mental health score and the physical component score for the SF-36v2 are 50Citation3. For the VI population, the mean SF-36v2 physical (PCS) and mental component scores (MCS) were 36.1 ± 8.5 and 47.9 ± 10.9, respectively, at baseline. These scores improved slightly at 30 days post-procedure, but the differences were not clinically significant. Additionally, these scores did not vary significantly at subsequent follow-ups.
Table 4. Patient health-related QoL over time (VI population).
Similarly, patient health-related QoL, as measured using the EQ-5D-3L, remained relatively stable over time. The mean patient utility score was 0.71 at baseline, improving slightly to 0.75 at the 30-day, post-procedure follow-up, and decreasing slightly to 0.71 at 2 years (see )Citation20.
Length of stay and resource usage analyses
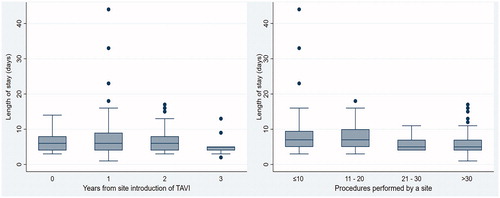
presents the hospital LOS by the number of years of experience in performing TAVI and the number of TAVI procedures performed. In the first year post TAVI, the average LOS was 6.7 ± 2.9 days. In the second year, the average LOS increased to 7.4 ± 6.2 days. In the third year, the average LOS decreased to 6.6 ± 3.6 days, and then to 5.4 ± 3.0 in year 4. Of the 11 sites that performed TAVI, eight were considered to perform a low volume of procedures (≤20 TAVI); the average LOS was 8.4 ± 6.4 days for these sites. For sites performing fewer than 10 procedures, the average hospital LOS was 9.0 ± 7.7 days compared with an average of 6.0 ± 3.2 days for sites performing more than 30 TAVI procedures.
The results of the exploratory costing analyses are presented in Supplementary Appendix C. Briefly, based on micro-cost data obtained from the major study sites, the median total cost of TAVI in year 1 was $61,642 (IQR: $50,014–$70,077), decreasing to $55,400 (IQR: $49,647–$60,162) in year 4 for the Fiona Stanley Hospital (FSH) and the Royal Perth Hospital (RPH). TAVI procedural costs remained relatively stable over time. The average cost of a prosthesis was $29,574 ± $5,514 and varied across the major sites and over the four years of the trial depending on price-volume-service agreements with Edwards Lifesciences.
The number of ED attendances and outpatient visits up to 1 year post-procedure are presented in . The cumulative number of ED attendances were heavily skewed for both non-admitted (median attendances: 2; IQR: 1–2; range: 1–5) and admitted patients (median: 3; IQR: 2–4; range: 1–9) at the 1-year, follow-up period. The cumulative number of outpatient visits per patient were also heavily skewed at the 1-year follow-up period (median: 3; IQR: 3–5; range: 1–10). Costs attributed to ED attendances and outpatient visits were correspondingly skewed; at the 1-year, follow-up period, the median cumulative cost of ED attendances for non-admittted patients was $540 (IQR: $483, $1,029), and $2,074 (IQR: $1,041–$3,072) for admitted ED patients (see Supplementary Appendix C). The median cumulative cost of outpatient visits at the 1-year, follow-up period, after allowing for estimates in temporal trends, varied from $859 (IQR: $859–$1,432) to $1,163 (IQR: $1,163–$1,938).
Table 5. ED attendances and outpatient visits over time (VI population).
Discussion
Valve safety and performance
Following the success of the PARTNER 2 A trial that demonstrated the safety and efficacy of TAVI using balloon-expandable THV in patients with severe AS at intermediate operative riskCitation12, the SOLACE-AU trial determined the initial experience of TAVI using the second-generation SAPIEN XT THV for similar patients in Australia. It should be noted that the third-generation Sapien 3 valve is now approved for use in AustraliaCitation7. Based on the primary safety outcome, the majority of patients experienced no significant safety event at 30 days post-procedure. The safety of TAVI using the SAPIEN XT for this intermediate-risk patient population was further supported by KM analyses of key secondary safety outcomes.
Data pertaining to patient NYHA class and valve hemodynamic characteristics were considered in the evaluation of TAVI efficacy. Overall, the rate of successful device implantation was high, and the majority of patients saw improvement in NYHA functional class at 30 days post-procedure. Improvements to patient NYHA functional class were maintained through 1 year following implantation; importantly, the proportion of patients with NYHA functional Class III heart failure symptoms in the cohort declined drastically at 30 days post-procedure. The frequency and rate of PVL were broadly comparable with data from the PARTNER 2A trial, with the majority of patients having only mild PVL over timeCitation12.
It should be noted that recent trials have demonstrated that TAVI was at least non-inferior to AVR for patients at low risk of surgical complications (STS-PROM <4)Citation14,Citation15. In the recent PARTNER 3 trial comparing TAVI using a balloon-expanded prosthesis with AVR, the rate of occurrence of the composite outcome of all-cause death, stroke, or rehospitalization was considerably lower for patients treated with TAVI relative to patients treated using AVR at 1 year (hazard ratio (HR): 0.54; 95% CI: 0.37–0.79) (p = .001)Citation14. A separate trial comparing TAVI using a self-expanding prosthesis with AVR, concluded that TAVI was non-inferior based on the reduction in composite outcome of all-cause death or disabling stroke at 24 monthsCitation15. Such promising results highlight the potential utility in expanding guidelines for TAVI to include the AS population at low surgical risk of complications in Australia.
Patient quality of life and length of stay
Slight variations around patient QoL over time were observed. Based on the EQ-5D-3L, there was a numerical improvement to patient QoL at 30 days post-procedure compared with baseline estimates, and a gradual return to baseline levels over 2 years. It is expected that elderly patients (mean age of 86 years) would have a background decrease in quality of life. The results from the EQ-5D-3L provide support that TAVI improves QoL as patients had a similar EQ-5D-3L score at 2 years follow-up as they had a baseline (i.e. an initial gain that declined over time). Without TAVI these patients likely would have died or had substantially lower QoL. However, these variations were not clinically significant, and overall, patients QoL remained stable over time. Similarly, PCS and MCS scores measured using the SF-36v2 questionnaire varied slightly through time, and overall, reflect the stable patient QoL measured using the EQ-5D-3L. It should be noted that utility values estimated using the SF-36v2, through conversion to the short form, 6-dimension questionnaire, may not be appropriate for the severe, symptomatic AS population. This is based on a prior study that found that the SF-6D was less discriminative in the setting of severe cardiovascular disease conditionsCitation21.
Concerning patient index hospitalizations, the average hospital LOS for patients in the SOLACE-AU cohort was favorable in comparison with the initial reports from the PARTNER 2 trials, where the average LOS was 8 daysCitation3,Citation10. Furthermore, the results of the SOLACE-AU study demonstrated that as operators become more experienced (i.e. more procedures performed and more years of experience), the average LOS markedly decreased (6.0 days from 30+ procedures), whereas those with low throughput (<10 procedures) had an average LOS of 9.0 ± 7.7 days. Operators with low throughput were the new adopters of TAVI and becoming familiar with the procedure. This familiarity is not just a training matter for the interventionalist, but requires a major shift in management throughout the hospital care pathway for TAVI patients. For example, the nursing staff learn how to manage TAVI patients; likewise coronary care staff may be extra cautious in managing TAVI patients and be more vigilant with these patients, detaining them longer. Reduced LOS increases bed availability and enables greater throughput of patients in a hospital and a subsequent reduction in hospital resources utilization. Importantly, based on data from the PARTNER 3 trial, the median length of index hospitalization was 3.0 (IQR: 2.0–3.0). Hence, further reductions in resource use may be possible upon the introduction of the latest generation of balloon-expandable devices, and the inclusion of low-risk AS patients for TAVI in Australian guidelinesCitation14.
Healthcare resources usage
In the exploratory costing analyses, it was noted that the key driver of index hospitalization costs was the prosthesis itself. While minor variations to prosthesis costs were noted over time and across hospitals, prosthesis costs drove 51.0% of the costs of index hospitalisation (Supplementary Appendix C). Costs associated with the index TAVI procedure decreased between the first and fourth year of introducing the TAVI procedure to TPCH, the FSH and the RPH; this was in line with decreasing LOS at these sites. The mean cumulative cost of outpatient visits per patient and the cumulative cost of ED attendances at the 1 year follow-up were heavily skewed. This was attributed to the large degree of variance around the number of ED attendances per patient for a given period. Furthermore, healthcare costs are typically positively skewed, with the majority of costs being attributed to a minority of individualsCitation24.
As part of this analysis on health resources usage, the rate of PPM implantation was explored (see Supplementary Appendix D). The incidence of PPM implantation in the SOLACE-AU trial was consistent with other trials evaluating TAVI using balloon-expanding prosthesesCitation9,Citation11,Citation12. Further, in comparison with TAVI studies using self-expanding prostheses, patients in the SOLACE-AU trial had markedly lower rates of PPM implantationCitation22,Citation25. This was in line with literature comparing self-expanding and balloon-expandable prostheses, and highlights the potential cost-savings associated with the use of balloon-expandable prosthesesCitation23,Citation25.
Limitations
This study had some limitations. Firstly, this was an open-label, non-randomized study without a comparator arm, with patients selected for a procedure following an assessment of risk for AVR. Hence, it was not possible to assess the performance and safety of transfemoral TAVI compared with AVR or transapical TAVI. It should be noted that the comparisons between SOLACE-AU and current literature on AVR were indirect in nature, and should be interpreted in this light. Furthermore, the number of patients recruited across hospitals over time was small with half of the patient population being recruited by three hospitals in Australia (TPCH/FSH/RPH). Patient recruitment across other sites contributed to 2.0% (Royal North Shore) to 24% (Alfred Hospital) of the total patient cohort.
The estimates pertaining to health care resource use were exploratory. Furthermore, the accuracy of costs estimated for ED attendances and outpatient visits in the analyses are limited by the application of a general, nationwide cost per admission from NHCDC data. A key strength of this study is the bottom-up, micro-costing analyses using hospital cost data from TPCH and FSH/RPH is that the direct costs attributed to a procedure may be estimatedCitation26. Importantly, micro-cost data from TPCH and FSH/RPH comprised 99 patients (as-treated population) and represents approximately 50% of the SOLACE-AU as-treated cohort. Further, follow-up data was available up to 2 years from procedure, allowing for the assessment of long-term safety and performance of TAVI.
Conclusions
The SOLACE-AU study demonstrated that the TAVI procedure for intermediate-risk patients was associated with a favorable safety and device performance profile. Additionally, patient QoL remained stable over time. It is likely that TAVI will lead to a reduction in health care resources usage and costs. However, the cost savings estimated in this study are exploratory and may not be applicable outside of the local setting. Ultimately, a randomized, controlled trial of this procedure compared with AVR within the local clinical context is essential to define the true value of this novel technology in Australia.
Transparency
Declaration of funding
The SOLACE-AU trial was funded by Edwards Lifesciences.
Declaration of financial/other relationships
PL and PS were funded by Edwards Lifesciences to provide this report. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose. All authors met the ICMJE criteria for authorship.
Appendices
Download MS Word (38.4 KB)Acknowledgements
Tracey Fine, MS, ELS of Edwards Lifesciences provided medical writing services.
References
- Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis. J Am Coll Cardiol. 2012;60(19):1854.
- Joseph J, Naqvi SY, Giri J, et al. Aortic stenosis: pathophysiology, diagnosis, and therapy. The Am J Med. 2017;130(3):253–263.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607.
- De Sciscio P, Brubert J, De Sciscio M. Aortic stenosis in Australia: disease prevalence and patient eligibility for aortic valve replacement. Heart, Lung and Circulation. 2015;24:S409.
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Euro Heart J. 2017;38(36):2739–2791.
- Borger MA, Moustafine V, Conradi L, et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. The Ann Thorac Surg. 2015;99(1):17–25.
- Nelson AJ, Montarello NJ, Cosgrove CS, et al. Transcatheter aortic valve implantation: a new standard of care. The Med J Austr. 2018;209(3):136–141.
- Walters DL, Webster M, Pasupati S, et al. Position statement for the operator and institutional requirements for a transcatheter aortic valve implantation (TAVI) program. Heart, Lung and Circulation. 2015;24(3):219–223.
- Fearon WF, Kodali S, Doshi D, et al. Outcomes after transfemoral transcatheter aortic valve replacement: a comparison of the randomized PARTNER (Placement of AoRTic TraNscathetER Valves) trial with the NRCA (Nonrandomized Continued Access) registry. JACC:Cardiovasc Interv. 2014;7(11):1245–1251.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198.
- Thourani VH, Suri RM, Gunter RL, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. The Ann Thorac Surg. 2015;99(1):55–61.
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620.
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2017;135(25):e1159–e1195.
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–1705.
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706–1715.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349.
- Webb JG, Altwegg L, Masson JB, et al. A new transcatheter aortic valve and percutaneous valve delivery system. J Am Coll Cardiol. 2009;53(20):1855–1858.
- Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Euro J Cardio-Thorac Surg: Offic J Euro Assoc Cardio-Thorac Surg. 2012;42(5):S45–S60.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292.
- Viney R, Norman R, King MT, et al. Time trade-off derived EQ-5D weights for Australia. Value in Health: J Int Soc Pharmacoecon Outc Res. 2011;14(6):928–936.
- Kularatna S, Byrnes J, Chan YK, et al. Comparison of the EQ-5D-3L and the SF-6D (SF-12) contemporaneous utility scores in patients with cardiovascular disease. Qual Life Res. 2017;26(12):3399–3408.
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790–1798.
- Barbanti M, Gulino S, Costa G, et al. Pathophysiology, incidence and predictors of conduction disturbances during transcatheter aortic valve implantation. Exp Rev Med Dev. 2017;14(2):135–147.
- Mihaylova B, Briggs A, O’Hagan A, et al. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20(8):897–916.
- Wijeysundera HC, Qiu F, Koh M, et al. Comparison of outcomes of balloon-expandable versus self-expandable transcatheter heart valves for severe aortic stenosis. The Am J Cardiol. 2017;119(7):1094.
- Tan SS, Rutten FF, van Ineveld BM, et al. Comparing methodologies for the cost estimation of hospital services. Eur J Health Econ. 2009;10(1):39–45.