Abstract
Background and aim: A non-inferiority cost analysis was performed to assess if the early capsule approach would incur higher costs than the standard of care approach in patients presenting with non-hematemesis gastrointestinal bleeding.
Methods: A prospective non-inferiority cost analysis was performed on patients receiving either an early video capsule as the first diagnostic procedure or an endoscopic procedure as determined by gastroenterology staff that were not involved in the study. Primary outcome was total direct costs incurred in both groups.
Results: Forty-five patients and 42 patients were enrolled into the early capsule and standard of care arms, respectively. There was no difference in total direct cost per inpatient case in both groups ($7,362 vs $7,148, p = 0.77 [CI = −2,285–2,315, equivalent margin = –$3,100]). Localization of a bleeding source after the first diagnostic procedure was identified more frequently in the early capsule group (69.2% vs 27.9%, p = 0.0003). If patients were discharged after their last non-diagnostic evaluation, then length of stay could be decreased by 50% in both groups (58.5 to 31.6 h, p = 0.02 in the early capsule group and 69.4 to 39.2 h in the standard of care group p = 0.001). Projections indicate the fastest a patient with non-diagnostic evaluations could be discharged is 0.88 days in the early capsule group vs 1.63 days in the standard of care group (p = 0.0005).
Discussion: In patients with non-hematemesis bleeding, video capsule endoscopy may be a more efficient diagnostic approach than the standard of care approach, since it detects bleeding significantly more often without an increase in healthcare costs.
Introduction
Acute gastrointestinal bleeding (GIB) is a common presentation in many US emergency department (ED) settings, and is a significant factor in healthcare costs. According to the Health Care Cost and Utilization Project, there were roughly 545,000 US based hospital admissions for gastrointestinal bleeding (GIB) in 2008, of which 246,000 were found to have an upper GI source, 130,000 were found to have a lower GI source, and 165,000 were unspecified or indeterminateCitation1. Costs associated with upper GIB (UGIB) alone have been estimated to exceed $2.5 billion annually, and attempts have been made to reduce these costsCitation2.
Several scoring systems have been developed based on clinical features of upper gastrointestinal bleeding (UGIB), including the Blatchford Glasgow and Rockall scoresCitation3–8. However, these clinical assessments alone are non-diagnostic and do not allow bleeding localizationCitation9,Citation10. A recent randomized study suggests that the early use of video capsule endoscopy may reduce hospital admissions, because it was reliably possible to assess who was bleeding and from whereCitation11.
For patients with non-hematemesis gastrointestinal bleeding, the presenting complaints have poor localization value, necessitating a sequence of procedures that take time. Melena has traditionally been associated with an UGI source, prompting most gastroenterologists to perform an EGD as the first diagnostic procedureCitation8–13. However, recent studies have suggested a shift in the epidemiology of gastrointestinal bleeding with a decrease in the true incidence of UGIB and the rising incidence of bleeding sources in other areas of the GI tractCitation14–16. Melena can be associated with bleeding anywhere from the oropharynx to the right colonCitation17,Citation18. As a result, in a retrospective study we have shown low diagnostic yields of EGD (esophagogastroduodenoscopy) and/or COL (colonoscopy) and a high diagnostic yield for VCE when done as a first diagnostic procedure in patients presenting with NHGIBCitation19.
Based on the need to improve the diagnostic yield in this patient population, we performed a randomized control trial comparing rate of bleeding localization in NHGIB patients with early capsule (EC) deployment versus standard of care (SOC). In phase one of our analysis we found EC deployment to be superior to SOC in detecting location of bleedingCitation20. However, questions remain on whether using the EC algorithm would increase total costs, since a higher rate of bleeding detection would necessitate an increase in a subsequent therapeutic procedure.
The two major contributors to costs associated with gastrointestinal bleeding are the procedures required to diagnose/treat the bleed and hospital length of stayCitation21. Since phase one of our RCT successfully demonstrated NHGIB can occur anywhere in the GI tract, requiring multiple procedures, re-evaluation of associated costs needs to be undertaken. Literature in this regard is sparse. Phase 2 of our analysis looks to evaluate costs associated with applying the EC algorithm compared to SOC in patients with NHGIB. In addition, we investigated which clinical practices are major contributors to these costs. We hypothesize effective detection of bleeding via the EC algorithm would not incur higher costs (non-inferior) when compared to SOC.
Methods
Study design
Phase one of our study was a randomized control trial from April 2015 to July 2017 at the University of Massachusetts Medical School. Phase two of our study, which is the scope of this article, uses the same cohort to perform a cost analysis after following these patients over that period. VCE is an easily administered, less invasive diagnostic tool used for evaluating luminal GI pathology. Phase one of our study demonstrated its higher efficacy in localizing the site of bleeding when compared to standard of care. However, we wanted to demonstrate that deployment of VCE would not be inferior to standard of care with regards to incurred costs, and thus designed a non-inferiority study. The study was approved by The University of Massachusetts Medical School Institutional Review Board (IRB Protocol ID H00006661, which was registered with ClinicalTrials.gov (NCT02442830)).
Patients/intervention
Patients were included in the study if they were at least 18 years old, able to provide informed consent, hemodynamically stable (defined as blood pressure greater than 100/60 mm Hg or pulse less than 100 beats per minute at the time of consent), and admitted for evaluation of new onset, non-hematemesis gastrointestinal bleeding (i.e. melena, hematochezia with anemia, or symptomatic, iron deficiency anemia without a prior diagnosis).
Excluded patients were those that were unable to provide informed consent; were prisoners; were pregnant; had a prior history of gastroparesis; presented with symptoms of colitis; had dysphagia; were intubated at the time of consultation; had anorectal bleeding concerning for hemorrhoids; had an allergy to metoclopramide or erythromycin; had a code status of do not resuscitate; had a history of Crohn’s disease; had a history of abdominal radiation or intestinal surgery; had an implanted cardiac device (e.g. pacemaker or defibrillator); had clinical findings concerning for an acute abdomen or bowel obstruction; and/or if they received an American Society of Anesthesiologists score of 4 or greater.
Informed consent for included patients was obtained prior to randomization. A detailed description of the process of randomization was provided in phase one of our studyCitation19. Phase two of our study involved using this same cohort to perform our cost analysis. Patients were randomized into the EC arm or SOC arm. Patients randomized to the EC arm ingested an Olympus EC-S10 VCE (Olympus Corporation; Tokyo, Japan) within 10 min of consent. Immediately following ingestion of the VCE, a research staff member activated the VCE real-time viewer (RTV) on the Olympus RE-10 recorder (Olympus Corporation; Tokyo, Japan) to look for blood in the stomach. If the RTV demonstrated evidence of blood the gastroenterology consult team was notified of the findings. If no blood was seen on the RTV, it was rechecked 60 min later to ensure that the capsule had entered the small bowel. It was the responsibility of research staff to upload the complete VCE after at least 8 h of run time. A gastroenterologist with expertise in reading VCE did an initial read within 1 h of download and a complete read within a few hours. The primary gastroenterology team was immediately notified of findings by a study staff member. With the VCE information available to them the gastroenterology team performed further endoscopic examination at their discretion. Once the results of the VCE were delivered to the primary team, research staff did not provide further clinical management recommendations to limit bias. In addition, protocols for timing of subsequent procedures and criteria for discharge were not established to allow for accurate depiction of current clinical tendencies and behaviors. Of note, the primary goal of the VCE was to determine location, and not the specific source of bleeding. Once localization was obtained, targeted therapy based on location could be performed. Moreover, we wanted to provide an easier, less invasive method to quickly determine the location of bleeding. Thus, bowel preparations were not given to patients. All VCE were deemed appropriate quality for visualization of blood.
Outcomes
Phase one of our study compared the rate of localization of bleeding as the primary outcome between both groups. Patients that had localization of bleeding were identified as patients with endoscopic signs of bleeding (ESOB) or identification of a lesion with associated high-risk stigmata of recent hemorrhage (SRH). For the purposes of this study, if ESOB or a lesion with SRH were seen, it was considered a positive or a diagnostic test. Patients without ESOB or a lesion with low risk SRH were determined to have undergone complete diagnostic evaluation without a source of bleed identified or a negative test. Detailed descriptions on how we defined localization of bleeding and etiologic diagnosis are described in our concurrent manuscriptCitation19.
Phase two of our study was the primary focus of this manuscript. It involved providing a descriptive analysis of overall cost incurred by both algorithms. These costs were the actual calculated costs determined by our financial department at the University of Massachusetts Medical Center (UMMC). Specific costs that were evaluated in detail were total charges incurred, direct and indirect costs, and specific costs of each diagnostic and therapeutic entity used. Total Charges are the amounts that UMMC charges the patient. Estimated Net Revenue is the amount UMMC receives from the payor based upon our current contracts. Total Direct Expenses are expenses that are directly related to the encounter (supplies, labor, etc.), which is generally the measure used to determine the impact on healthcare costs. Contribution Margin is the Est Net Revenue less Total Direct Expenses. Fixed Indirect Costs are the expenses that are allocated to the encounter (admin fees, department, etc.). Total Margin is the Contribution Margin less the Fixed Indirect Costs. The cost of the study capsule was included in final direct costs.
A second analysis was performed on length of stay (LOS). Actual and projected LOS were calculated to determine the most efficient time to discharge. Length of stay was determined by time patient presented to the emergency room to the time the discharge order was written. We stratified LOS in patients with positive and negative test results as both patient populations are expected to be managed differently, i.e. patients with positive test results are likely to undergo further procedures and more unpredictable hospital stays. Re-bleeding rates were calculated if the patient presented to our hospital for re-bleeding within 1 year.
Statistical analysis
Patient characteristics and demographics were described as means with standard deviation (SD) and proportions. Descriptive analyses (p-values for means) were used to compare costs and length of stays in both arms. Univariate analysis involved Mann-Whitney tests for continuous variables and Chi Square or Fisher’s Exact tests for categorical variables. A non-inferiority margin of –$3,100 was calculated based on our hospital’s determination that a difference greater than this value would be of significant clinical value. A difference below this value would be deemed clinically insignificant. Based on this margin, if there was truly no difference between the SOC and VCE arm, then 84 patients (sample size) were required to be 90% sure that the lower limit of a one-sided 95% confidence interval would be above the non-inferiority limit of –$3,100 (Sealed Envelope Ltd. 2012. Power calculator for continuous outcome non-inferiority trial. https://www.sealedenvelope.com/power/continuous-noninferior).
Results
Of the 206 patients screened, 87 patients were enrolled into the study and 119 were excluded (Supplementary Figure S1). Phase one analysis included 42 patients in the EC arm and 45 patients in the SOC arm. For phase 2 of our analysis we excluded one patient in the EC arm due to the inability to identify costs and LOS. Two patients in both groups were excluded with LOS > 18 days. Eighteen days was selected as the cut-off to exclude outliers as this has been determined by the University of Massachusetts Medical Center Financial department as standard outliers in previous calculations for LOS. Final cost and LOS analysis included 39 and 43 patients in the EC and SOC arms, respectively.
Baseline patient demographics are depicted in Supplementary Table S1. Most patients in the study were male (58.6%) and had an average age of 68.7 years old. One patient in the EC arm and two in the SOC arm died secondary to decompensated liver disease and > 10 days post-enrollment (p = 1.00). Ten patients (24%) required ICU level care in the EC arm compared to seven patients (15.6%) in the SOC arm. In these ICU patients, all but one patient had a positive test in both groups. The percentage of patients presenting with melena (60% vs 75.6%, p = .11), hematochezia (26.1% vs 20%, p = .49), and guaiac + stool/anemia (14.4% vs 4.4%, p = .11) were similar in both EC and SOC groups, respectively. On average, both groups underwent a similar number of procedures (1.9 in EC vs 1.6 in SOC).
The results of our phase one analysis are described in detail in our RCTCitation20. An overall total positive test result was identified in 69.2% (n = 27) in the EC group compared to 32.6% in the SOC group (n = 14). A positive test result was identified on the first diagnostic procedure 69.2% of the time in the EC group, compared to only 27.9% of the time in the SOC group (). When EGD (n = 33), COLO (n = 2), or VCE (n = 4) was chosen as the first diagnostic choice in the SOC arm, the rate of testing positive was 27.3%, 50%, and 50%, respectively. The EC group, on average, required 1.9 procedures, compared to 1.6 in the SOC arm. Of the patients who had negative testing on VCE, none required further endoscopic investigation while in the hospital. Detailed etiologic findings are described in Phase one of our study and in Supplementary Table S3.
Table 1. Ability of various procedures to detect (positive) bleeding/lesions with stigmata of recent hemorrhage (SRH).
Total charges and cumulative costs for both groups are depicted in . Per inpatient case, there was no difference in total charges to the hospital ($25,023 vs $25,880, p = .78), direct costs to the hospital ($7,362 vs $7,148, p = .77 [CI = −2,285–2,315, equivalent margin = –$3,100]), or direct cost contribution margin ($3,445 vs $4,259, p = .88) for both the EC and SOC groups, respectively. The largest contributors to total costs in both groups were occupying a hospital bed (emergency room, ward bed, or ICU bed) and gastrointestinal procedure costs (Supplementary Table S2).
Table 2. Cumulative and per inpatient case charges after exclusion.
Patients in the EC group completed their diagnostic evaluation (whether the test was positive or negative) quicker than the SOC group (24.5 vs 34.0 h, p = .02) (). Overall LOS () in the EC group was 70.0 h (2.79 days), compared to 78.9 (3.29) in the SOC group (p = .76). Sub-group analysis demonstrated, when comparing LOS in EC patients with negative and positive test results, patients that tested negative were discharged quicker than patients who tested positive (58.5 vs 80.4 h, p = .02) (). Similar findings were seen between SOC patients with negative and positive test results (69.4 vs 98.5, p = .04).
Figure 1. Average time taken to end of diagnostic evaluation (Detection and No Detection). Abbreviations. EC, Early Capsule; SOC, Standard of care.
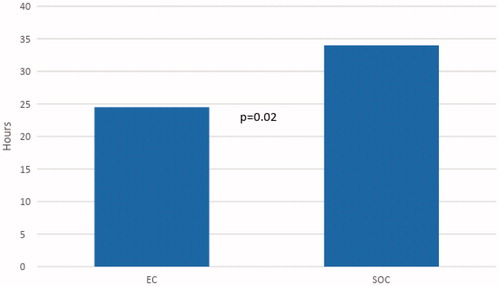
Figure 2. Length of stay (LOS) for all patients and stratification based on patients with bleeding detection (+) and no detection (–).
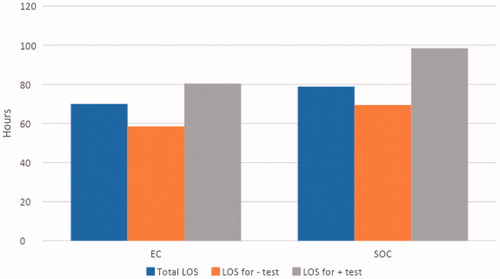
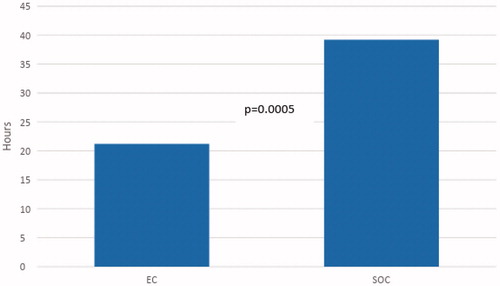
However, if patients with negative test results had been discharged after their last diagnostic test and were not bleeding, our projections () indicate LOS for this subset population could decrease significantly in both groups (58.5 to 31.6 h, p = .02 in the EC group and 69.4 to 39.2 h, p = .001 in the SOC group). Moreover, in this subset population, the average time from admission to ER to deployment of EC was 10.4 h, as deployment of VCE was dependent on gastroenterology staff as per the protocol. If it was possible to deploy an EC in an ED setting by any physician, we projected the LOS for EC patients with negative test results could be decreased further to 21.2 h, if the EC was deployed on arrival to the ER. With these projections, LOS in patients with negative test results would be lower in the EC group compared to SOC (21.2 vs 39.2 h, p = .0005) (). This suggests that, for patients with non-diagnostic testing, EC deployment could ideally discharge a patient in 0.88 days vs 1.63 days for a SOC patient. Extrapolating these projections over a year, EC deployment would allow the hospital to service an additional 190 patients per patient bed for patients with non-diagnostic test results (or an 84.8% increase).
Figure 3. Actual vs Projected LOS (length of stay) for patients with negative testing if discharged after negative diagnostic evaluation. Abbreviations. EC, Early Capsule; SOC, Standard of care.
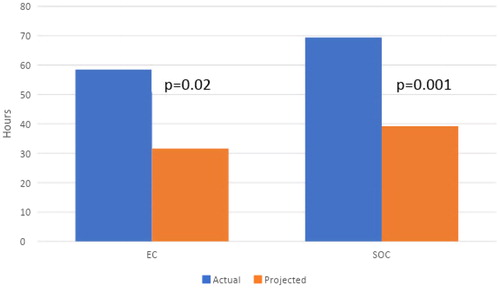
Figure 4. Projected LOS (length of stay) for patients with negative testing in both groups if capsule deployed in the emergency room. Abbreviations. EC, Early Capsule; SOC, Standard of care.
When specifically looking at patients with positive test results in the EC group, all underwent a subsequent planned therapeutic procedure. Three (11.1%) patients in the EC group were found to have high risk lesions (peptic ulcers with visible oozing, visible vessel, or active bleeding) that would necessitate a 72 h hospital stay compared to six (42.9%) patients in SOC groupCitation22,Citation23. There was no difference in LOS between the EC and SOC groups who had positive test results (80.4 vs 98.5 h, p = .26).
Discussion
Our previous RCT demonstrated an increasing rate of bleeding detection in non-hematemesis GIB patients who underwent early capsule deployment compared to standard of care. Our current study shows this increase in bleeding localization by the EC algorithm does not lead to increased total direct costs.
Numerous studies have been performed evaluating the cost burden of managing patients with upper GIB, and methods to minimize those costs have been exploredCitation2,Citation24,Citation25. VCE has been considered a cost-effective tool to help risk stratify patient with UGIBCitation26. However, these studies are based on the premise that the bleed is originating from the upper GI tract, and do not portray an accurate representation of costs associated with finding the true bleeding source in a patient presenting with non-hematemesis bleeding. Our study is the first to compare total incurred costs as applied to each arm of a randomized controlled trial with comparison between early capsule endoscopy and standard of care. Analysis of the data from this trial provides an accurate representation of the potential economic burden associated with finding the true location and source of bleeding without predetermining the location based on symptoms alone.
Given its ability to determine the location of bleeding more accurately, the EC approach would generate a larger cumulative need for subsequent therapeutic procedures, as VCE has no therapeutic role. Consequently, the EC approach could be thought of as costlier than the SOC approach. However, our results indicate this is not the case. The reasons for the similarities in cost are multifactorial, and likely revolve around the most financially meaningful variables in managing GIB: gastrointestinal procedures chosen and hospital beds.
Both groups on average underwent more than one cumulative gastrointestinal procedure, which was expected given the lower rate of positive test results by the end of the first procedure in the SOC arm and the necessity to perform a subsequent therapeutic procedure in the EC arm. The second procedure performed in the EC group, however, was primarily for a therapeutic measure whereas in the SOC group it was for determining the location of bleeding. From an economic standpoint, undergoing a total of two endoscopic procedures to diagnose and treat a bleeding source seems ideal, but performing two procedures without detecting the bleeding source seems less efficient. Our results indicated that in the SOC arm a significant portion of patients undergo multiple invasive non-diagnostic procedures. Consequently, although there is no difference in cumulative costs between both groups, a significant portion of the costs in the EC group are focused on detecting and potentially treating the bleeding lesion, whereas SOC patients experience similar costs without obtaining a definitive diagnosis. In addition, a non-diagnostic VCE has the added benefit of ruling out any high-risk bleeding lesion within most of the GI tract in one procedure, whereas the SOC approach uses an EGD first and then colonoscopy without evaluation of the midgut, until much later after index bleeding time.
Although this study did not test this hypothesis, EC may actually improve healthcare utilization over the long run when compared to SOC, by increasing the number of patients with positive test results and allowing for more focused evaluations of the GI tract involved, even during recurrent hospital admissions. The best evidence of this effect is with vascular lesions, which EC can detect more frequently than SOCCitation20. Since this type of lesion bleeds intermittently without leaving much mucosal footprint they are difficult to find with the traditional endoscopic evaluation algorithm. It is of no surprise then that Prakash and ZuckermanCitation27 found acute small bleeding to be more costly than other types of bleeds given the number of diagnostic tests that need to be performed and the number of hospital stays required to do so. For these reasons, it seems EC could have a much larger economic impact in the long run by increasing the number of positive findings yet still allowing for quick patient disposition if it is non-diagnostic. Future protocol driven studies need to be undertaken to test this hypothesis.
The second major variable in costs associated with diagnosing and treating GIB are the number of hospital days. There was no difference in hospital LOS in both of our patient populations, hence similar total direct costs. Previous studies have evaluated LOS in patients with presumed upper GIB and average LOS have been reported to be around 2–3 days, which is similar to our findings in both groupsCitation28,Citation29. However, our study is the first to evaluate LOS in all comers presenting with NHGIB without the presumption of an UGI bleeding source. Although there was no difference in LOS between both groups, closer examination with simple projections reveal LOS could be further reduced in both groups.
Our results indicate that patients in both groups, on the whole, stay in the hospital twice as long after the end of their diagnostic evaluation is complete (whether bleeding was found or not found). In patients with positive testing this is not alarming since various forms of treatments may be needed and some patients need 72 h of proton pump inhibitor therapy for lesions with high risk of re-bleedingCitation22. However, in patients with no evidence of bleeding lesions, the need for long hospitalizations seems less than ideal. Our projections indicate if patients with negative test results would have been discharged after their last negative evaluation, LOS in this patient population could be decreased by 50% in both groups in its most ideal setting. This discrepancy may reflect clinician behavior, as research staff did not intrude on timing of discharge, and suggests clinicians do not feel comfortable discharging patients when no source of bleeding is identified. This time lag cannot be explained by complicated admissions, as the majority of patients requiring ICU level care were patients with bleeding lesions. Given the low readmission rate in both groups, this reluctancy seems conservative and if aggressive, yet safe, measures were taken, significant reductions in LOS and ultimately cost may be seen. However, it is important to emphasize that further protocoled long-term follow-up studies are needed to corroborate this hypothesis, but nevertheless depicts potential room for cost savings.
Extending these projections further, in patients with non-diagnostic tests, if EC was dropped in the ER rather than waiting for the gastroenterology team to deploy it, than the maximum reduction in LOS in the EC group could decrease to 21 h. Projections on further reduction of LOS in the SOC group are difficult since EGD/COLO require patients to be medically stable for sedation and require a gastroenterologist to perform the study. Thus, in its most ideal process, EC could discharge a patient with no evidence of bleeding on average in 0.88 days, whereas the best the SOC approach could do is 1.63 days. From an economic standpoint, the EC may not only decrease direct cost since each patient would occupy less time in the hospital, but allow for quick turnaround of that one hospital bed. Over a years’ time, this quick turnover could allow for substantial improvement in healthcare utilization, since more patients could be serviced.
There are several limitations of our study. First, the primary aim of this study was to show the EC approach did not prove to be an economic burden during a patient’s initial hospital encounter. Making the claim EC is overall more cost effective than SOC would be premature since calculation of costs incurred for long-term outcomes such as re-bleeding rates and overall mortality would ultimately decide on global cost effectiveness. Despite our attempts we were unable to consistently follow-up with patients who were discharged, since getting in communication with them was quite challenging. Consequently, follow-up EGD and COLO could not be performed in most patients with non-diagnostic VCE. Thus, long-term economic effects are still unknown regarding both strategies. In addition, these findings can only be generalized within a United States payor system. Other countries may have stronger financial implications for “non-diagnostic” hospitalizations, and the use of EC in those institutions needs to be studied extensively. Finally, conclusions made regarding LOS are purely projections, but do illustrate a potential cost saving area in GIB management. All three of these limitations, however, can be addressed in our future prospective study where discharge times will be under protocol and long-term follow-up would be required.
In conclusion, the results of our previous randomized control trial and the current cost analysis indicate, in patients with their first episode of NHGIB, EC may be a more efficient diagnostic approach than the SOC approach since it detects bleeding significantly more often without an increase in costs during their initial hospital encounter. Long-term follow-up will be needed to determine overall cost-effectiveness. However, if our projections hold true in future studies, deployment of the VCE upon presentation to the ER could allow for quick risk stratification within 24 h and allow safe discharge from the hospital. In the era of optimizing healthcare economics, the EC approach may have significant implications.
Transparency
Declaration of funding
Olympus Corp. (Tokyo, Japan) provided an unrestricted grant and non-financial support for this trial.
Declaration of financial/other relationships
Dr David Cave is a consultant for Boston Scientific and an investigator for clinical trials supported by Medtronic, Celgene, and Pfizer. Dr Kanishka Bhattacharya is an investigator for a clinical trial supported by Olympus Corporation. Drs Salmaan Jawaid, Neil Marya, and Christopher Marshall have no disclosures to report. Michelle Hicks has no disclosure to report. A peer reviewer on this manuscript has disclosed that they have participated in an Advisory Board for Olympus Corp. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Salmaan Jawaid – study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, and statistical analysis. Neil Marya – study concept and design, acquisition of data, analysis and interpretation of data, and drafting of manuscript. Christopher Marshall – study concept and design, critical revision of the manuscript for important intellectual content. Michelle Hicks – acquisition of data. Kanishka Bhattacharya – study concept and design, critical revision of the manuscript for important intellectual content. David Cave – study concept and design, critical revision of the manuscript for important intellectual content.
Acknowledgements
None reported.
Supplemental Material
Download MS Word (45.7 KB)References
- Zhao Y, Encinosa W. Hospitalizations for GIB in 1998 and 2006. Rockville, MD: Agency for Healthcare Research and Quality; 2008. (HCUP Statistical Brief #65).
- Johanson JF. Curbing the costs of GI bleeding. Am J Gastroenterol. 1998;93(8):1384–1385.
- Rockall TA, Logan RF, Devlin HB, et al. Risk assessment after acute upper gastrointestinal hemorrhage. Gut. 1996;38(3):316–321.
- Blatchford O, Murray WR, Blatchford MA. A risk score to predict need for upper-gastrointestinal hemorrhage. Lancet. 2000;356(9238):1318–1321.
- Church NI, Dallal HJ, Masson J, et al. Validity of the Rockall system after endoscopic therapy for bleeding peptic ulcer: a prospective cohort study. Gastrointest Endosc. 2006;63(4):606–612.
- Das A, Wong RC. Prediction of outcome of acute GI hemorrhage review of risk scores and predictive models. Gastrointest Endosc. 2004;60(1):85–93.
- Cappell MS, Friedel D. Initial management of acute upper gastrointestinal bleeding: from initial evaluation up to gastrointestinal endoscopy. Med Clin North Am. 2008;92(3):491.
- Srygley FD, Gerardo CJ, Tran T, et al. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072–1079.
- Ibach MB, Grier JF, Goldman DE, et al. Diagnostic considerations in evaluation of patients presenting with melena and nondiagnostic esophagogastroduodenoscopy. Digest Dis Sci. 1995;40(7):1459–1462.
- Zuccaro G. Management of the adult patient with acute lower gastrointestinal bleeding. Am J Gastroenterology. 1998;93(8):1202–1208.
- Sung JJ, Tang RS, Ching JY, et al. Use of capsule endoscopy in the emergency department as a triage of patients with GI bleeding. Gastrointest Endosc. 2016;84(6):907–913.
- Witting MD, Magder L, Heins AE, et al. ED predictors of upper gastrointestinal tract bleeding in patients without hematemesis. Am J Emerg Med. 2006;24(3):280–285.
- Peura DA, Lanza FL, Gostout CJ, et al. The American College of Gastroenterology Bleeding Registry: preliminary findings. Am J Gastroenterol. 1997;92(6):924–928.
- Van Leerdam ME, Vreeburg EM, Rauws EAJ, et al. Acute upper GIB: did anything changing. Am J Gastroenterol. 2003;98(7):1494–1499.
- Whelan CT, Chen C, Kaboli P, et al. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141–147.
- Loperfido S, Baldo V, Piovesana E, et al. Changing trends in acute upper-GI bleeding: a population-based study. Gastrointest Endosc. 2009;70(2):212.
- Boonpongmanee S, Fleischer D, Pezzullo J, et al. The frequency of peptic ulcer as a cause of upper-GI bleeding is exaggerated. Gastrointestinal Endosc. 2004;59(7):788–794.
- Enestvedt BK, Gralnek IM, Mattek N, et al. An evaluation of endoscopic indications and findings related to nonvariceal upper-GI hemorrhage in a large multicenter consortium. Gastrointest Endosc. 2008;67(3):422.
- Jawaid S, Gondal B, Singh A, et al. The epidemiology of gastrointestinal bleeding in an academic emergency department as a basis for reconfiguring the conventional approach to its diagnosis and management. Gastrointest Endosc. 2013;77(5):AB483.
- Marya N, Jawaid S, Foley A, et al. A randomized controlled trial comparing efficacy of early video capsule endoscopy with standard of care in the approach to nonhematemesis GI bleeding. Gastrointest Endosc. 2018;89(1):33.e4–43.e4
- Campbell HE, Stokes EA, Bargo D, et al. Costs and quality of life associated with acute upper gastrointestinal bleeding in the UK: cohort analysis of patients in a cluster randomised trial. BMJ Open. 2015;5(4):e007230.
- Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009;7(1):33–47.
- Lin HJ, Perng CL, Lee FY, et al. Clinical courses and predictors for rebleeding in patients with peptic ulcers and non-bleeding visible vessels. Gut. 1994;35(10):1389–1393.
- Cryer BL, Wilcox CM, Henk HJ, et al. The economics of upper gastrointestinal bleeding in a US managed-care setting: a retrospective, claims-based analysis. J Med Econ. 2010;13(1):70–77.
- Viviane A, Alan BN. Estimates of cost of hospital stay for variceal and non-variceal upper gastrointestinal bleeding. Value Health. 2008;11(1):1–3.
- Meltzer AC, Ward MJ, Gralnek IM, et al. The cost-effectiveness of VCE compared to other strategies to manage acute upper gastrointestinal bleeding. Am J Emerg Med. 2014;32(8):823–832.
- Prakash C, Zuckerman GR. Acute small bowel bleeding: a distinct entity with significantly different economic implications compared with GI bleeding from other locations. Gastrointest Endosc. 2003;58(3):330–335.
- Hay JA, Lyubashevsky E, Elashoff J, et al. Upper gastrointestinal hemorrhage clinical guideline determining optimal hospital length of stay. Am J Med. 1996;100(3):313–322.
- Hay JA, Maldonado L, Weingarten SR, et al. Prospective evaluation of a clinical guideline recommending hospital length of stay in upper gastrointestinal tract hemorrhage. JAMA. 1997;278(24):2151–2156.
