Abstract
Aims: Estimate the direct costs of high-risk patients presenting with coronary artery disease (CAD) or peripheral artery disease (PAD) in France.
Materials and methods: This retrospective cohort study used a representative claims database, the “Echantillon Généraliste de Bénéficiaires” (EGB), to identify patients presenting with CAD or PAD between 2011 and 2016. Among those, patients meeting the COMPASS trial selection criteria were selected, as well as controls matched on age and sex. Direct costs (Euros 2016) were estimated in a societal perspective by comparing case and controls.
Results: The adult population presenting with CAD or PAD in the EGB in 2016 was estimated at 29,888 individuals, representing a crude prevalence rate of 5.44%. After using the documented selection criteria of the COMPASS study, this population (COMPASS-like) was estimated at 17,369 individuals (58.1% of the CAD and/or PAD total population). Among them, a proportion of 11.5% presented with CAD + PAD. Compared with the original COMPASS population, patients were older (76.5 vs 68.2 years) and with a lower male predominance (60.0% vs 78.2% males). Compared with controls, the COMPASS-like population was characterized by a higher annual mortality (5.9% vs 3.5%) and the presence of more comorbidities on top of CAD and/or PAD. The annual per capita extra direct cost of the COMPASS-like population was estimated at €4,284, with a main contribution from inpatient care (58.9%). This extra cost was higher in the PAD ± CAD sub-group (€5,552) and the CAD + PAD sub-group (€8,067).
Limitations: The EGB had limitations about several clinical features defining high-risk patients that may lead to bias in our estimates.
Conclusions: Due to the high prevalence of CAD and/or PAD and the associated high unit costs, this population generates a significant economic burden, which is higher among patients with PAD and in those presenting simultaneously with both conditions.
Introduction
Atherosclerosis, with its main clinical complications, coronary artery disease (CAD), peripheral artery disease (PAD), and cerebrovascular disease, constitutes a major public health issue in terms of mortality worldwideCitation1,Citation2. Despite considerable improvement in its management over the last decades, atherosclerosis remains a leading cause of death, as estimated in 2015Citation3. In France, cardiovascular diseases constitute the second leading cause of death, accounting for 27% of the 535,000 deaths in 2011 (i.e. a standardized rate of 216/100,000)Citation4, with ischemic heart disease accounting for 7% of deaths. Despite the importance of these diseases, and apart from disease registries, few large-scale prevalence studies and cost estimates have been performed. This situation is especially true for PAD for various reasons, including notably the lack of standardization of the definitions usedCitation5, and despite the magnitude of its burden as suggested by the few existing international estimates, for example from JapanCitation6 and the USCitation7–10. Several authors have noticed the likely situation of under-diagnosis and under-treatment of patients presenting with PADCitation11–13. The management of cardiovascular risk factors in patients with established cardiovascular disease has been described in the REACH (Reduction of Atherothrombosis for Continued Health) registry and suggested a general under-use of antiplatelet and lipid-lowering agents in patients with PADCitation14, despite their higher risk of myocardial infarction, ischemic stroke or cardiovascular death. Another factor in the under-treatment of PAD may be the lack of cardiovascular therapies specifically targeting this population. The recent approval in July 2018 by the Committee for Medicinal Products for Human Use (CHMP) of an extension of indication for rivaroxaban 2.5 mg twice daily co-administered with acetylsalicylic acid (ASA), for the prevention of atherothrombotic events in adult patients with CAD or symptomatic PAD at high risk of ischemic events has brought a novel therapeutic option to this domainCitation15–18. The transferability of the results of clinical trials to real-life national situations is often raised as a problem by Health Technology Assessment bodies for two reasons: the first relates to selection bias due to the over-representation of younger and less severely ill patients than the overall eligible population. The second relates to the international variability of cardiovascular risk. This study was designed to document these aspects for France, as well as to provide a description of the prevalence and economic burden of CAD and PAD fitting the profile of French patients in this new indication.
Methods
Source of data
The national public health insurance in France, first established in 1945, is now composed of several schemes constructed around various occupational sectors. Salaried workers of the private sector and their dependents are covered by the so-called “general scheme” (Caisse nationale d’Assurance Maladie [CNAM]) (approximately 76% of the 66 million inhabitants of France in 2016). This scheme also covers civil servants, employees of territorial administrations and public hospitals, and students (approximately 11% of the population). The self-employed workers scheme (“régime social des indépendants” [RSI]) covers craftsmen, shopkeepers, liberal professions, and their dependents and represents 6% of the population. The agricultural scheme (“mutualité sociale agricole” [MSA]) covers farmers and agricultural workers and their dependents (5% of the population). Retired persons, the unemployed, and minimum welfare beneficiaries are covered by the scheme corresponding to their former occupation.
The “Système national d’information inter-régimes de l’Assurance maladie” (SNIIRAM) claims database comprehensively records all outpatient prescriptions and healthcare procedures reimbursed to beneficiaries of the three main health schemes but does not comprise any clinical information concerning results related to consultations, prescriptions, or examinations. However, patients presenting with chronic severe diseases, which qualify for exemption of copayment, benefit from a so-called long-term disease (LTD) status after validation by a national health insurance physician. For those patients, their International Classification of Diseases 10th Edition (ICD-10) diagnosis codes are documented in the database. An anonymous and unique identification number for each beneficiary allows this information to be linked to the data collected by the “Programme de médicalisation des systèmes d’information” (PMSI; the French national hospital discharge database) during hospital stays in all types of facilities, whether public or private. Hospital diagnoses are also coded according to the ICD-10 codes, in the same way as the diagnoses allowing attribution of LTD statusCitation19. A random representative 1/97 sample of approximately 600,000 people from SNIIRAM called the “Echantillon Generaliste de Beneficiaires” (EGB) database was constituted and has been used in this study.
Identification of the populations of interest
The selection of patients was performed in a stepwise process consisting first of identifying patients with CAD and/or PAD over a retrospective period of 5 years from 2011 to 2015 to define the prevalent population on 1 January 2016 and then over the year 2016 to define the incident population (new cases diagnosed in 2016). The resulting total population (prevalent plus incident) is used in all subsequent sub-groups and cost analyses.
Patients with CAD and/or PAD
In a first step, the following algorithm was used to identify CAD and/or PAD patients:
Patients aged 18 years and over on 1 January 2016 recorded in the EGB database (3 main schemes) between 1 January 2011 and 31 December 2016 and alive on 1 January 2016
AND
With at least one hospitalization for CAD and/or PAD in primary, related, or secondary diagnosis (I20–I25 and/or I70–I79 ICD-10 codes) between 1 January 2011 and 31 December 2016
OR
With at least one reimbursement linked to an LTD status for CAD and/or PAD (I20–I25 and/or I70–I79 ICD-10 codes) between 1 January 2011 and 31 December 2016
Among this population, incident patients in 2016 were selected as presenting with CAD and/or PAD for the first time in 2016, i.e. with no history of CAD and/or PAD during the period 1 January 2011 to 1 January 2016 (pre-study period).
COMPASS-like population
Within this population, the so-called COMPASS-like population was defined in a second step as fitting as much as possible the inclusion/exclusion criteria of the COMPASS study.
CAD patients were restricted as following:
Age ≥65 years on 1 January 2016
OR
<65 years but presenting with at least 2 of the following identifiable conditions over the pre-study period (other risk factors like smoking were not documented in the database):
Diabetes mellitus (Type 1 or 2)
Chronic kidney disease (CKD) stages 3 to 4 or hospitalization with diagnosis ICD-10 codes N18.3 and N18.4
Heart failure: hospitalization with diagnosis ICD-10 codes I11.0, I13.0, I13.2, and I50
Non-lacunar ischemic stroke having occurred more than 1 month before 1 January 2016 or before date of diagnosis in 2016: hospitalization with ICD code for ischemic stroke (I63, I64, I693), and not lacunar stroke G46.5, G46.6 coded in secondary diagnosis
CAD or PAD patients presenting with any of the following conditions were excluded:
Stroke (ischemic or hemorrhagic) within 1 month before 1 January 2016 or before date of diagnosis in 2016 OR any history (over pre-study period) of hemorrhagic OR lacunar stroke. These criteria were considered through hospitalization for hemorrhagic stroke (ICD-10: I60–I61–I62) or lacunar stroke (ICD-10: G46.5, G46.6)
Estimated glomerular filtration rate (eGFR) <15 ml/min (CKD stage 5): hospitalization with diagnosis ICD-10 code N18.5 or kidney transplant ICD-10 code Z94.0
Systemic treatment (over the pre-study period) with strong inhibitors of both cytochrome P450 (CYP) 3A4 and P-glycoprotein (P-gp) (e.g. systemic azole antimycotics, such as ketoconazole, and HIV-protease inhibitors, such as ritonavir), or strong inducers of CYP3A4, i.e. rifampicin, rifabutin, phenobarbital, phenytoin, and carbamazepine: ATC classes: J02AB, J02AC, J05AE, N03AB02, N03AF01, N03AA02
Use of dual antiplatelet therapy, other non-ASA antiplatelet therapy, or oral anticoagulant therapy (over the pre-study period)
Any known hepatic disease associated with coagulopathy (over the pre-study period): hospitalization with diagnosis ICD-10 code D65–D69
COMPASS-like Sub-groups
Three sub-groups of CAD and/or PAD patients were constituted according to usual epidemiological perspectives:
“CAD with or without PAD” (CAD ± PAD) comprising CAD only patients or patients with CAD and PAD, whichever occurred first
“PAD with or without CAD” (PAD ± CAD) comprising PAD only patients or patients with PAD and CAD, whichever occurred first
“CAD + PAD” patients presenting with both diseases
Controls
Different control groups were identified to estimate the incremental costs attributable to CAD and/or PAD in 2016 in each group. To construct the control groups, we used a stratified sampling approach consisting of distributing the CAD and/or PAD cases to exclusive sub-groups defined by age group and gender. We then identified controls for each sub-group by randomly selecting from the database individuals without CAD and/or PAD according to our algorithm (three controls for each sub-group case as far as possible) and alive on 1 January 2016.
In total, four control groups were constituted for the COMPASS-like population globally and for each sub-group.
Healthcare resource consumption
The following items relating to healthcare consumption are documented in the database and were used in the estimation of costs over the calendar year 2016. Current 2016 prices in Euros were used. Cost estimates were all measured in a societal perspective (reimbursed by public healthcare insurance and out of pocket expenses). It may be noted that patients with CAD and/or PAD all benefitted from full coverage (LTD) because of the severity of their conditions for all expenses deemed to be associated with CAD or PAD:
Medical fees: visits (including primary care general practitioner and specialist visits) and technical procedures performed by physicians
Pharmacy: medicinal products and other human origin products
Laboratory tests
Auxiliary care: nursing care, physiotherapists, speech therapists, orthoptists
Medical devices
Transportation: ambulances, light medical vehicles, taxis, mobile emergency medical services, mobile emergency units
Other community care
Hospitalization: any private or public hospital (acute care)
Hospital unit costs estimates
Hospital cost estimation was restricted in this study to stays in acute care facilities (medicine, surgery, obstetrics) and did not include rehabilitation, post-acute care facilities, and “hospital at home”. There are two possible ways in France to estimate hospital costs according to available sources: (i) the data from the ENCC (National cost study), based on retrieval of hospital accounting data by Diagnosis Related Groups (DRGs) collected in a national representative sample of facilities, and (ii) the data from the fees charged by DRGs, which must be viewed as tariffs. In this study, we used the first approach as recommended by the current French guidelinesCitation20.
To avoid biases due to the absence of information on individuals whose death was not recorded or who were living in nursing homes or changed their insurance scheme for any reason, the population of the present study excluded those individuals for whom no hospital or outpatient care expenditure was reimbursed during the calendar year 2016. This group represented about 4–5% of the population, varying according to age and gender.
Statistical analysis
Continuous data are presented as mean values ± standard deviation (SD) or as median values, and categorical data as frequency counts and percentages. The occurrence of comorbidities in patients with CAD and/or PAD was compared with the control group using non-parametric Mann–Whitney tests, because our sampling method did not use any individual matching. Other categorical data were compared using the χ2 test. For cost comparisons, the Wilcoxon test was used. All reported p-values can only be interpreted in an exploratory manner, i.e. are nominal. All statistical analyses were performed using SAS software Version 9.4 (Cary, USA).
Ethical considerations
Because this study was a retrospective analysis of an anonymized database and had no influence on patient care, ethics committee approval was not required. Access to the EGB database has been authorized by CNIL (Decision DR-2018-035 dated March 02, 2018).
Results
Prevalence and incidence of CAD and/or PAD
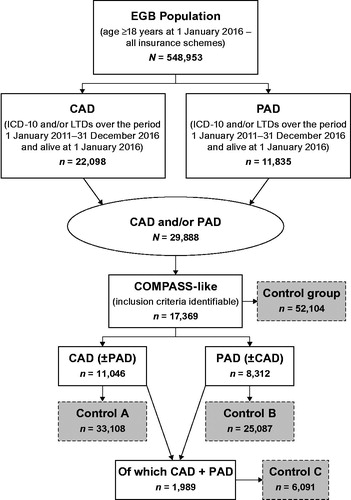
shows the flowchart of population selection. The adult population presenting with CAD or PAD in France in the EGB database in 2016 was estimated at 29,888 individuals, which represented a crude 2016 prevalence rate of 5.44%. The crude prevalence rates of the CAD (22,098 cases) and the PAD population (11,835) were estimated at 4.02% and 2.15%, respectively, in 2016. After using the inclusion/exclusion criteria of the COMPASS study, this population (COMPASS-like) was then estimated at 17,369 individuals (58.1% of the CAD and/or PAD total population), representing an extrapolated number of about 1.8–1.9 million patients in France. Among them, 11,046 individuals (63.6%) presented with CAD (±PAD) and 8,312 (47.9) with PAD (±CAD). Both populations overlapped by 1,989 individuals (11.5%), which constituted the CAD + PAD group, meaning that 88.5% of the COMPASS-like population was presenting with a single disease. It should be noted that no control could be found for 1 patient, explaining why the number of controls for the COMPASS-like population was 52,104.
Figure 1. Flowchart of populations selection. Abbreviations. CAD, coronary artery disease; EGB, Echantillon Généraliste de Bénéficiaires; LTD, long-term disease; PAD, peripheral artery disease.
shows the demographic profile of the COMPASS-like population as well as those of the sub-groups. The mean age was 76.5 years and varied from 72.6 years in the PAD ± CAD sub-group to 80.0 years in the CAD ± PAD sub-group. Male patients were predominant, with 60.0–68.7% depending on the sub-group. Among the COMPASS-like population, 14.4% of patients were diagnosed in 2016 (incident patients) with a higher percentage (21.1%) in the CAD + PAD subgroup. The corresponding crude incidence rate for COMPASS-like patients was 0.45% (2,497/548,953) of the total adult population.
Table 1. Demographics of the COMPASS-like population and sub-groups and list of ICD-10 diagnoses used for selection.
The CAD and/or PAD diagnoses recorded in the EGB database used for patient selection are shown in . These diagnoses were recorded in the hospital discharge database as well as causes for benefiting from an LTD status. Both sources closely overlapped, as can be seen in . Among patients with CAD, the most frequent diagnosis was chronic ischemic heart disease (I25) recorded in 84.4% of cases, followed by angina pectoris (ICD-10: I20) in 32.6%. Among patients with CAD, the most frequent diagnosis was chronic ischemic heart disease (ICD-10: I25) recorded in 84.5% of cases, followed by angina pectoris (ICD-10: I20) in 32.6%. Among patients with PAD, the most frequent diagnosis was atherosclerosis (ICD-10: I70) recorded in 72.7% of cases followed by chronic ischemic heart disease in 21.9%. It should be noted that a same patient may have several diagnoses during the pre-study period.
Comparison with COMPASS-enrolled (aspirin alone arm) profile
compares the demographic characteristics and some selected diagnoses and procedures from the medical history of the COMPASS-like population and from the original COMPASS trial as presented by Darmon et al.Citation21. Compared with the original COMPASS population, the patients in our sample were older (mean age of 76.5 vs 68.2 years) and had a lower male predominance (60.0% vs 78.2% males). In terms of medical history, the percentage of patients presenting with unstable angina or history of myocardial infarction were rather similar (20.7% vs 18.5% and 53.7% vs 57.9%, respectively), whereas the presence of diabetes mellitus (23.3% vs 38.1%) and congestive heart failure (4.4% vs 21.7%) was notably lower in our population. The proportions of patients with a history of percutaneous coronary intervention, coronary artery bypass grafting, or lower extremity revascularization procedures were also lower, although our figures only related to a retrospective period of a maximum 5 years (pre-study period).
Table 2. Comparison of the characteristics of the COMPASS-like and the original COMPASS (aspirin alone arm) population.
Profile and healthcare resources consumption of the COMPASS-like population versus controls
In , the profile of the COMPASS-like population is compared with controls matched on age and sex in terms of comorbidity and mortality. Comorbidities were identified only on basis of ICD-10 diagnoses recorded in the list of eligibility for health insurance full coverage (LTD status). The percentage of patients benefitting from LTD status for any cause was 90.5% in the COMPASS-like population compared with 44.1% of the controls. This percentage included a large proportion of patients with CAD and/or PAD (65.3%) within the COMPASS-like population, but it appeared that other comorbidities were also more frequent among them, such as diabetes mellitus (22.4% vs 12.3%), severe hypertension (8.1% vs 4.8%) or heart failure (4.0% vs 2.5%). This excess comorbidity translated into all-cause mortality in 2016 of 5.9% vs 3.5% in controls. Over the 3-year period 2016–2018, the mortality rates were 15.4% vs 9.8%, respectively.
Table 3. Comparison of the profile of the COMPASS-like population proportions versus controls (matched for age and gender).
The annual direct per capita costs in both populations are shown in with a disaggregation by item of healthcare consumption. The mean total annual extra cost was €4,284 as measured in a societal perspective. The fraction of this total cost that was not reimbursed by the public health insurance was minimal (€8 = €4,284 – €4,276). The main contribution to this extra cost was related to inpatient care (€2,525 or 58.9%), then to pharmaceuticals (€500 or 28.4% of outpatient care cost) and physician’s visits (€374 or 21.3% of outpatient care cost).
Table 4. COMPASS-like population versus controls: comparison of annual per capita health expenditures per item of consumption (mean [SD] and median) in Euros 2016 – (societal perspective).
Similar results were obtained for each subgroup (). The mean total annual extra cost was substantially increased in the PAD ± CAD sub-group compared with the CAD ± PAD sub-group (€5,552 vs €4,008), but the difference peaked in the CAD + PAD sub-group (€8,067). However, the distribution of the extra cost by category of healthcare use remained quite similar, with a major contribution by hospitalization.
Table 5. Comparison of extra annual per capita costs versus controls per item of consumption in Euros 2016 – (societal perspective).
Discussion
Our crude 2016 prevalence estimates for CAD and PAD of 4.02% and 2.15%, respectively, can be compared to similar results issued for 2013 from the global claims database of the general insurance schemeCitation22. The corresponding standardized prevalences (population aged >20 years) were 3.5% and 1.33%, respectively, showing a difference most probably due to the standardization method. The cost estimates could not be compared to our results because they were not presented on a per capita basis. Another study estimated the economic burden of PAD in the European Union as substantial with 2-year vascular-related hospitalization costs for patients with PAD estimated at €3,182 in France in 2012Citation23.
This study used a claims database, which may have several limitations. One limitation pertains to the reliability of the ICD-10 codes used for cardiovascular diseases, which has not yet been studied in a comprehensive manner in France, with some exceptionsCitation24–27. Another limitation is that the database contains limited clinical data. Information about smoking status, body mass index, and biological tests (such as eGFR for renal dysfunction) were not recorded and could not be used in accordance with the inclusion/exclusion criteria in the COMPASS trial. In addition, we could not identify patients at high risk of bleeding and those presenting with severe heart failure with ejection fraction <30% or New York Heart Association class III or IV symptoms – these were other exclusion criteria. Consequently, our results establishing that the COMPASS-like population accounted for 58.1% of the total CAD or PAD population were probably overestimated and should, therefore, be used with caution. A similar attempt was performed on the basis of the large international observational REACH registryCitation21, which had a substantially more comprehensive set of clinical information. The result of this analysis was that 52.9% of patients fitted the COMPASS inclusion criteria, suggesting that our study might have overestimated the size of the COMPASS-like population, assuming that the profile of French patients was similar. Our mean age of 76.5 years compared with 71.1 years in the REACH registry could explain why our population had more severe comorbidities. A possible explanation is that the diagnosis of CAD and PAD based on hospitalization or LTD status may have led to overestimation of the severity of these conditions. On the other hand, it may have ensured the specificity of the diagnosis compared with a primary care setting.
The LTD status provides for the full coverage of healthcare costs related to the disease; therefore, it is unlikely that patients with CAD or PAD or their physician would not request such a status. We observed a very important overlap between hospital discharge and LTD diagnoses. Other limitations of ICD-10 coding for conditions such as limb ischemia or transient ischemic attack or the use of nonspecific codes such as 170.2 (atherosclerosis of arteries of extremities) and 173.9 (peripheral vascular disease unspecified) could also lead to underestimate the CAD and/or PAD prevalenceCitation7.
Despite those limitations, our results illustrate the discrepancies observed between the profiles of patients enrolled in COMPASS and the COMPASS-like French patients, due to the selection bias resulting from the over-representation of younger patients with fewer comorbidities than the overall eligible population.
Regarding the inpatient care cost analysis, the EGB database documented only acute care hospital stays. Hospital stays for post-acute care or rehabilitation were not included in our analysis, leading to an under-estimation of the costs, which could be considered as substantial in this population. As explained in the Methods section, we excluded from the analysis all patients with no healthcare reimbursement in 2016. This method was intended to avoid bias with patients who might be living in a nursing home without medical consumption recorded in the database or the death of whom was not recorded. Considering the age range of this population, such a decision seemed justified but might have led to under-estimation of the prevalent population and the economic burden.
One main conclusion about the economic cost of disease in the COMPASS indication was the substantially higher cost of the subgroup presenting with PAD as compared to CAD (€5,552 vs €4,008), and especially in those patients presenting with CAD and PAD (€8,067 vs €4,008).
Transparency
Declaration of funding
This study was funded by an unrestricted grant from Bayer AG to CEMKA.
Declaration of financial/other interests
JB Briere, T Evers are employees of Bayer AG. C Emery, E Torreton, F Fagnani are employees of CEMKA. Sponsorship: Bayer AG. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
The analysis was performed by C Emery, E Torreton and F Fagnani. Interpretation of results was carried out by JB Briere, T Evers, F Fagnani and C Emery. F Fagnani and C Emery are responsible for writing of the article.
Acknowledgements
None reported.
References
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322.
- Townsend N, Nichols M, Scarborough P, et al. Cardiovascular disease in Europe 2015: epidemiological update. Eur Heart J. 2015;36(40):2696–2705.
- Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25.
- Aouba AJ, Eb M, Rey G. L’évolution de la mortalité et des causes de décès entre 1990 et 2009. Actualité et Dossier en Santé Publique. 2012;80:24–28.
- Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Eur Heart J. 2018;39(9):763–816.
- Hosaka A, Miyata T, Onishi Y, et al. Clinical and economic burden in patients with diagnosis of peripheral arterial disease in a claims database in Japan. Clin Ther. 2014;36(8):1223–1230, 1230 e1221–1224.
- Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60(3):686–695.
- Marrett E, DiBonaventura M, Zhang Q. Burden of peripheral arterial disease in Europe and the United States: a patient survey. Health Qual Life Outcomes. 2013;11(1):175.
- Margolis J, Barron JJ, Grochulski WD. Health care resources and costs for treating peripheral artery disease in a managed care population: results from analysis of administrative claims data. JMCP. 2005;11(9):727–734.
- Mahoney EM, on behalf of the Reduction of Atherothrombosis for Continued Health (REACH) Registry Investigators*, Wang K, Keo HH, et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3(6):642–651.
- Fowkes FG, Aboyans V, Fowkes FJ, et al. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14(3):156–170.
- Hiatt WR, Rogers RK. The treatment gap in peripheral artery disease. J Am Coll Cardiol. 2017;69(18):2301–2303.
- Berger JS, Ladapo JA. Underuse of prevention and lifestyle counseling in patients with peripheral artery disease. J Am Coll Cardiol. 2017;69(18):2293–2300.
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180–189.
- European Medicines Agency. Summary of opinion (post authorisation): Xarelto. 2018. (EMA/CHMP/515065/2018). [cited 27 September 2019] Available from: https://www.ema.europa.eu/documents/smop/chmp-post-authorisation-summary-positive-opinion-xarelto-ii-58_en.pdf.
- Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319–1330.
- Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):219–229.
- Hiatt WR, Rogers RK. The development of therapeutics for peripheral artery disease: a unique cardiovascular risk population. J Am Coll Cardiol. 2016;67(23):2729–2731.
- Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: From the systeme national d’information interregimes de l’Assurance Maladie (SNIIRAM) to the systeme national des donnees de sante (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65 (Suppl 4):S149–S167.
- Haute Autorité de Santé. Choices in methods for economic evaluation. 2012. [cited 27 September 2019] Available from: https://www.has-sante.fr/upload/docs/application/pdf/2012-10/choices_in_methods_for_economic_evaluation.pdf.
- Darmon A, Bhatt DL, Elbez Y, et al. External applicability of the COMPASS trial: an analysis of the reduction of atherothrombosis for continued health (REACH) registry. Eur Heart J. 2018;39(9):750–757a.
- Tuppin P, Riviere S, Rigault A, et al. Prevalence and economic burden of cardiovascular diseases in France in 2013 according to the national health insurance scheme database. Arch Cardiovasc Dis. 2016;109(6-7):399–411.
- Smolderen KG, Wang K, de Pouvourville G, et al. Two-year vascular hospitalisation rates and associated costs in patients at risk of atherothrombosis in France and Germany: highest burden for peripheral arterial disease. Eur J Vasc Endovasc Surg. 2012;43(2):198–207.
- Bosco-Lévy P, Duret S, Picard F, et al. Diagnostic accuracy of the International Classification of Diseases, Tenth Revision, codes of heart failure in an administrative database. Pharmacoepidemiol Drug Saf. 2019;28(2):194–200.
- Prat M, Derumeaux H, Sailler L, et al. Positive predictive values of peripheral arterial and venous thrombosis codes in French hospital database. Fundam Clin Pharmacol. 2018;32(1):108–113.
- Bezin J, Girodet PO, Rambelomanana S, et al. Choice of ICD-10 codes for the identification of acute coronary syndrome in the French hospitalization database. Fundam Clin Pharmacol. 2015;29(6):586–591.
- Cohen S, Jannot AS, Iserin L, et al. Accuracy of claim data in the identification and classification of adults with congenital heart diseases in electronic medical records. Arch Cardiovasc Dis. 2019;112(1):31–43.
