Abstract
Aims: To assess the cost-utility of cladribine tablets versus fingolimod in patients with highly active relapsing-remitting multiple sclerosis (RRMS) in Portugal.
Methods: A 1-year cycle cohort-based Markov state transition model was developed to simulate disease progression, measured by Kurtzke Expanded Disability Status Scale (EDSS), relapses, and conversion to secondary-progressive MS (SPMS). Patients were assumed to remain on treatment until progression to EDSS level 7, conversion to SPMS, or complete loss of efficacy due to waning effect. Natural history was based on British Columbia Multiple Sclerosis registry, London Ontario database, UK MS Trust, and cladribine tablets clinical trial (CLARITY). Portuguese all-cause mortality was adjusted for the MS associated increased mortality. Clinical inputs for active treatments (disability progression and relapse rate) were estimated on a network meta-analysis. Utility weights were derived from UK-MS Survey and published literature. Resource consumption by EDSS and due to relapses was based on published literature, National DRG microdata and expert opinion. Unit costs were obtained from official sources. The analysis was conducted from payers’ perspective, time horizon of 50 years and discount rate of 5%, for both costs and benefits. Uncertainty was assessed via probabilistic and deterministic sensitivity analyses.
Results: Compared to fingolimod, cladribine tablets were associated with a delay in progression, resulting in a gain of 0.85 quality adjusted life years (QALYs) and a cost decrease of 25,935 €. Probabilistic sensitivity analysis resulted in a mean ICER of −31,781 € per QALY and was dominant in 98.7% of the simulations. Cladribine tablets were dominant across the scenario analyses tested.
Conclusions: Treatment of highly active RRMS with cladribine tablets was less costly and more effective than treatment with fingolimod. Hence, it is a dominant strategy in the Portuguese setting. No conclusions can be drawn from the present study regarding other treatment options, in particular natalizumab and alemtuzumab.
Introduction
Multiple sclerosis (MS) is an immune-mediated inflammatory demyelinating disease of the central nervous system (CNS) with loss of motor and sensory functionsCitation1. The incidence and prevalence rates of MS vary substantially between geographic regionsCitation2. In Europe, the overall estimated incidence and prevalence rates are 5.5 and 108 per 100,000, respectivelyCitation2. Lower estimations have been reported for Portugal (4.48 and 56.20 per 100,000 for incidence and prevalence rates, respectively)Citation3,Citation4. MS is twice as common in women as men, with an average age of onset of about 30 years. Disease progression is variable and influenced by both environmental and genetic factorsCitation5.
Different subtypes of MS have been identified, based on clinical presentation and disease course. The most common MS type is relapsing-remitting MS (RRMS; around 85% of MS cases), which is characterized by clearly defined relapses (attacks of new or increasing neurologic symptoms) followed by remissions (periods of partial or complete recovery). By definition, in RRMS there is no apparent progression of the disease during remission. However, most RRMS cases will eventually transit to another MS type designated secondary progressive MS (SPMS), in which there is a progressive worsening of neurologic function (accumulation of disability) over timeCitation6,Citation7. Patients with RRMS may be further classified as having or not a highly active disease characterized by frequent clinical relapses and/or magnetic resonance imaging (MRI) activity, either when untreated or on a disease-modifying drug (DMD)Citation8. DMDs are indicated for most cases of RRMS, with the aim of modifying disease progression, either reducing the number of relapses or delaying the progression of disabilityCitation9,Citation10. In RRMS, the current treatment options include interferon-β, glatiramer acetate, natalizumab, fingolimod, teriflunomide, alemtuzumab and dimethyl fumarate. Second-line treatment with natalizumab, fingolimod or alemtuzumab is recommended to patients with active disease and an insufficient response to at least one drug, as well as to patients with rapidly evolving severe RRMSCitation11–14. In Portugal, these second-line treatments are used in the treatment of patients with highly active disease.
Cladribine tablets have recently been approved in Europe for the treatment of adult patients with highly active RRMS as defined by clinical or imaging featuresCitation15. This decision was based on the positive results from a large randomized, double-blind, placebo-controlled clinical study (CLARITY)Citation16,Citation17 and its extension phase (CLARITY EXT)Citation18. The recommended dose of cladribine tablets is 3.50 mg/kg over 2 years, administered orally in one treatment course of 1.75 mg/kg per year (each treatment course comprises two treatment weeks during the first 2 months).
The objective of this study is to estimate the cost-utility of cladribine tablets compared to fingolimod in the treatment of highly active RRMS in Portugal from the payer’s perspective.
Methods
Model structure
The analyses were conducted using an existing cohort-based Markov model that simulated the costs and effectiveness of RRMS treatment, following the structure used in previous economic modelsCitation19,Citation20.
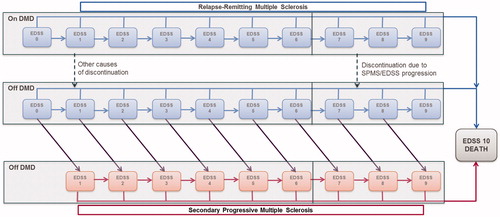
Disease progression was modelled using the Kurtzke’s Expanded Disability Status Scale (EDSS) and comprises 21 states: 10 EDSS states for RRMS, 10 EDSS states for SPMS and an all-cause death state (). At each yearly cycle, patients are at risk of experiencing disability progression (moving to a higher EDSS state); improving in disability status (moving to a lower EDSS state); remaining at their current EDSS state; converting to SPMS with a simultaneous disability progression; or die. Patients are assumed to remain on treatment until progression to EDSS level 7, conversion to SPMS, treatment discontinuation, or complete loss of efficacy due to waning effect on disability progression and relapse rate. The analysis assumed a time horizon of 50 years and a discount rate of 5%, for both costs and benefits, as specified by the Portuguese guidelines for the economic evaluation of pharmaceuticalsCitation21.
Model inputs
The cohort considered in the model replicates the subgroup of patients included in the CLARITY trial with high disease activity (HDA), defined as: (a) at least one relapse in the previous year while on DMD and at least one T1 gadolinium-enhancing lesion or at least nine T2 lesions, or (b) two or more relapses in the previous year whether on DMD or not. As observed in CLARITY trial, the mean age is 37.1 years and the female to male ratio is 1.7. shows the initial distribution amongst EDSS states.
Table 1. Initial HDA patient distribution, by EDSS state.
Disease progression and relapse occurrence with either treatment were modelled by applying a relative treatment effect to the disease natural history. Assuming that the latter represents what would occur if patients were on placebo, this allows use of the results of a network meta-analysis that compares the treatment effect of each DMD and placebo.
No prospective registry data for MS patients is available in Portugal. Therefore, the disease natural history was based on the British Columbia Multiple Sclerosis registry ([BCMS, Canada]Citation16). This registry includes a large representative MS population, recording EDSS scores prospectively with a long-term follow-up (28 years). In this research, transition probabilities differ between those patients whose age at disease onset are equal or greater, or less than 28 years old. Given that the mean age at CLARITY baseline was 38 years old, the latter transition matrix was considered. Moreover, as the original BCMS analysis was performed on a cohort that covers a broad group, including patients with mild and less progressive forms of RRMS alongside people with highly active or rapidly evolving disease, an acceleration factor that accounts for a faster rate of progression on HDA population was included. A hazard ratio (HR) of 1.38 was estimated by comparing the proportion of progression-free at week 96, for the placebo arm, in the HDA-RRMS (71.7%) subgroup of CLARITY with its complement (non HDA-RRMS, 78.6%). Due to the lack of information by EDSS level, we assumed that the HR was constant across all EDSS levels. The resulting annual transition probabilities are shown in .
Table 2. Annual transition probability matrix by EDSS state (MS age of onset ≥28 years), after adjustment for HDA-RRMS.
Besides EDSS transition, between RRMS health states, patients were at risk of converting from RRMS to SPMS. Annual transitions to SPMS, by EDSS RRMS state, were based on the median time to event estimated using the London Ontario registryCitation23, as this registry allows estimation of a conversion rate dependent on EDSS state. The annualized conversion probabilities are shown in .
Table 3. SPMS conversion probabilities, by EDSS state.
The transition progression matrix for SPMS based on data from London Ontario is presented in .
Table 4. Annual transition probability matrix by EDSS state for SPMS.
The annualized relapse rate was obtained from the UK MS Trust, which allows to estimate different relapse rates according to EDDS state for both RRMS and SPMSCitation24 ().
Table 5. Annualized relapse rate by EDSS state and MS classification.
All-cause mortality rate, averaged by gender and age, was derived from general population mortality reported in national life tablesCitation25, and inflated by the authors of the modelCitation20 to account for the excess mortality associated with MS. The standardized mortality ratio by EDSS state was modelled using data from a prospective survey of MS people reported in Sadovnick et al. Citation26 and PokorskiCitation27. The mortality multiplier applied to both populations, RRMS and SPMS, is showed in . The original study by Sadovnick et al.Citation26 did not differentiate between MS subtypes. Therefore, we assumed the same multiplier for both populations.
Table 6. MS standardised mortality ratios by EDSS state.
Comparative efficacy of cladribine tablets was obtained from a systematic review and network meta-analysis (NMA)Citation28, which included aggregated data from 44 clinical trials assessing 12 DMDs, and provided data for the subgroup of patients with HDA. The clinical efficacy of DMDs in the subgroup with HAD was modelled for the relative reduction in annualized relapse rate (ARR) and a reduction in the hazard rate for EDSS progression, defined as 3-month confirmed disability progression. Although the original publication from Siddiqui et al.Citation28 presents only the results for the overall ITT population and for confirmed disability progression at 6 months, the technical report of the NMA (not published) and the model used provided data specifically for the HDA subgroup and for the 3-month period. shows the results for the interventions assessed in the present study (cladribine tablets and fingolimod).
Table 7. Relative clinical efficacy of cladribine tablets and fingolimod, vs. placebo.
The risk of treatment-related adverse events was based in the systematic review and NMACitation28 and published literatureCitation29–31.
Waning effect assumptions were based on CLARITY and the CLARITY extension study (CLARITY EXT) for cladribine tablets, and NICE submissions for fingolimodCitation32,Citation33. According to CLARITY EXT, treatment with cladribine tablets for two years resulted in a durable clinical response for at least two additional treatment-free years, with no significant differences in ARR or relapse-free proportion compared to the response achieved at the end of CLARITY. Therefore it was assumed that the full effect of cladribine tablets was sustained through the first four years, waning from 66.7% (fifth year) to 33.3% (sixth year) of drug effect, with no effect assumed beyond year 6. For fingolimod, it was assumed 100% effect on the first 2 years, 75% from second year until fifth year and 50% thereafter.
Annual probability of treatment withdrawal was estimated based on CLARITY trial data for cladribine tablets (4.9%, applied until 2 years of treatment) and based in the NMACitation28 for fingolimod (10.3%, applied while on treatment).
Adverse events were assumed to occur at the start of the simulation. The list of adverse events was defined following revision of the summary of product characteristics for each drug. This information was validated by experts’ opinion. Model inputs related to adverse events are presented on .
Utility scores
Utility weights for patients and caregivers, as well as the disutility due to relapses, were derived from the UK MS Survey and published literatureCitation34. The scores used in the model are presented in . Adverse event disutility (ranging between 0.110 and 0.400) and duration were obtained from published literatureCitation35.
Table 8. Mean utility scores.
Resource utilization and costs
Resource consumption estimates were based on relevant national published literatureCitation36. The estimates reported were revised by clinical experts from the National MS society. Hospital resource utilization and costs were directly sourced from the Portuguese 2015 diagnostic related group (DRG) microdata. Multiple sclerosis inpatient episodes were identified through International Classification of Diseases, 9th Revision (ICD9: 340).
Resources were valued according to Portuguese official sources: Portaria 234/2015Citation37 Catalogue for Public Health Procurement (SPMS, 2016) and InfomedCitation38. Only direct costs were computed.
presents the estimated annual costs by EDSS state. In contrast with most previous cost-effectiveness analyses on MS, the base-case scenario considered different costs for relapse, according to EDSS state. The reason being because the Portuguese experts consistently identified different relapse resource consumption according to EDSS state (including differences on the number of hospitalizations, on rehabilitation and technical aids.
Table 9. Estimated annual costs, by EDSS.
Cladribine tablets price was made available by the market authorization holder. The dose depends on body weight (3.5 mg/Kg), so drug costs were estimated according to the weight distribution published in the Portuguese National Health Survey 2014Citation25,Citation39. Both cladribine tablets and fingolimod are oral treatments, therefore no administration costs were considered.
Costs associated with rescue therapy among cladribine-treated patients were considered in the model as one-off cost. A logistic-regression model with fixed effects for trial group and region was adjusted to estimate the probability of receiving rescue therapy for cladribine tablets versus placebo (OR = 0.40 [0.19–0.81]). It was estimated that rescue therapy with interferon beta-1a was given to 2.6% of patients in cladribine arm.
Costs associated with treatment-related adverse events are presented in .
Table 10. Model inputs related to adverse events.
Sensitivity analysis
Deterministic and probabilistic sensitivity analyses were performed. One-way deterministic sensitivity analyses were undertaken on the following parameters: transition probabilities, mortality risk, discontinuation, treatment waning effect, utility weights, discount rate, time horizon and costs (EDSS disability, relapse and adverse events). For costs and utility weights parameters, the deterministic sensitivity analyses considered the estimates for PortugalCitation40 from the recent cross-sectional studyCitation41, conducted in 16 European countries. A total of 535 Portuguese patients (54% with RRMS) were included in the European cross-sectional study.
Probabilistic sensitivity analysis (PSA) was performed to account for uncertainty in the estimate of the parameters. The simulation included 10,000 iterations. The parameters considered in the PSA and the corresponding distributions are described in .
Table 11. Parameters and distributions used in probabilistic sensitivity analysis.
Results
Base-case scenario
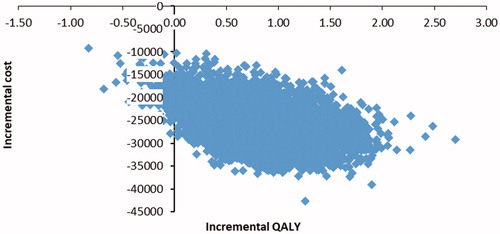
Treatment with cladribine tablets was associated with a delay in disease progression, resulting in a gain of 0.85 quality adjusted life years (QALYs). From an economic viewpoint, this delay allowed for a decrease in total follow-up costs that amount to 25,935 € (time-horizon of 50 years). Paradoxically, as the relative relapse risk was higher in lower EDSS states, it implies an increase in relapse costs. These results showed that cladribine tablets was dominant when compared to fingolimod ().
Table 12. Results for estimated costs and consequences (base-case scenario).
Sensitivity analysis
The sensitive analysis performed () showed that base-case results were robust, with cladribine tablets remaining a dominant strategy compared to fingolimod.
Table 13. One-way deterministic sensitivity analyses results.
All the assumptions regarding costs had a minor impact on results. Considering an 11-state model, without distinction between RRMS and SPMS, resulted in higher cost savings. Although CLARITY EXT trial supports the durability of cladribine tablets effect, if we assume that treatment effect doesn’t sustain after the third year (worst case scenario), the dominance was maintained.
Probabilistic sensitivity analysis resulted in a mean ICER of −31,781 € per QALY. These results confirmed the dominant profile of cladribine tablets compared to fingolimod. Cladribine tablets was dominant in 98.7% of the 10,000 simulations ().
Discussion and conclusion
In spite of the low incidence and prevalence rates, MS is one of the leading causes of non-traumatic disability in young adults (World Health Organization [WHO] 2013). Interestingly, the global MS burden of disease, assessed by disability-adjusted life years (DALY), has been recently reported to have a global downward trendCitation43. This trend was found for both developed and developing countries, although MS DALY rates are about 25 higher in developed countries compared to developing ones. According to the authors, the reason for this decline, especially in European countries, was that by increasing level of health care (and budget) for MS patients, the age of death had risen significantly in recent years. Overall, this positive effect outweighs the negative impact on the number of years that MS patients live with disability.
Our study showed that cladribine tablets was a dominant strategy in patients with HDA-RRMS, being a less costly and more effective treatment than fingolimod. Cladribine tablets demonstrated to have an incremental benefit on disease progression when compared to fingolimod. However, this benefit was not observed when relapse rate was considered, possibly due to the fact that estimated relapse rates were lower in higher EDSS states.
Our study had several limitations. First, this study does not include other current treatment options for MS patients with HAD, in particular natalizumab and alemtuzumab. The reasons for not having included these comparators are: (1) Fingolimod is the first treatment option in Portugal for MS patients with HAD, representing about 2/3 of the overall second-line treatments; (2) Alemtuzumab was not an option at the time of the study (Q1, 2018) because it was not reimbursed for use in Portuguese NHS hospitals; (3) When fingolimod was evaluated by the national HTA Authority, the selected comparator was natalizumab because natalizumab was, at that time (Q1, 2012), the only treatment option for patients with HAD and an insufficient response to first-line treatments, as well as for patients with rapidly evolving severe RRMS. The national HTA Authority considered that, for this population, fingolimod had an added therapeutic value in comparison to natalizumab due to safety and convenience aspects. Fingolimod proved cost-effectiveness in a minimization cost analysis due the lower treatment costs; and (4) Both fingolimod and cladribine tablets are oral treatments, and mode of administration is considered a relevant factor associated with patient preferences.
Second, the 21-health state model structure is commonly used in multiple sclerosis assessments but it should be stressed that the RRMS matrix was based on BCMS data which also includes patients with SPMS (16%). Although this was not expected to influence results, it should be addressed as a limitation. Third, after discontinuation, patients were assumed to switch to Best Supportive Care, with progression and relapse rates based on disease natural history. Although treatment switching may occur in clinical practice, this was not considered in our study, once no clinical data after switch was available.
Moreover, according to the perspective followed, only direct costs were included. Although it could be argued that the inclusion of indirect costs would have provided a more comprehensive analysis, our approach can be potentially viewed as a conservative one, as it could be assumed that that the inclusion of indirect costs may demonstrate a stronger dominance of cladribine tablets.
The results from the sensitivity analyses showed that the cost-effectiveness estimates on cladribine tablets were robust. The major source of uncertainty was the efficacy of DMDs beyond clinical trial duration. In line with previous studiesCitation32,Citation33, we considered a reduced effect of DMD beyond year 2 through the waning effect. Although CLARITY-EXT justifies the waning effect assumed for cladribine tablets over 4 years, there was no data to support the assumptions beyond the fourth year. Considering no cladribine tablets effect after the fourth year impacted the estimated QALYs (reduced from 0.85 to 0.44).
Recently, Hettle et al.Citation20 published a cost-effectiveness analysis comparing cladribine tablets with alemtuzumab and natalizumab in the treatment of relapsing-remitting multiple sclerosis with HDA in England. The authors found that cladribine tablets was a dominant alternative. However, no comparison regarding fingolimod was done. Of note, in this study the authors used an 11-health state structure that tracks the disability status of a combined cohort of people with RRMS and SPMS, instead of the 21-health state model used in the present case.
NICE appraised the cost-effectiveness of cladribine tablets, compared to alemtuzumab, daclizumab, fingolimod and natalizumab, for two separate subgroups of HDA-RRMS (patients with rapidly evolving severe and sub-optimally treated), not providing data for the overall HDA-RRMS population. Despite the uncertainty in the available data, NICE concluded that cladribine tablets could be considered a cost-effective use of National Health Service (NHS) resources in both subgroups of HDA-RRMSCitation44.
To the best of our knowledge, our study was the first to assess the cost-effectiveness of cladribine tablets versus fingolimod in patients with HDA-RRMS. The results showed that cladribine tablets were a cost-effective alternative to fingolimod in the treatment of HDA-RRMS from the portuguese payers' perspective.
Transparency
Declaration of funding
The study was funded by Merck S.A. Funding was independent of the study outcome. The sponsor did not participate in the design, analysis or interpretation of the data. The drafting and final version of the paper is the solely responsibility of the authors.
Declaration of financial/other relationships
All authors are members of the Evidence Based Medicine (EBM) Center at Lisbon School of Medicine, Portugal. This research centre is devoted to pre‐ and postgraduate medical education. Since 2002, the Lisbon EBM Center has undertaken several clinical, epidemiological and phamacoeconomic research projects, which had unrestricted funding from over 20 different pharmaceutical companies. One of those was Merck S.A., which developed cladribine tablets. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Pinheiro B, Costa J and Miguel LS were involved in all phases of the work, including conception and design, analysis and interpretation of the data, the drafting of the paper and critical revision for intellectual content.
Guerreiro R participated in the conception, the drafting of the paper and critical revision for intellectual content.
All authors approved the final version of the published manuscript and agree to be accountable for all aspects of the work.
Acknowledgements
The authors thank the participation of the expert MD José Vale.
References
- Peixoto S, Abreu P. Magnetic resonance imaging conversion predictors of clinically isolated syndrome to multiple sclerosis. Acta Med Port. 2016;29(11):742–748.
- World Health Organisation. Atlas of multiple sclerosis. Multiple Sclerosis International Federation, 2013.
- de Sá J, Alcalde-Cabero E, Almazan-Isla J, et al. Incidence of multiple sclerosis in Northern Lisbon, Portugal: 1998–2007. BMC Neurol. 2014;14(1):249.
- de Sá J. Captura-recaptura como método epidemiológico a aplicar à Esclerose Múltipla. Universidade de Lisboa: Lisboa. 2014b.
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517.
- Harrison DM. Multiple sclerosis. Ann Intern Med. 2014;160(7):ITC4-2–ITC4-18.
- Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286.
- Scolding N, Barnes D, Cader S, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol. 2015;15(4):273–279.
- Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–788.
- Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96–120.
- Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89(2):225–240.
- Tsivgoulis G, Katsanos AH, Grigoriadis N, et al. The effect of disease modifying therapies on disease progression in patients with relapsing-remitting multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2015;10(12):e0144538.
- Goodin DS, Frohman EM, Garmany GP, Jr, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002;58(2):169–178.
- Infarmed. Orientações da Comissão Nacional de Farmácia e Terapêutica (CNFT). Utilização de fármacos para o tratamento da esclerose múltipla 2017 [updated 10 March 2017]. p. 1–3. Available from: http://www.infarmed.pt/documents/15786/1816213/4_CNFT_Esclerose+M%C3%BAltipla/69b03432-969c-4976-8ab0-7ed3f83f7940.
- European Medicines Agency. New recommendations to minimise risks of the rare brain infection PML and a type of skin cancer with Gilenya: EMA/688187/2015. 18 December 2015. Available from: https://www.ema.europa.eu/en/news/new-recommendations-minimise-risks-rare-brain-infection-pml-type-skin-cancer-gilenya.
- Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416–426.
- Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011;10(4):329–337.
- Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. 2018;24(12):1594–1604.
- Chilcott J, McCabe C, Tappenden P, et al. Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. Commentary: evaluating disease modifying treatments in multiple sclerosis. BMJ. 2003;326(7388):522–522.
- Silva EA, Pinto CG, Sampaio C, et al. Orientações metodológicas para estudos de avaliação económica de medicamentos. Lisboa: Ministério da Saúde, Infarmed; 1998.
- Palace J, Bregenzer T, Tremlett H, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open. 2014;4(1):e004073.
- Ebers GC. Natural history of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;71(Suppl 2):ii16–ii19.
- Warwick Evidence. Beta interferon and glatiramer acetate for treating multiple sclerosis (review of TA32) [ID809], 2016. Available from: https://www.nice.org.uk/guidance/GID-TAG529/documents/assessment-report.
- Instituto Nacional de Estatística (INE). Tábuas completas de mortalidade 2014-2016. 2017. Available from: https://www.ine.pt/xportal/xmain?xpgid=ine_main&xpid=INE&xlang=pt
- Hettle R, Harty G, Wong SL. Cost-effectiveness of cladribine tablets, alemtuzumab, and natalizumab in the treatment of relapsing-remitting multiple sclerosis with high disease activity in England. J Med Econ. 2018;21(7):676–686.
- Sadovnick AD, Ebers GC, Wilson RW, et al. Life expectancy in patients attending multiple sclerosis clinics. Neurology. 1992;42(5):991–994.
- Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med. 1997;29(2):101–106.
- Siddiqui MK, Khurana IS, Budhia S, et al. Systematic literature review and network meta-analysis of cladribine tablets versus alternative disease-modifying treatments for relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2018;34(8):1361–1371.
- European Medicines Agency. New recommendations to minimise risks of the rare brain infection PML and a type of skin cancer with Gilenya: EMA/688187/2015. 18 dezember 2015.
- Multiple Sclerosis Trust. Multiple Sclerosis Trust: Progressive multifocal leukoencephalopathy (PML). 2016. 2016.
- Pakpoor J, Disanto G, Altmann DR, et al. No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine. Neurol Neuroimmunol Neuroinflamm. 2015;2(6):e158.
- National Institute for Health and Clinical Excellence (NICE). Technology appraisal guidance. Daclizumab for treating relapsing–remitting multiple sclerosis (TA441). [cited 2017 April 26]. Available from: https://www.nice.org.uk/guidance/ta441
- National Institute for Health and Clinical Excellence (NICE) Technology appraisal guidance. Dimethyl fumarate for treating relapsing-remitting multiple sclerosis (TA320). [cited 2014 Aug 27]. Available from: https://www.nice.org.uk/guidance/ta320.
- Orme M, Kerrigan J, Tyas D, et al. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health. 2007;10(1):54–60.
- Acaster S, Perard R, Chauhan D, et al. A forgotten aspect of the NICE reference case: an observational study of the health related quality of life impact on caregivers of people with multiple sclerosis. BMC Health Serv Res. 2013;13(1):346.
- Mateus MdCC. Contributos para a avaliação económica de medicamentos em Portugal: Escola Nacional de Saúde Pública, Universidade Nova de Lisboa, Lisboa. 2010.
- Ministério da Saúde. Diário da República n° 153/2015 - Série I, Portaria n° 234 de 07 de agosto de 2015.
- Infarmed. Infomed databse [Infarmed website]; [cited 2017 Nov]. Available from: http://app7.infarmed.pt/infomed/
- Instituto Nacional Estatística (INE), Inquérito Nacional de Saúde 2014. 2016. Available from: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpub_boui=263714091&PUBLICACOESmodo=2&xlang=pt.
- Sá MJ, Kobelt G, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe: results for Portugal. Mult Scler. 2017;23(2_suppl):143–154.
- Kobelt G, Thompson A, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23(8):1123–1136.
- Hawton A, Green C. Health Utilities for Multiple Sclerosis. Value Health. 2016;19:460–468.
- Mansouri S, Zayeri F. Global and regional trends of multiple sclerosis disability-adjusted life years rates: a 25-year assessment. Neuroepidemiology. 2019;52(1–2):17–24.
- Lambe T, Duarte R, Mahon J, et al. Cladribine tablets for the first-line treatment of relapsing-remitting multiple sclerosis: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2019;37:345–357.