Abstract
Aim: To estimate the cost-effectiveness of atezolizumab compared with docetaxel and nivolumab for the treatment of advanced non-small cell lung cancer (NSCLC), as a second-line treatment, in a French setting.
Materials and methods: A three-state partitioned-survival model was developed (progression-free survival, post-progression survival, death) based on the phase IIIOAK trial on a 10-year time horizon. The comparison between nivolumab and atezolizumab came from a network meta-analysis. Utilities were estimated from the OAK trial EQ-5D applying the French utility tariffs. Overall survival (OS), progression-free survival (PFS), and treatment duration were estimated using parametric models selected using Akaike and Bayesian information criterion. Extrapolation beyond the trial duration followed NICE DSU TSD 14. Economic perspective was the one of all payers, discount rate fixed at 4% on benefits and costs. This analysis was aligned with French Haute Autorité de Santé recommendations. Results were expressed in total cost (2019) and €/QALY (Quality Adjusted Life Year). Model robustness was checked through sensitivity analyses, and a probabilistic sensitivity analysis was conducted.
Results: In comparison to docetaxel, atezolizumab costs 49,429€ more and increased life expectancy by 8 months, generating 0.47 QALY. Incremental cost-effectiveness ratio was estimated at 104,835€/QALY. When comparing nivolumab to atezolizumab, a cost minimization analysis was conducted since no clear evidence supporting a difference in terms of survival benefit was reported. Using list price, and the Market Access Authorization regimens, atezolizumab saved approximately 6,000€, 9.5% of its total costs. Sensitivity analyses confirmed the robustness of our findings.
Conclusion: Atezolizumab is more efficient and more costly than docetaxel in the second-line treatment of NSCLC of stage IIIB or IV, in France, with results consistent to previous French authorities’ evaluation of immunotherapies in similar indication. Lastly, atezolizumab is a cost saving alternative to nivolumab, based on list price.
Introduction
Lung cancer remains an important public health issue in France. It is the first cause of mortality by cancer in men and the second in womenCitation1 and most of the cases are attributable to tobaccoCitation2.
In 2017Citation3, 49,109 incident cases of lung cancer were diagnosed in France, and incidence rate was still expected to increase in the coming yearsCitation4. Seventy-five percent of them were non-small cell lung cancer (NSCLC)Citation5 and most of them were diagnosed at an advanced stage (stages IIIb or IV)Citation6. Among those diagnosed at a less advanced stage, 40% had a recurrenceCitation6 and therefore the population of incident NSCLC patient needing a first-line systemic treatment could be estimated between 22,980 and 26,950 patients yearly, in a 67 m inhabitant country. A second-line treatment is initiated in about 40–50% of these patients, depending on histology and mutational statusCitation1,Citation7,Citation8. Overall survival (OS) in advanced lung cancer remains short: at 1 year, survival rate was 54.2%, with strong inequities between socioeconomics groupsCitation9, although French heath care insurance relied on universal coverage.
Latest standards of care in France for second-line NSCLC are described in AURA 2019Citation10 recommendation accounting for histology (squamous versus non-squamous) and molecular markers. In squamous NSCLC, atezolizumab, nivolumab, docetaxel, erlotinib, and pembrolizumab (only in PD-L1 positive) are the main validated treatment options after first-line treatment failure. Erlotinib is recommended only if other alternatives have failed or are inappropriate. In non-squamous NSCLC, pemetrexed and bevacizumab plus paclitaxel were added to the above list, as a 2nd+ line treatment. In case of Epidermal Growth Factor Receptor (EGFR) mutation or Anaplastic lymphoma kinase (ALK) rearrangement, specific targeted treatments are available.
Atezolizumab is a programmed death-ligand 1 (PD-L1) blocking antibody. As monotherapy, it is indicated for the treatment of adult patients with locally advanced or metastatic NSCLC after prior chemotherapy (Market access authorization: July 21st, 2017). Patients with EGFR activating mutations or ALK-positive tumor mutations should also have received targeted therapy before receiving atezolizumab as a second-line treatments. Other anti-PD-1 checkpoint inhibitors are available in the treatment of NSCLC: nivolumab and pembrolizumab. NivolumabCitation11 is given regardless of PD-L1 expression, similarly to atezolizumabCitation12, while pembrolizumabCitation13 is only indicated to patients whose tumours have a PD-L1 tumour proportion score >1%.
The benefit risk evaluation of atezolizumab versus docetaxel in second-line NSCLC treatment is supported by the results of the OAK phase III trialCitation14,Citation15. Atezolizumab demonstrated an OS benefit versus docetaxel [hazard ratio (HR) = 0.75, 95% confidence interval: 0.64–0.89, p = .0006] after a minimum follow-up time of 26 months. Improvement of survival was observed across all programmed death-ligand 1 and histological subgroups. Fewer patients receiving atezolizumab experienced stages III or IV treatment-related adverse events (AEs) (14.9%) than those treated with docetaxel (42.4%).
Innovation in the field of oncology are often perceived as expensive and cost-effectiveness analysis plays a major role when making price decisionCitation16. Cost effectiveness analysis of atezolizumab in NSCLC second-line treatment has already been reported. In the USA, based on four RCTs, in comparison to docetaxel, the Quality adjusted life years (QALY) gain for atezolizumab was 0.354 and the incremental cost-effectiveness ratio (ICER) was $215,802Citation17. Based on the results of the OAK study and using a network meta-analysis, Ondhia et al.Citation18 evaluated efficiency of atezolizumab in Canada. They reported a gain QALYs of 0.60 for atezolizumab compared with docetaxel at an incremental cost of $85,073 based on a 10 years horizon, resulting in an ICER of $1,42,074/QALY. Atezolizumab was more effective and less costly than nivolumab although based on a modest difference in QALYs (+0.04) and costs (−$4,275), as stated by the authors. These results favouring atezolizumab are here driven by a fractional polynomials NMA specifically used for this analysis, showing no clear difference regarding survival benefits. Differentiating atezolizumab from nivolumab in this indication on the basis of an indirect comparison remains difficult regarding available data.
Access to the French market for innovative drugs and/or resulting in an important budget impactCitation19 are conditioned to a cost effectiveness analysis evaluated by the French Haute Autorité de Santé (HAS) before going through price negotiation with the CEPS (Comité économique des produits de santé)Citation20. Health economic evaluations should follow recommendations specified by the CEESP (Commission Évaluation Économique et de Santé Publique)Citation8. This was the case for atezolizumab which was evaluated both by Transparency CommissionCitation21 and the CEESPCitation22.
The objective of our study was to estimate the cost-effectiveness of atezolizumab against nivolumab and docetaxel, as a NSCLC second-line treatment, in France.
Methods
Type of economic analysis
This cost-utility analysis was performed according to a collective perspective in line with the recommendations of the HASCitation20. A cost-minimization was used when the incremental QALY was not big enough to justify an ICER calculation.
The model
A partitioned survival model was developed in Microsoft Excel (release 2010, Microsoft Inc, the USA) comprising of three distinct health states: progression-free survival (PFS), post-progression survival (PPS), and death as the absorbing state. Overall survival was partitioned into PFS or progression. This type of model was used and published in several NSCLC economic evaluationCitation17,Citation23–26. All patients started at free of progression (PFS) on treatment, then transitioned to progression. Death could occur from any state. Patients under atezolizumab or nivolumab continued treatment until loss of clinical benefit, independently of the progression status since immune checkpoint inhibitors may provide additional clinical benefit beyond progression. The time horizon of the model was 10 years allowing to capture more than 95% of the deaths. Model cycle was 1 week aligned with treatment administration frequencies and half cycle corrections were implemented.
Population simulated in the model
The target population from the multinational OAK study was simulated in this model and considered being an acceptable proxy of the French patients treated for a locally advanced or metastatic NSCLC with a previous chemotherapy. External validityCitation21 was checked with real world data from PMSI (Programme de médicalisation des systèmes d’information) database (a French nationwide exhaustive in-patient claim data base) and the OAK patients were found similar to the French patient population (age, gender, smoker) although OAK patients had a better ECOG score.
Comparators
The comparators used in this analysis were docetaxel and nivolumab. Erlotinib was excluded as HAS recently gave a negative opinion to its reimbursement in this indicationCitation27. Pemetrexed and bevacizumab plus paclitaxel were not considered as being indicated only in non-squamous NSCLC; moreover, a majority of patients with advanced non-squamous NSCLC are treated with a pemetrexed-based combination in first-line setting. Pembrolizumab was not considered as an alternative as being indicated only in patients whose tumours cells harbour PD-L1 expression. These decisions were driven by the “all comers” targeted population defined in the market authorization label. Other chemotherapies were excluded due to marginal utilization in French practice and lack of validation and comparative data in literature.
Effectiveness estimates
Atezolizumab, docetaxel and nivolumab efficacy (PFS and OS) were estimated through a network meta-analysis based on 19 RCTs, as one source, using the results of fractional polynomials method and proportional hazard fixed effects method. Full details are available on the HAS websiteCitation21 Use of polynomial method allowed a better fit for treatments with delayed onset of treatment effect and long term OS and PFS for which proportional hazard assumption is violated. Thus, fractional polynomial method can be considered as a better approach than traditional NMA relying on proportional hazard assumption to evaluate relative efficacy of immunotherapiesCitation28. Extrapolation of survival functions beyond the time horizon of the trial followed NICE DSU TSD 14Citation29. The best fit against Kaplan–Meier curves was selected using Akaike’s Information Criterion (AIC) and Bayesian information criterion (BIC), penalty functions, visual inspection, and independent expert opinion. In order to have an even better fit, use of Kaplan–Meier curves followed by a parametric extrapolated tail among the following classic survival function (exponential, Weibull, log-normal, log-logistic Generalized Gamma, and Gompertz) was tested. A standard parametric Weibull extrapolation was also tested. Kaplan–Meier curve plus Generalized Gamma tail distribution was retained for PFS and log-logistic tail for OS. External validity was checked with nivolumab clinical trialsCitation30,Citation31, INCa dataCitation32 and POPLAR trialCitation14 and demonstrated acceptable fits.
Atezolizumab treatment duration was extrapolated using the above described methodology and was estimated from the OAK trial. A non-proportional hazard Weibull was used, as proportional hazard assumption did not hold mainly due to longer treatment duration with immunotherapies. In the absence of nivolumab information on treatment duration, atezolizumab function was used.
Safety estimates
Adverse events included into the model were those whose incidence rate was higher than 1% with a stages III or IV. Incidence rate came from OAK study for atezolizumab and docetaxel and from phase III RCTs for nivolumabCitation29,Citation30. Weekly probability was estimated using the exponential function described by Fleurence et al.Citation33
Resource utilization
Medical resources used in the context of an out-patient setting were collected through face to face expert interviews conducted in June 2016. A questionnaire was developed with a focus on medical resources dedicated for second and third NSCLC treatment line, end of life treatment, and transportations. The questionnaire was sent prior to the interview and estimates included into the model were validated by the experts.
Costing
All results were expressed in €2019. General inflation rate was used when costs were published before 2019Citation34.
The economic perspective was the collective. Production costs were used when available otherwise replaced by tariffs as a proxy. Costs of interest were (1) studied treatment (drug, administration, follow-up, AEs), (2) NSCLC related (disease under control, under progression), (3) next line treatment after studied treatment failure, and (4) end of life. Base case analysis was conducted with a discount rate of 4% both on cost and benefits in line with HAS recommendationsCitation20.
The French following sources were used for costing: (1) in-patient care: ENCCCitation35 (Étude Nationale des Coûts à méthodologie Commune). (2) expensive in-patient drugs: “liste en sus”Citation36 (3) Transportation: “Rapport cour des comptes 2010”Citation37 (4) out-patient drugs: Vidal websiteCitation38 (5) out-patient medical acts: NGAPCitation39,Citation40 (Nomenclature Générale des Actes Professionnelles) (6) out-patient biology: Biologie médicale nomenclature des actesCitation41,Citation42.
Utility estimates
Utilities associated with the status of NSCLC [PFS, post-progression survival (PPS)] were calculated from the OAK trial, selecting second-line treatment patients, and applying the French utility tariff to patient reported EQ-5D. Disutility associated to AEs came from a literature review. Average of documented utility of documented AE was applied when an AE utility was not reported in the literature.
Base case analysis
lists the main parameters used into the model.
Table 1. Main parameters of the cost-utility model, base case scenario.
Most of these parameters were subject to sensitivity analysis. This base case scenario was fully aligned with the recommendation from HASCitation20.
Sensitivity and probabilistic analyses
Several sensitivity and full stochastic analyses were conducted to estimate the robustness of our findings. This included other utility valueCitation43,Citation44 from the literature with a focus on patients progressing, use of a mixture cure model (less conservative approach needing long term OS data), various discounting rates. In a mixture cure model, a fraction of patients (the “cured fraction”) is expected to become long-term survivors, corresponding to stabilized disease, and not susceptible to die from cancer (but from other causes). Lastly, cost-effectiveness acceptability curves were constructed using a Monte-Carlo simulation.
Results
OS and PFS estimates used into the model are reported in .
Table 2. Overall survival and progression free survival of docetaxel, atezolizumab, and nivolumab.
In comparison to docetaxel, atezolizumab demonstrated a statistically significant difference for OS with an incremental 4.2 months gain for the median OS. The hazard ratio did not reach the statistical significance for PFS.
The comparison versus nivolumab was reported using different meta-analysesCitation21,Citation27. Results were quantitatively consistent for PFS (nivolumab showed PFS extended by about 2 months), but inconsistent for OS: adjustment on treatment switch made OS longer with atezolizumab while longer for nivolumab without adjustment. Moreover, the effect size was modest, less than one month. Also, previous analyses showed a slight difference either in favour (+0.04 QALY)Citation17 or in disfavour (−0.05 QALY)Citation21 of atezolizumab versus nivolumab, depending on the NMA used in these analyses.
Therefore, we decided to run a cost-utility analysis for docetaxel, and a cost minimization for nivolumab considering that superiority of one of the immuno-modulators was not clearly evidenced.
describes the AEs considered for the model.
Table 3. Adverse events included in the cost-effectiveness evaluation.
Docetaxel, as a cytotoxic agent, had a safety profile impacted by severe neutropenia, febrile or not. Anaemia and fatigue were more often reported with docetaxel. These findings support (1) the inclusion of the consequences and costs of AEs in the model, (2) the use of a cost-utility analysis when benchmarking against docetaxel, (3) the use of a cost-minimization when comparing to nivolumab.
Utilities used in the model and their sources are reported in .
Table 4. Utilities related to disease status and disutility related to adverse events.
No major differences in utility were found between patients in progression (0.701) and patients whose disease was controlled (0.704). Disutilities related to AEs ranged from −0.047 (diarrhoea) to −0.200 (neutropenia). Of note, AEs related to cytotoxicity had the most important disutilities.
Cost of chemotherapy courses are reported in .
Table 5. Treatment cost per injection.
Immuno-oncology drug was the most important chemotherapy course cost as shown in . Cost per injection was 3,569.52€ for atezolizumab and ranged from 3,143€ (240 mg every 2 weeks) to 5,649€ (480 mg every 4 weeks) for nivolumab, depending on the regimen.
reports atezolizumab and nivolumab costs over 10 years, based on list price.
Table 6. Cost breakdown for the base case scenario.
Anti-PD(L)-1 drug represented at least two-thirds of total cost. Administration cost was lower with atezolizumab (−4,100€) that was given once every 3 weeks while nivolumab was administered with a biweekly schedule. This resulted in −6,084€ savings when atezolizumab was used instead of nivolumab, representing about 9% of atezolizumab total cost. Drug regimen was also an important cost driver since infusion frequency is directly associated with the quantity of drug injected, in the one hand. However, on the other hand, more frequent injections lead to an increasing use of health care resources.
Docetaxel was the cheapest option, representing 25% of atezolizumab total cost. Atezolizumab reduced dramatically the costs related to AE management and costs after treatment failure.
describes the impact of treatment regimen on the results of the cost-minimization analysis, atezolizumab versus nivolumab.
Table 7. Cost comparison between atezolizumab versus nivolumab according to treatment regimens.
At the current list price, atezolizumab was a cost saving treatment in all cases in comparison to nivolumab.
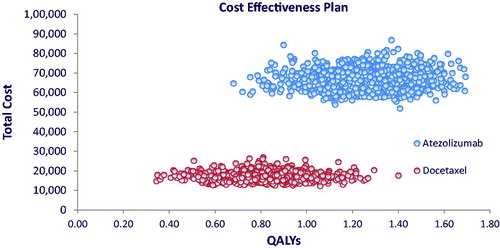
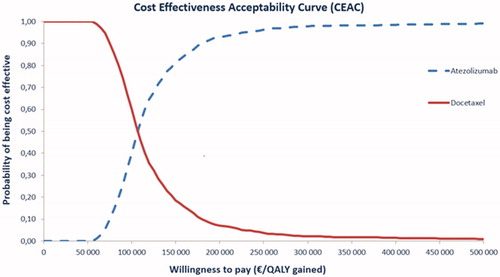
Cost-utility and sensitivity analyses versus docetaxel are reported in , cost effectiveness plan in , and ICER acceptability curves in .
Table 8. Base case cost-effectiveness analysis and sensitivity analyses. Atezolizumab versus docetaxel.
Over 10 years, discounted at a 4% rate both on benefits and costs, atezolizumab costed 49,429€ more than docetaxel, increased life expectancy by 8 months, generating 0.47 QALYs. ICER was estimated at 104,835€/QALY.
The cost-effectiveness plan does not show a strong association between utility and costs, for both treatments. While no overlapping between the two strategies appears on the cost dimension, this is less the case on the utility dimension, although the drugs clearly differentiated in terms of effectiveness.
According to the cost effectiveness acceptability curves, willingness to pay is about 150,000€/QALY to guarantee 80% to make the right decision based on efficiency.
Several sensitivity analyses were conducted on the most sensitive parameters of our model. ICER varied between −15 and 22% from the central scenario and never exceeded 121 K€/QALY. Most sensitive parameters were the use of the cure model, a shorter time horizon and sources of utilities. The results of our sensitivity analyses supported the robustness of our findings.
Discussion
Cost effectiveness analysis is one of the key drivers when allocating resources to fund innovation. Immunotherapy appears to be promising in terms of effectiveness in the treatment of NSCLC but also appears to payers and citizens as expensive alternatives to current standard of care. Both nivolumab and atezolizumab demonstrated clear evidence of an increase of life expectancy over current standard of care, docetaxel, with an improved safety profile.
Our model included the major dimensions of the respective benefit risk ratio of each compound, followed the recommendation of the HASCitation20, and estimated that an incremental of about 100 K€ should be paid to have one incremental QALY, for a switch from docetaxel to atezolizumab. This ratio is in line with that reported in Canada, a country where price is regulated by the state, and lower than in the USA, a health care private insurance country. It is also close to the value reported in France with other immuno-oncologic drugs in the same indication, i.e. pembrolizumabCitation24 and nivolumabCitation25.
Several fractional polynomial meta-analyses were conducted comparing atezolizumab with nivolumab to benchmark their respective effectivenessCitation18,Citation22,Citation28. Evidences varied according to the type of analyses, never reached a meaningful difference, and were always associated with a minor effect size (always less than 1 month). Safety profile based on their respective phase III programs did not show major differences between the two drugs, not likely to lead to either a loss of benefit or an increasing cost. Therefore, cost minimization was appropriate to make decision in such a situation.
In our base case scenario, we compared nivolumab and atezolizumab based on their current drug regimen as fixed by the EMA, and their list prices at the time of analysis: nivolumab is given every 2 weeks and is more expensive per injection than atezolizumab which is administered every 3 weeks. We found that atezolizumab saved approximately 6,000€ over a period of 10 years, representing about 10% of atezolizumab total cost. Most of the savings occurred during the treatment period (during the first year of the second-line treatment) resulting from a lower list price and a less frequent dose regimen. Of note, a comparison with nivolumab every 4 weeks still favoured atezolizumab every 3 weeks with a saving of −1,159€, assuming that this nivolumab schedule is not associated with a loss of benefit.
We performed several sensitivity and scenario analyses that confirmed the robustness of our findings. ICER was impacted by less than 25%, positively or negatively. Acceptability curves asymptotically reached 100% at a credible willingness to pay which confirms the validity of the choice of a cost-utility analysis. Payer accepting a risk of 20% would have to accept an ICER of 147 K€/QALY. Responder rate of atezolizumab at 2 years was 30.9% which is a notable improvement in terms of survival in comparison to chemotherapy. Confirmation of a longer survival among this subset of patients will be needed as being a major driver of cost-effectiveness.
Our model suffers from some limitations. We fixed parity between nivolumab and atezolizumab treatment duration, in the absence of real-world information. Number of injections was a key driver of total costs and prescription data collected through electronic medical records need to be analyzed to confirm or not this hypothesis.
We used list prices for PD1 drugs since discounts fixed at the level of CEPS or during hospital negotiation are not made public in France
Although patients from OAK trials were very similar to French NSCLC patients seen in routine clinical practice, the most severe patients were not included. This raises the issue of immunotherapy efficacy in some sub-groups of patients that were not eligible in OAK trial. Observational surveys and real-life studies will be helpful for confirming the results in terms of efficacy and tolerance of atezolizumab in a broader French population.
Lastly, we do not have any available head-to-head randomized clinical trial comparing atezolizumab and nivolumab, explaining that we had to develop network meta-analyses to build an indirect comparison. We recognize the limitations of this approach although the developed technology was in line with the recommendations of the HASCitation48 and reviewed by SEESPCitation21.
EQ-5D data from OAK patients did not report significant differences between patients under control or progressing. Although values were not likely to be affected by a floor effect (value close to 0.7, i.e. very far from 0), patients included in our trial were selected according to their ECOG status (0 or 1) and likely did not experience a drop in their utility function whatever the drug they received… Lastly, sensitivity analyses were conducted using the utility estimates reported by ChevalierCitation43 and Chouaid et al.Citation44 with a contained impact on the ICER.
French authorities have never fixed a willingness to pay threshold to define what is an acceptable ICER. While other European countries as the United Kingdom or Netherland applied a cost effectiveness threshold as guide to decision making, French authorities assessment of cost-effectiveness analysis is mainly methodological and has to comply with CEESP guidelines. Once validated by the CEESP, economic evaluations may be used as a basis for price negotiation. To date, ICER reported in previous evaluation validated by French authorities for immunotherapies in second line of NSCLC was all between 105,000Citation25 and 145,000Citation26 €/QALY. Hence, these results confirm that our analysis is consistent with previous analysis validated by the CEESP.
Lastly, PD1/PDL1 blocking antibodies are gaining market access authorization to first line treatment in NSCLC with important changes in the therapeutic guidelinesCitation9. New health economic evaluations will be required to account for these changes and therefore, ensure access to the patient of efficient therapies.
Conclusion
This analysis indicated that, following the recommendations of HASCitation20, atezolizumab is more efficient and more costly than docetaxel in the treatment of second line of NSCLC of stage IIIB or IV, in France, with results consistent to previous CEESP evaluation of immunotherapies in similar indication. Based on list price, aligned with the current MAA regimen, atezolizumab was less expensive than nivolumab, with most of savings observed in the 2 years after initiating immunotherapy treatment. These results were robust according to the sensitivity analyses but will need confirmation when real world data will become available.
Transparency
Declaration of funding
This manuscript received funding from Hoffmann-La Roche Limited.
Declaration of financial/other relationships
PN, FF, NP, and MB were Roche/Hoffman la Roche employees. SM and RS received an unrestricted grant to develop the model and generate the results. MP did expertise for Roche.
The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed receipt of honoraria from Roche (Hellas) SA (2018), Bristol-Myers Squibb (Hellas) SA (2017, 2018, 2019), and Merck Sharp & Dohme (MSD) Greece SA (2018, 2019). The reviewers have no other relevant financial relationships or otherwise to disclose.
Acknowledgements
The authors thank Gilles BERDEAUX, MD, BERDEAUX Consulting SAS, F-78240 Chambourcy, France, for the medical writing of this manuscript.
References
- HAS/Commission de la transparence. Avis OPDIVO – 3 février 2016. 2016. Available from: https://www.has-sante.fr/portail/upload/docs/evamed/CT-14655_OPDIVO_PIC_INS_Avis3_CT14655.pdf
- Bertuccio P, Alicandro G, Malvezzi M, et al. Cancer mortality in Europe in 2015 and an overview of trends since 1990. Ann Oncol. 2019;30:1356–1369.
- HAS. Pembrolizumab. Commission de la transparence. Avis 20 Février 2019. Available from: https://www.has-sante.fr/upload/docs/evamed/CT-17280_KEYTRUDA_PIC_EI_poumon_avec_pemetrexed_Avis3_CT17280.pdf
- INCa. Les cancers en France. Edition 2015. Avril; 2016.
- HAS; INCa. Guide du parcours de soins. Cancers broncho-pulmonaires. Juillet; 2013.
- Debieuvre D, Locher C, Neidhart AC, et al. [Ten-year evolution in non-small-cell lung cancer according to sex. Results of the KBP-2010-CPHG Study by the College of General Hospital Respiratory Physicians]. Rev Mal Respir. 2014;31:805–816. French.
- HAS/Commission de la transparence. Avis OPDIVO - 11 janvier 2017. 2017. Available from: https://www.has-sante.fr/upload/docs/evamed/CT-15261_OPDIVO_non_epidermoide_PIC_INS_Avis3_CT15261.pdf
- HAS. Guide méthodologique. Choix méthodologiques pour l’évaluation économique à la HAS. Available from: https://www.has-sante.fr/portail/upload/docs/application/pdf/2011-11/guide_methodo_vf.pdf
- Chouaïd C, Debieuvre D, Durand-Zaleski I, et al. Survival inequalities in patients with lung cancer in France: A nationwide cohort study (the TERRITOIRE Study). PLoS One. 2017;12:e0182798.
- Cancer bronchique non à petites cellules. Référentiels Auvergne Rhône-Alpes en oncologie thoracique®. Mise a jour 2019. Available from: C:/Users/user/AppData/Local/Microsoft/Windows/INetCache/Content.Outlook/R519313P/AURA%20CBNPC_2019_VDEF.pdf
- EMA. SmPC Nivolumab. Available from: https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf
- EMA. SmPC Atezolizumab. Available from: https://www.ema.europa.eu/en/documents/product-information/tecentriq-epar-product-information_en.pdf
- EMA. SmPC pembrolizumab. Available from: https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf
- Rittmeyer A, Barlesi F, Waterkamp D; OAK Study Group, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265.
- Fehrenbacher L, von Pawel J, Park K, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab vs docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:1156–1170.
- Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296:716–721.
- Aguiar PN Jr, Perry LA, Penny-Dimri J, et al. The effect of PD-L1 testing on the cost-effectiveness and economic impact of immune checkpoint inhibitors for the second-line treatment of NSCLC. Ann Oncol. 2017;28:2256–2263.
- Ondhia U, Conter HJ, Owen S, et al. Cost-effectiveness of second-line atezolizumab in Canada for advanced non-small cell lung cancer (NSCLC). J Med Econ. 2019;5:1–13.
- HAS. Définition de l’impact significatif sur les dépenses de l’assurance maladie déclenchant l’évaluation médico-économique des produits revendiquant une ASMR ou une ASA de niveaux I, II ou II. Available from: https://www.has-sante.fr/portail/upload/docs/application/pdf/2019-02/definition_de_limpact_significatif_sur_les_depenses_de_lassurance_maladie_declenchant_levaluation_medico-economique_des_prod.pdf
- Accord cadre du 31/12/2015 entre le Comité économique des produits de santé et les entreprises du médicament (Leem). Available from: E:/Gilles%20Pro/HEVA/accord_cadre_version_definitive_20151231.pdf.
- Commission de la transparence. Atezolizumab. Avis 30 mai 2018. Available from: https://www.has-sante.fr/upload/docs/evamed/CT-16457_TECENTRIQ_CBNPC_PIC_INS_Avis2_CT16457.pdf.
- HAS. Avis d’efficience. Tecentriq® (Atezolizumab). Roche. Available from: https://www.has-sante.fr/portail/upload/docs/application/pdf/2018-12/tecentriq_13122018_avis_efficience.pdf
- Ahn MJ, Tsai CM, Hsia TC, et al. Cost-effectiveness of bevacizumab-based therapy versus cisplatin plus pemetrexed for the first-line treatment of advanced non-squamous NSCLC in Korea and Taiwan. Asia Pac J Clin Oncol. 2011;7 :22–33.
- Carlson JJ, Garrison LP, Ramsey SD, et al. The potential clinical and economic outcomes of pharmacogenomic approaches to EGFR-tyrosine kinase inhibitor therapy in non-small-cell lung cancer. Value Health. 2009;12:20–27.
- HAS. Avis d’efficience. KEYTRUDA® (Pembrolizumab). Cancer bronchique non à petites cellules Epidermoïde (2ème ligne). Available from: https://www.has-sante.fr/upload/docs/application/pdf/2018-11/keytruda_cbnpc_ltt2_17012017_avis_efficience.pdf
- HAS. Avis d’efficience. Opdivo® (nivolumab) Cancer bronchique non à petites cellules Epidermoïde (2ème ligne). Available from: https://www.has-sante.fr/upload/docs/application/pdf/2017-12/opdivo_08122015_avis_efficience.pdf
- Commission de la transparence. Avis Erlotinib. 19 Mars 2018. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2008-10/compte-rendu_ct_19-03-08.pdf
- Schulz C, Gandara D, Berardo CG, Rosenthal R, et al. Comparative efficacy of second- and subsequent-line treatments for metastatic NSCLC: a fractional polynomials network meta-analysis of cancer immunotherapies. Clin Lung Cancer. 2019;20:451–460.e5.
- Latimer N. Nice. DSU technical support document 14. Survival analysis for economic evaluations alongside clinical trials – Extrapolation with patient level data. Decision support unit. 2011 [last updated March 2013]. Sheffield (UK): School of Health and Related Research, University of Sheffield.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–135.
- Données INCa : cancer métastatique stade IV, classe d’âge 55 65 ans : INCa. Survie des personnes atteintes de cancer en France métropolitaine 1989-2013 / Partie 1 - Tumeurs solides [Internet]. 2016. Février. (Etat des lieux et des connaissances/Epidémiologie). Disponible sur: http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Survie-des-personnes-atteintes-de-cancer-en-France-metropolitaine-1989-2013-Partie-1-Tumeurs-solides
- Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. PharmacoEconomics. 2007;25:3–6.
- Institut national de la statistique est des études économiques. Taux d’inflation en 2018 Données annuelles de 1991 à 2018. Available from: https://www.insee.fr/fr/statistiques/2122401
- Agence technique de l’information sur l’hospitalisation. Guide de l’étude nationale des coûts à méthodologie commune. Available from: https://www.atih.sante.fr/sites/default/files/public/content/3075/guide_enc_v2017_vdef.pdf
- Ministère des solidarités et de la santé. Référentiel des indications des spécialités pharmaceutiques inscrites sur la liste en sus. Available from: https://solidarites-sante.gouv.fr/soins-et-maladies/autres-produits-de-sante/dispositifs-medicaux/la-liste-en-sus/article/referentiel-des-indications-des-specialites-pharmaceutiques-inscrites-sur-la
- Rapport cour des comptes. Chapitre XI. Les transports de patients à la charge de l’assurance maladie. Cour des comptes. Sécurité sociale. Paris (France); 2012.
- Vidal web site. Available from: https://www.vidal.fr/
- Ameli.fr. Honoraires des professionnels de santé APE par région. Année 2014. [Internet]. [cité 31 août 2016]. Disponible sur: http://www.ameli.fr/l-assurance-maladie/statistiques-et-publications/donnees-statistiques/professionnels-de-sante-liberaux/honoraires/honoraires-totaux-et-moyens.php
- Ameli.fr. Activité des médecins APE par région. Année 2014. [Internet]. [cité 31 août 2016]. Disponible sur: http://www.ameli.fr/l-assurance-maladie/statistiques-et-publications/donnees-statistiques/professionnels-de-sante-liberaux/activite-et-prescriptions/activite-des-medecins.php
- CNAMTS. Table Nationale de codage de Biologie [Internet]. 2016. [cité 2013 14 Nov]. Disponible sur: http://www.codage.ext.cnamts.fr/codif/nabm/index.php?p_site=AMELI
- CNAMTS. Nomenclature des Actes de Biologie Médicale (NABM) [Internet]. Available from: http://www.codage.ext.cnamts.fr/f_mediam/fo/nabm/DOC.pdf
- Chevalier J, Lay KL, Pouvourville GD. Health state utility values in advanced non-small cell lung cancer patients. Value Health. 2013;16:A419.
- Chouaid C, Agulnik J, Goker E, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8:997–1003.
- Edwards S, Barthon S, Nherera L. Pixantrone monotherapy for the treatment of relapsed or refractory aggressive non-hodgkin(s) lymphoma. BMJ Technology Assesment Group; 2013 (Report No.: 10/59).
- Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84.
- Beusterien KM, Davies J, Leach M, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. 2010;8:50.
- Haute Aurorité de Santé. Indirect comparisons Methods and validity summary report. 2009 July. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2011-02/summary_report__indirect_comparisons_methods_and_validity_january_2011_2.pdf