?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim: This study aimed to quantify the healthcare burden of clinically significant tricuspid regurgitation (TR) in patients with and without heart failure (HF).
Materials and Methods: Data were from the IBM MarketScan Research Databases from October 2011 to September 2016. Eligible patients met the following inclusion criteria: age ≥18 with a TR diagnosis, 12 months pre (baseline), and 6 months post (landmark) medical enrollment. The landmark period was used to categorize TR severity, defined as a record of pulmonary hypertension with ascites, lower extremity edema or hepatic insufficiency, or tricuspid valve surgery. Cohorts were defined based on TR etiology and severity: (1) no HF and no clinically significant TR; (2) HF with no clinically significant TR; (3) no HF with clinically significant TR; and (4) HF with clinically significant TR. Outcomes of interest were all-cause hospitalizations, hospital days, and expenditures. Multivariable models were fit for each of the annualized outcomes and adjusted for patient demographics, comorbidities, and other concomitant valve diseases.
Results: There were 92,994 patients eligible for analysis. Patients with no HF and no clinically significant TR had the annualized healthcare burden of 0.20 all-cause hospitalizations (approximately one inpatient hospitalization every 5 years), 1.07 hospital days, and $17,478 in expenditures. The presence of clinically significant TR, alone or with HF, significantly increased healthcare utilization and expenditures. For patients with no HF with clinically significant TR, the annualized economic burden increased to 0.41 all-cause hospitalizations, 3.13 hospital days, and $29,985 in expenditures. For patients with HF and clinically significant TR, the annualized economic burden was even greater with 0.59 all-cause hospitalizations, 4.31 hospital days, and $42,255 in expenditures.
Conclusion: The presence of clinically significant TR is associated with an increase in healthcare utilization and expenditures, irrespective of the presence of HF.
Introduction
The epidemiology of tricuspid valve disease (TVD) regurgitation is not well described. Incidence and prevalence estimates of the population affected by tricuspid regurgitation (TR) are not known. One commonly cited figure from a 2006 study estimated that 1.6 million people in the United States have moderate to severe TR.Citation1 Given the aging population and the numbers of individuals with conditions associated with TR including heart failure (HF), pulmonary hypertension, lung disease, and liver disease, it is possible that the prevalence of TR is much larger than estimated. There is also a large gap in evidence regarding the treatment of TR. However, in 2014, the American College of Cardiology and American Heart Association Guidelines included a Class I recommendation of tricuspid valve surgery for patients with severe functional TR undergoing surgery for left-sided valve disease.Citation2
Due to the increasing attention on what was previously referred to as the “forgotten valve,” there is a small but growing body of literature on outcomes following tricuspid valve surgery, be it repair or replacement.Citation3 However, there is little published research on the healthcare burden of TR, especially within the larger context of HF. The objective of this study is to understand and quantify the healthcare burden (hospitalizations, hospital days, and expenditures) of TR on patients and the healthcare system. Due to the physiological interaction of HF on TR, the cohorts were defined by both TR severity and HF diagnosis.
Methods
Data from the IBM MarketScani Research Databases from 1 October 2011 through 30 September 2016 were used in this analysis. These data are fully integrated, de-identified, individual-level healthcare claims. They include complete payment records (insurance payments and patient payments), specialty pharmacy, and mail-order records for individuals covered by a variety of health plans located throughout the United States. Data from individual patients are integrated from all provisions of care, maintaining all healthcare utilization and cost record connections at the patient level. This database is inclusive of 150 employers and 21 health plans, representing 130 unique national carriers.Citation4
All data used to perform this analysis were de-identified and accessed in compliance with the Health Insurance Portability and Accountability Act. As a retrospective analysis of a de-identified database, the research was exempt from IRB review under 45 CFR 46.101(b)(4).
Inclusion criteria
Eligible patients included adults greater than 18 years of age with a diagnosis of TVD (Supplementary Appendix A), 12 months of medical enrollment before their first TVD diagnosis, and 6 months of enrollment post-index. The 6 months post-index period was considered a landmark period for the purpose of categorizing TVD severity. To ensure the focus was TR and not all TVD, patients were excluded from this study if they had a record of tricuspid stenosis, rheumatic tricuspid insufficiency, rheumatic mitral insufficiency, primary organic disease (endocarditis, congenital Ebstein’s anomaly, tricuspid atresia, carcinoid valve disease, or cleft valve), cardiac tumors, double orifice tricuspid valve, iatrogenic (right ventricular biopsy induced) valve disease, or valve prolapse anytime in the database (Supplementary Appendix B).
Cohort definitions
Patients with TR meeting the initial criteria above were further sub-divided by a record of HF diagnosis (Supplementary Appendix C). The TR cohort was further divided into three mutually exclusive, collectively exhaustive groups: (1) no presence of an HF diagnosis anytime in the database, (2) a minimum of one inpatient or two outpatient diagnoses of HF either during the 12-month baseline or 6-month landmark periods, or (3) only one outpatient diagnosis of HF during the 12-month baseline or 6-month landmark periods. The third group was considered to be inconclusive for a true HF diagnosis and thus subsequently dropped from the analysis. The two remaining groups, (1) no record of HF anytime in the database and (2) those with a minimum of one inpatient or two outpatient records of HF in the baseline or landmark period, were then further classified by the severity of TR. Patients were considered to have severe or clinically significant TR if during the baseline or landmark period they had a record of pulmonary hypertension with ascites, edema, or hepatic insufficiency. Additionally, patients who underwent tricuspid valve surgery during the landmark period were also considered to have clinically significant TR and included within this cohort (Supplementary Appendix D).
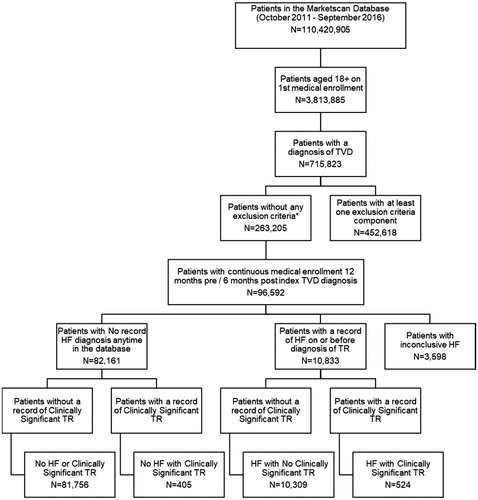
Therefore, the final cohorts were labeled based on TR etiology and severity as follows: (1) No HF or Clinically Significant TR––These patients had no record of HF anytime in the database and their TR was not known to be clinically significant; (2) HF with No Clinically Significant TR––These patients had a minimum of one inpatient or two outpatient visits with a diagnosis of HF on or before their diagnosis of TR, however, their TR was not known to be clinically significant; (3) No HF with Clinically Significant TR––These patients had clinically significant TR but no record of an HF diagnosis anytime in the database; and (4) HF with Clinically Significant TR––These patients had a minimum of one inpatient or two outpatient visits with a diagnosis of HF on or before their diagnosis of TR and their TR was considered clinically significant. See for the complete attrition diagram and sample size of each cohort.
Figure 1. Attrition diagram. Abbreviations. HF, heart failure; TR, tricuspid regurgitation; TVD, tricuspid valve disease. *A patient had a record of tricuspid stenosis, rheumatic tricuspid insufficiency, rheumatic mitral insufficiency, primary organic disease (endocarditis, congenital Epstein anomaly, valve hypoplasia, valve cleft), cardiac tumors, double orifice tricuspid valve, iatrogenic (right ventricular biopsy), or valve prolapse anytime in the database.
Statistical analysis
All 4 cohorts were described by patient characteristics (). Descriptive analytics were presented as mean and standard deviation for continuous variables or count and percentage for categorical variables. The Elixhauser Comorbidity Index (ECI) was used to quantify patient baseline comorbidities.Citation5 The ECI is a validated set of 31 categories of comorbidities that are associated with mortality. These comorbidities were identified using diagnosis codes that appeared in the 12-month period before a patient’s index diagnosis of TR. The composite ECI score is reported in . A table with counts and percentages for all 31 comorbidities that make up the ECI, as well as their complete coding detail, can be found in Supplementary Appendix E.
Table 1. Patient characteristics by cohorts.
Three main outcomes of interest were used to quantify the economic burden of patients with TR: all-cause hospitalizations, hospital days, and total expenditures. The outcome period started the day after the 6-month landmark and ended up to 12 months afterward, with no additional continuous enrollment criteria applied. Since patients had differing lengths of follow up, each outcome variable was annualized, resulting in an annual estimated rate per patient.
Multivariable models were fit for each of the annualized outcome variables of interest (all-cause hospitalizations, hospital days, and total expenditures) using ordinary least-squares (OLS) regression. The two independent variables of interest were HF and clinically significant TR (ClinSigTR); with patient demographics (age, sex, region, and insurance type), the composite ECI score (to represent the patient’s comorbidities), and an indicator for other valve disorders (aortic and mitral) as covariates in each model (OVD):
Interactions were tested between the main independent variables (HF and ClinSigTR). By using OLS regression, after the models were fit, the coefficients for HF and ClinSigTR were used to estimate the incremental effect for each outcome variable (all-cause hospitalizations, hospital days, and expenditures) while holding all covariates constant. This allowed for individual estimates for each cohort.
Parameter estimates and p-values were calculated as measures of association strength and significance, respectively, for each of the models. Testing for outliers and distribution assumptions were performed. All statistical analyses in this study were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
displays the attrition diagram and cohort counts for this study. Between 1 October 2011 and 30 September 2016, of the 110,420,905 patients in the MarketScani Databases, 715,823 patients 18 years of age or older had a diagnosis of TVD. The requirements outlined in the methods section regarding confounding cardiomyopathies and enrollment were used to further refine the TVD patients, resulting in 96,592 TR patients.
Patients who met inclusion and enrollment criteria were separated into cohorts by TR severity and HF diagnosis. Eighty-five percent (n = 82,161) had no record of a diagnosis of HF anytime in the database, and of those, 81,756 (99.5%) were classified as not having clinically significant TR. This group of patients made up the No HF or Clinically Significant TR cohort. Patients with no HF and meeting the requirements of clinically significant TR (n = 405) made up the No HF with Clinically Significant TR cohort.
While 85% (82,161/96,592) of our sample did not have a record of HF anytime in the database, 10,833 (11%) did meet the HF criteria on or before their TR diagnosis. Ninety-five percent (n = 10,309) of these patients had no record of clinically significant TR, and these patients were classified as the HF with No Clinically Significant TR cohort. The remaining 524 patients who met the criteria for HF on or before their TR diagnosis and had clinically significant TR were assigned to the HF with Clinically Significant TR cohort.
The remaining 3,598 (4%) of the 96,592 TR patients were dropped from the analysis because they had one outpatient visit for HF in the baseline or landmark period which did not qualify them for either of the two definitions for HF: (1) no diagnosis anytime in the database or (2) a minimum of one inpatient or two outpatient visits in the baseline or landmark period.
Patient demographics (age, sex, region, and insurance type), valvular severity, and ECI are reported for the four cohorts in . Not unexpectedly, the No HF or Clinically Significant TR cohort was considerably younger and healthier than the other three cohorts. The mean (SD) age of the No HF or Clinically Significant TR cohort was 57.6 (15.5) years compared to the other three cohorts: HF with No Clinically Significant TR 71.1 (14.5); No HF with Clinically Significant TR 64.6 (15.1) and HF with Clinically Significant TR 71.6 (13.8). A similar pattern was seen in the ECI Score. The mean (SD) ECI of the No HF or Clinically Significant TR cohort was 3.6 (1.9) compared to the other three cohorts: HF with No Clinically Significant TR 6.9 (2.6); No HF with Clinically Significant TR 6.0 (2.4) and HF with Clinically Significant TR 8.6 (2.8).
reports the OLS regression results for each of the annualized outcome variables of interest (total expenditure, all-cause hospitalization, and hospital days). In all three models, there were no statistically significant interactions between clinically significant TR and HF. The presence of other valvular diseases (aortic and mitral) was not statistically significant and, therefore, was not included in the final model results. The two main independent variables, HF and clinically significant TR, were statistically significant across all three outcomes of interest. A diagnosis of HF was associated with an increase of $12,270 (p<.0001) in annualized expenditures, of 0.18 (p<.0001) in annualized rate of hospitalizations, and of 1.18 (p<.0001) in annualized hospital days. Patients with clinically significant TR had an increase of $12,507 (p = .0197) in annualized expenditures, of 0.2 (p<.0001) in annualized rate of hospitalizations and of 2.1 (p<.0001) in annualized hospital days.
Table 2. Regression results.*
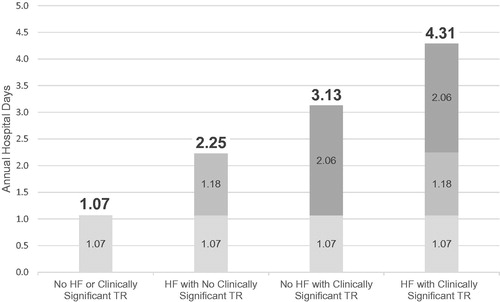
show the independent association of each cohort with the outcomes of interest. The figures display each cohort in the following order: (1) No HF or Clinically Significant TR (2) HF with No Clinically Significant TR, (3) No HF with Clinically Significant TR, and (4) HF with Clinically Significant TR. The No HF or Clinically Significant TR in each figure can be interpreted as the starting point or baseline and is calculated by taking the average for each outcome of interest (total expenditures, hospital days, and annualized all-cause hospitalizations) for patients with no record of HF and TR that did not meet the criteria of clinically significant. This represents the baseline utilization and expenditures in this analysis. For example, in , the estimated annual hospital days for the patients with No record of HF or Clinically Significant TR is 1.1 days. When patients have HF without clinically significant TR, their annualized hospital days increase by 1.2 days, thus estimating their overall annual burden at 2.3 days (1.1 starting point + incremental increase of 1.2 days). Patients with no HF with Clinically Significant TR and HF with Clinically Significant TR have even higher annualized hospital days with incremental increases from the starting point and total burden of 2.1 (total 3.1) and 3.2 (total 4.3), respectively.
Figure 4. Estimated annual rate of all-cause hospitalizations. Note: All estimates are based on multivariable models adjusting for patient demographics, comorbidities, and other valve disease.
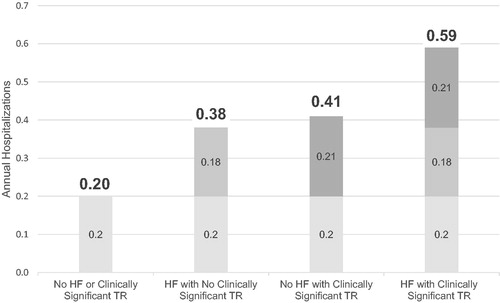
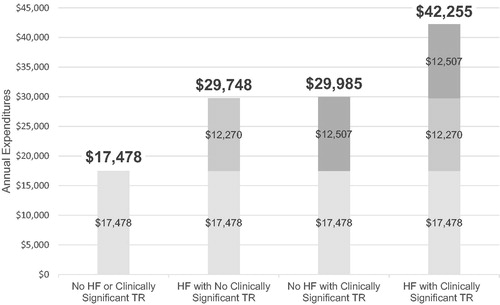
In , the baseline annualized expenditures are $17,478. The same pattern of annualized expenditures was evident in annualized hospital days, whereby estimated annualized expenditures increased with each cohort from HF with No Clinically Significant TR ($29,748) to No HF with Clinically Significant TR ($29,985) to HF with Clinically Significant TR ($42,255). The same pattern was consistently observed for the annualized rate of hospitalizations () whereby No HF or Clinically Significant TR is 0.2, followed by HF with No Clinically Significant TR 0.4, No HF with Clinically Significant TR 0.4 and HF with Clinically Significant TR 0.6.
Discussion
In this retrospective, real-world analysis of patients with TVD, as coded through automated sources of data, patients were divided into cohorts based on TR severity and HF diagnosis. Most TVD patients in this claims database (85%) did not have clinically significant TR and had not been diagnosed with HF. This large cohort (n = 81,756) was used as a baseline to illustrate the incremental burden of clinically significant TR with and without HF on estimated annual hospital days, annual expenditures, and annual all-cause hospitalizations. These important estimates of burden were based on multivariable models, which included patient demographics, comorbidities, and other valve diseases.
TR is often a marker of advanced cardiac pathology and is associated with a worse prognosis after cardiac surgery. However, medical therapy for the management of TR is often limited to diuretics and is commonly ineffective.Citation6 Severe TR is associated with worse survival, independent of left ventricular ejection fraction and pulmonary hypertension.Citation7 Patients with severe symptomatic TR have been shown to have prolonged hospitalizations and higher rates of re-hospitalization.Citation8
The etiologic spectrum of TR and the commonly associated right ventricular dysfunction is complex. The tricuspid valve is a component of the right ventricular structure and can be adversely affected by chronic left-sided valvular heart disease or advanced diastolic dysfunction. Post-capillary pulmonary hypertension (or pulmonary venous hypertension) plays an important role in the development of right ventricular dysfunction and associated TR, where the venous contribution to pulmonary vascular resistance may be as high as 40%.Citation9
TR is associated with an increased healthcare burden, and currently, a very low percentage of patients affected with this condition are receiving treatment. It is estimated that only 5% of patients with significant TR undergo surgical intervention.Citation10 Emerging therapies, particularly minimally invasive percutaneous therapies, are being evaluated to assess their impact on patient-reported outcomes, hospitalizations, and survival.
Our analysis demonstrates that clinically significant TR is associated with an incremental healthcare burden commensurate to the presence of HF. This is illustrated in , whereby the annualized incremental healthcare burden is 2.1 versus 1.2 hospital days, $12,507 versus $12,270 in total expenditures, and 0.21 versus 0.18 hospitalizations for clinically significant TR and HF groups, respectively. For patients with HF and clinically significant TR, the combined incremental annualized healthcare burden is 3.2 hospital days, $24,777 in expenditures, and 0.4 hospitalizations. These patients had a minimum of one inpatient or two outpatient visits with a diagnosis of HF and clinically significant TR (as determined by pulmonary hypertension, ascites, edema, hepatic insufficiency, or a record of TV surgery).
Our data build upon the implications of prior analyses suggesting that the tricuspid valve may be amenable to surgery or percutaneous techniques that could lead to overall improvements in clinical outcomes such as HF hospitalization or cardiovascular death.Citation11 It is possible that correction of TR could result in improved right heart function and reduced hepatic congestion, edema, and improved renal function in such a meaningful way that the causal pathway for hospitalization in such patients is fundamentally changed.Citation12 As the case-mortality rate of TV surgery continues to improve, the opportunity for this new therapeutic target grows.Citation11,Citation13 Only future prospective, comparative, and hopefully, randomized trials of tricuspid valve intervention—aimed to reduce tricuspid insufficiency, reduce the right-ventricular volume overload, and relieve systemic venous congestion—hold the prospect of defining the independent contribution of TR to the natural history of HF.Citation13
Limitations
These results should be interpreted with caution for several reasons. We recognize that TVD was ascertained through sources of automated data that rely on coding. This could have been biased in terms of over- or under-coding. For example, since information on New York Heart Association (NYHA) functional classification was not available in the claims data, we relied on stringent coding detail whereby a patient had to have a minimum of one inpatient, or two outpatient claims for HF to be classified as having the disease. This strict requirement may have biased our sample to patients with more severe HF and worse functional status by NYHA. We could only control for known confounders largely of generalized illness and lacked the ability to control for unknown confounders ideally by randomization, stratification, matching, etc. Statistical modeling was used to control for the potential confounding effect of known variables with between-group differences. While statistical models controlled for several factors, models could not control for some variables which are not included in an administrative database, including, for instance, echocardiography results. We recognize that we relied upon proxies in automated data that suggested significant TR without having the quantitation of TR from imaging data. The use of proxies could have significantly reduced the proportion of patients with severe TR, and our data analysis should not be interpreted as a prevalence study. A strength of the present study, however, is that the data reflect real-world patient characteristics and outcomes across the country from different hospitals and physicians as compared to evidence from controlled clinical trials.
Conclusion
The presence of clinically significant TR, based on a retrospective claims database, significantly increased healthcare utilization and expenditures. For patients with HF and clinically significant TR, the overall annualized healthcare burden equated to a rate of all-cause hospitalizations of 0.6 and 4.3 hospital days per year with annualized expenditures totaling $42,255. This represents a significant annual incremental increase from patients without clinically significant TR and no record of HF, who had an overall annualized healthcare burden of 0.2 hospitalizations, 1.1 hospital days, and $17,478 in healthcare expenditures. Clinically significant TR and HF individually carried similar healthcare burdens. Much work remains to identify the optimal subsets for benefit and groups to avoid in terms of risk of harm in the future of TVD intervention. Our data suggest that, if such patients could be selected, meaningful changes in the economic burden and healthcare utilization of patients with TR could be realized with specific interventions designed for the tricuspid valve.
Transparency
Declaration of Funding
This study was sponsored by Edwards Lifesciences.
Declaration of financial/other relationships
DPC, PAM, HSM, and CMB, have consulting relationships with Edwards Lifesciences. CMB is an advisory board member for Medtronic and Boston Scientific. DPC has a consulting relationship with Abbott Laboratories and participates in a speaker’s bureau for Boston Scientific. HSM has a consulting relationship with Abbott Laboratories, Boston Scientific and participates in a speaker’s bureau for Actelion Pharmaceuticals, Bayer Healthcare Pharmaceuticals and Bristol-Myers Squibb Company. MPR and ERB are employees of and CG is a consultant to CTI Clinical Trial & Consulting Services, which is a consultant to Edwards Lifesciences. JV, SM and PV are employees of Edwards Lifesciences, the study sponsor. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous Presentation
This study was presented as a poster at TCT in San Francisco AC, September 25–29, 2019.
Author contributions
All authors contributed to the concept, study design and interpretation of the data, Ryan MP and Gunnarsson C contributed to the data analysis. Initial manuscript draft was developed by Ryan MP, Gunnarsson C, and Baker ER, which was substantially improved by all authors. We confirm that the manuscript has been read and approved by all authors.
The external authors and study sponsors participated in the study design, data analysis, data interpretation, and development of the report, and gave approval to submit for publication.
Supplemental Material
Download PDF (292.1 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
Data Sharing Statement
The data that support the findings of this study are available from IBM® MarketScan® Research Databases, but restrictions apply to the availability of these data and were used under license for the current study; therefore, they are not publicly available.
Note
Notes
i IBM Watson Health, Armonk, New York.
References
- Stuge O, Liddicoat J. Emerging opportunities for cardiac surgeons within structural heart disease. J Thorac Cardiovasc Surg. 2006;132(6):1258–1261.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2014;148(1):e1–e132.
- Braunwald NS, Ross J Jr, Morrow AG. Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation. 1967;35(4 Suppl):I63–69.
- Danielson E. Health research data for the real world: the MarketScan® Databases. 2014.
- Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- Beckhoff F, Alushi B, Jung C, et al. Tricuspid regurgitation––medical management and evolving interventional concepts. Front Cardiovasc Med. 2018;5:49.
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43(3):405–409.
- Lee JW, Song JM, Park JP, et al. Long-term prognosis of isolated significant tricuspid regurgitation. Circ J. 2010;74(2):375–380.
- Hahn RT, Waxman AB, Denti P, et al. Anatomic relationship of the complex tricuspid valve, right ventricle, and pulmonary vasculature: a review. JAMA Cardiol. 2019;4(5):478–487.
- Agarwal S, Tuzcu EM, Rodriguez ER, et al. Interventional cardiology perspective of functional tricuspid regurgitation. Circ Cardiovasc Interv. 2009;2(6):565–573.
- Veen KM, Etnel JRG, Quanjel TJM, et al. Outcomes after surgery for functional tricuspid regurgitation: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes. 2020;6(1):10–18.
- Maslow A, Abisse S, Parikh L, et al. Echocardiographic predictors of tricuspid ring annuloplasty repair failure for functional tricuspid regurgitation. J Cardiothorac Vasc Anesth. 2019;33(10):2624–2633.
- Pingpoh C, Nuss S, Kueri S, et al. Adding tricuspid repair to standard open heart surgery does not increase risk but improves right ventricular function. Interact Cardiovasc Thorac Surg. 2019;29(3):416–421.