Abstract
Aim: Hemophilia A is a genetic, chronic disorder classified by deficient or defective coagulation factor VIII (FVIII) that puts those affected at risk for spontaneous bleeding episodes, which lead to joint damage and chronic pain over time. Currently, most severe hemophilia A patients are treated with prophylactic FVIII, which requires costly and frequent infusions and life-long adherence to medication. A gene therapy (valoctocogene roxaparvovec) is currently in development for the treatment of severe hemophilia A. This model assessed the potential cost-effectiveness of treating patients with valoctocogene roxaparvovec rather than prophylactic therapy.
Materials and methods: We developed an individual-based, state-transition microsimulation model for assessing the likely cost-effectiveness of valoctocogene roxaparvovec compared to prophylactic FVIII. Men aged 30 with severe hemophilia A were modeled over a lifetime horizon, and costs were reported from the perspective of the United States health care system. Through microsimulation, patient-level heterogeneity was captured in starting weight, starting Pettersson score (PS), durability of valoctocogene roxaparvovec, and annual bleed rate (ABR).
Results: The model projects that treatment with single-administration valoctocogene roxaparvovec would be cost-saving to people with hemophilia A at a price point comparable to other currently available gene therapy products due to its potential to reduce FVIII utilization, direct medical costs, lifetime bleeds, and accumulated joint damage.
Limitations: The model relies upon evidence-based assumptions for clinical inputs due to limited data availability. Such uncertainty was mitigated by modeling heterogeneity across the population, specifically with regards to long-term gene therapy durability, lifetime bleed rates, and joint damage progression.
Conclusion: Valoctocogene roxaparvovec was found to be cost-saving—on average by about $6.8 million per patient—and more effective than prophylactic therapy for treatment of hemophilia A. The comparative benefit of gene therapy was observed across a broad range of simulated patients that were representative of the real-world severe hemophilia A population.
Introduction
Hemophilia A is a rare, X-linked hereditary bleeding disorder marked by a deficiency in coagulation factor VIII (FVIII) that is estimated to affect approximately 18,000 predominantly male individuals in the USCitation1,Citation2. FVIII deficiency causes a predisposition to uncontrolled spontaneous and traumatic bleeding. In high-income countries, 46% of males with hemophilia A are reported to have a severe phenotype of the disease (FVIII <1 IU/dL), which carries a stronger propensity than the mild and moderate forms for debilitating complications including hemophilic arthropathy, joint destruction, and brain bleedsCitation3–5.
The clinical burden of hemophilia A is well established. In the short term, even with treatment, hemophilia A may cause joint pain and excessive bleeding from injuries and surgeriesCitation4. Chronic bleeds over time induce inflammation and joint degeneration, leading to chronic pain, limitations in mobility, and joint replacement surgeryCitation6. Consequently, patients with hemophilia A typically endure significant comorbidities, increased mortality, poorer health-related quality of life (HRQoL), and greater work-related impairment than the general populationCitation7,Citation8.
In the US, the current standard of care is prophylactically administered recombinant FVIII proteinCitation1,Citation9. Compared to on-demand FVIII replacement, prophylactic administration has been associated with improved HRQoL in some patients; however, its clinical and economic burden of treatment is substantialCitation10. Prophylactic treatments can require regular intravenous infusions, up to 2–3 times per week, and can cost hundreds of thousands of dollars per yearCitation1,Citation9,Citation11. Adherence to prophylactic therapy regimens can be challenging due to the frequency of infusions: one analysis of pooled trial data estimated that approximately 25% of patients do not follow frequency guidelinesCitation10. Divergence from recommended dosing schedules has been associated with a greater frequency of spontaneous bleeds and comorbidities, additional medical costs, and increased work or school day absencesCitation10,Citation12,Citation13.
The life-long management of hemophilia A places significant economic burden on patients, their families, and the health care system. In a recent analysis, the projected annual drug cost of prophylactic treatment in the US ranged from nearly $700,000 to $750,000 for standard and extended half-life FVIII products, respectivelyCitation9. Separate studies have shown patients with hemophilia A incur higher costs due to greater rates of hospitalization, ER visits, office visits, medical procedures, and laboratory tests compared with the general populationCitation7,Citation14,Citation15. Greater indirect costs for these patients are driven by absenteeism and productivity lossCitation7,Citation15.
Given the burden imposed by FVIII prophylactic dosing requirements and the associated costs, as well as the negative impact on patient HRQoL, alternative therapies are under development. In 2017, the US Food and Drug Administration approved emicizumab-kxwh (HEMLIBRA; Genentech, Inc., South San Francisco, CA), a bispecific factor IXa- and factor-X-directed antibody, for prophylactic use in hemophilia A patients with inhibitors and, in 2018, its indication was expanded to include those without inhibitorsCitation16. Emicizumab-kxwh greatly lessens treatment burden by reducing administrative frequency to as little as once every four weeks in order to maintain FVIII equivalency levels above 1 IU/dL, but still requires that patients adhere to a lifetime of treatment, including FVIII for treatment of breakthrough bleedsCitation16. Another emerging area of development is single-administration gene therapy, which has the potential to eliminate the need for frequent, repeated prophylactic infusions. Valoctocogene roxaparvovec is a hemophilia A gene therapy currently in clinical development by BioMarin Pharmaceutical Inc. (Novato, CA). This investigational product combines a viral capsid with a FVIII gene to create a therapeutic vector that can deliver FVIII DNA to patient cells, where it can be expressed as FVIII protein to significantly decrease bleeding rate and utilization of exogenous FVIII replacement therapiesCitation17. At the time of this publication, pivotal phase 3 trials were underway with a primary completion date expected in December 2022Citation18.
Economic value in combination with clinical effectiveness is becoming increasingly important to health care decision-makersCitation19. Cost-effectiveness analyses can provide insightful projections that leverage clinical data to inform the reimbursement decisions that ultimately affect patient access to careCitation19. Here, we present a microsimulation model that assesses the potential cost-effectiveness of gene therapy as compared with prophylactic FVIII replacement therapy.
Methods
Decision model
We developed an individual-based, state-transition microsimulation model for assessing the likely cost-effectiveness of valoctocogene roxaparvovec compared to FVIII prophylaxis (referred to as the comparator). To reflect the valoctocogene roxaparvovec trial population, we simulate a hypothetical cohort of males who are 30 years of age (phase 2 clinical trial median age), have a severe phenotype of hemophilia A, previously received prophylactic FVIII therapy, and have no history of inhibitors to FVIII proteinCitation20. The following outcomes are considered: total costs (incurred by treatment with FVIII, bleed management, and other medical costs), total number of FVIII infusions, total number of bleeding events (joint and non-joint), life-years gained, and quality-adjusted life-years (QALYs) gained. The analysis simulates 10,000 individual patient experiences using R version 3.3.3 based on a template for microsimulation modeling provided by Krijkamp et al. Citation21. This generates a distribution with a mean and variance: the mean is interpreted as the average effect for a single patient.
Model structure and overall assumptions
General structure
The model structure consists of four primary patient health states: “no bleed”, “joint bleed”, “non-joint bleed”, and “dead” (). Joints are defined as shoulders, elbows, wrists, hips, knees, and ankles. Non-joints are defined as any other body compartment. All patients begin in the “no bleed” state. In every weekly cycle, every individual has a probability of remaining in their current health state or moving to one of the other three. Based on the frequency of joint bleeds, individuals move irreversibly through progressive stages of arthropathy. Accordingly, state-specific utilities and the potential for surgical costs are modified and are tied to a corresponding increase in Pettersson score (PS). The PS ranges from 0 (indicating that no joints have signs of arthropathy) to 78 (indicating severe arthropathy in all affected joints)Citation22. In addition to arthropathy progression, patients who receive valoctocogene roxaparvovec transition to the comparator arm upon loss of response to gene therapy, described in more detail below. Following standard US modeling practices, future costs and health utilities were discounted at 3% annually and individuals were tracked until their health state reached “dead” according to disease- or age-specific causes of mortalityCitation23. Further details on model inputs can be found in .
Figure 1. Model structure. Within a range of Pettersson scores, individual patients remain in a health state (curved arrows) or move from one health state to another (straight arrows). The microsimulation models patients through weekly Markov cycles, and the patients' health state within a single cycle is a function of their assigned joint bleed rate, non-joint bleed rate, and age-adjusted mortality. An accumulation of joint bleeds progress patients to higher Pettersson scores and reduced utility for each health state. Patients in the gene therapy arm whose modeled endogenous FVIII levels drop below the response threshold are assumed to receive prophylactic FVIII for the remaining model horizon until death.
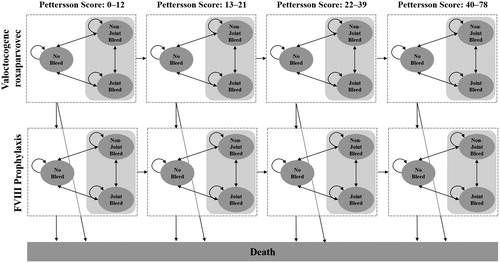
Table 1. Key model structure estimates and ranges for initial patient characteristics.
Table 2. Key bleed event estimates and ranges for valoctocogene roxaparvovec population.
Table 3. Key bleed event estimates and ranges for prophylaxis population.
Table 4. Key cost estimates.
Valoctocogene roxaparvovec
The model assumes that all patients receive prophylactic exogenous FVIII protein for the first four weeks following gene therapy administration. As assumed in a prior cost-effectiveness analysis of a hypothetical gene therapy for hemophilia A, after four weeks, 10% of individuals were assumed to have FVIII levels that did not prevent all spontaneous bleeds and subsequently resumed prophylactic therapyCitation25, while FVIII levels for 90% of individuals in the valoctocogene roxaparvovec were assumed to be the mean level from the publicly available trial data up to Year 3 (N = 7)Citation26. After Year 3, patients followed an individual-specific rate of annual decline in endogenous FVIII levels, drawn randomly from a log-normal distribution with a mean linear decline in FVIII levels of 3.7 IU/dL per year (standard deviation: 2) until they lost response to the therapy or diedCitation26. Loss or absence of response was defined as having an endogenous level of FVIII protein activity below 5 IU/dL and patients were assumed to resume exogenous FVIII prophylaxis at this point.
FVIII prophylaxis
Individuals in the comparator arm due to initial assignment or reassignment from the treatment arm received three weight-based doses of standard half-life exogenous FVIII protein during each weekly cycle for the remainder of their lives.
Clinical inputs
Patient-level bleed propensity
For the valoctocogene roxaparvovec arm, we simulated a patient-specific, time-invariant annual bleed rate (ABR) to reflect the mean ABRs and proportion of patients that are bleed-free reported at Year 3 of the clinical trialCitation20. Of the individuals who were administered valoctocogene roxaparvovec, 20% were assumed to experience bleeds even while responding to valoctocogene roxaparvovec and were assigned ABRs drawn from a normal distribution (mean 5, SD 1) and 80% were assumed to experience zero bleed events while responding to valoctocogene roxaparvovec.
For the prophylaxis arm, bleed propensity was modeled to represent the diversity of experiences of patients receiving prophylactic treatment. Patients were assigned to one of three bleed propensity categories: (1) patients experiencing bleeds with very low frequency; (2) patients experiencing bleeds with moderate frequency; and (3) patients experiencing bleeds with high frequency. Based on published data, 40% of patients were assigned to category 1, 33% of patients were assigned to category 2, and 27% of patients were assigned to category 3Citation27. Individuals within each category were then randomly assigned a bleed-likelihood parameter that indicated the likelihood that the patient experiences a higher bleed rate in a given week. Patients in category 1 experienced the equivalent of 0–1 ABR, while patients in category 2 experienced the equivalent of 1.7–5 ABR. Patients in category 3 experienced approximately 6–22 ABR. The overall prophylaxis population was assumed to have a mean ABR of 5 and a distribution which mirrors the distribution seen in real-world bleed rates for patients receiving prophylactic FVIIICitation27.
Bleed events
All ABRs were converted to weekly probabilities. Weekly bleed rate for individuals in the valoctocogene roxaparvovec arm was dependent on their modeled endogenous level of FVIII. Although a correlation between FVIII level and bleed risk has not been established in severe hemophilia A patients who have sustained factor levels above 1 IU/dL, a modest relationship was assumed in order to determine transition probabilities for bleed states (joint and non-joint). Here, 15 IU/dL was selected as a conservative threshold level, above which patients were assumed to experience 0 joint bleeds to align with observational data from prophylactic patients who showed a marked decline in joint bleeds above a factor level of 10 IU/dLCitation28,Citation29. For patients with a factor level greater than 15 IU/dL, all bleeds simulated in the model were assumed to be non-joint bleeds and thus the patient’s simulated ABR reflects only non-joint bleeds. When individuals have FVIII levels less than or equal to 15 IU/dL, their simulated ABR is assumed to reflect both joint and non-joint bleeds.
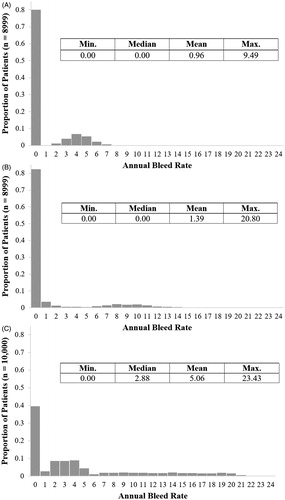
The distributions of patient-level ABRs for each model arm are presented in . The model assumes there is no correlation between a patient’s ABR when receiving valoctocogene roxaparvovec and when they are receiving FVIII prophylaxis.
Figure 2. Distribution of annual bleed rate. (A) Patient-level ABRs for the valoctocogene roxaparvovec arm during the time when patients are responding to treatment and have FVIII levels greater than 15 IU/dL; (B) Patient-level ABRs for the valoctocogene roxaparvovec arm during the time when patients are responding to treatment and have FVIII levels greater than 5 IU/dL and less than or equal to 15 IU/dL; (C) Patient-level ABRs for the prophylaxis arm during the entire model horizon.
Mortality
Given the lack of any measured differential impact on mortality in the trial, the model assumes identical mortality rates in both arms. To reflect the model’s lifetime horizon, this probability of death was determined by converting age-specific annual life tables for the male US population to weekly probabilitiesCitation30. These probabilities were adjusted to reflect the increased mortality rates exhibited by individuals with moderate to severe hemophilia A based on published standardized mortality ratiosCitation31.
Arthropathy
Each individual enters the model with a starting level of arthropathy drawn from an empirically derived distribution of simulated PSCitation32. An individual’s level of arthropathy progressed unidirectionally through the model by assuming that experiencing 12.6 cumulative joint bleeds was associated with a one-point increase in the PSCitation33. Furthermore, if an individual reached a PS of 28, they were assumed to have joint damage sufficient to require joint replacement surgery if they are below the age of 80 yearsCitation34,Citation35. Individuals who underwent surgery were assumed to require follow-up surgery every 20 years for as long as they remained below the age of 80Citation36.
Weight
Age range-specific patient body weights were based on Centers for Disease Control and Prevention data and are incorporated to calculate the appropriate dosage for both treatment armsCitation37. Individuals enter the model with a starting weight and weight percentile drawn from a log-normal distribution fitted to data for ages 30–39 years. As individuals progress through the model, they are assumed to remain within the same age-specific weight percentile for their age (i.e. a patient in the 90th percentile of the age 30–39 distribution at age 35 will be in the 90th percentile of the age 60–69 distribution at age 65).
Costs
Perspective
Costs are expressed in real 2019 USD based on a US health system perspective. Thus, historical event or unit costs (i.e. not provided in 2019 USD) were inflated using either the Personal Health Care Expenditure indexCitation38 or the Personal Consumption Expenditure indexCitation39 as recommended by the Second PanelCitation23.
Treatment, bleed management, and surgery costs
The price of valoctocogene roxaparvovec has not yet been established, so the model assumes a price comparable to currently available gene therapies at $2 M for a patient of average weight. This amount was converted to a one-time, per-kilogram (kg) price of $22,173. Additional costs associated with valoctocogene roxaparvovec therapy are based on the phase 1/2 clinical trialCitation24. These costs include outpatient facility utilization costs incurred during the one-time infusion, a tapered regimen of corticosteroids (prednisolone or prednisone), and an average adeno-associated virus serotype 5 (AAV5) diagnostic test cost, which was adjusted to account for individuals who may have incurred test costs but were not eligible for gene therapy. The model does not assume there is a FVIII level at which disease management would need to be intensified for patients who received valoctocogene roxaparvovec. Furthermore, the clinical management between treatment arms was not considered to be meaningfully different and therefore was not included in the model. We assume that individuals in the comparator arm use three weekly doses of exogenous FVIII protein at $65.33 per kg per dose (based on a median prescribed dose of 40 IU/kg cost of standard half-life FVIII prophylaxis, with a median wholesale acquisition cost [WAC] of $1.63 per IU)Citation9. Both joint and non-joint bleeds were assumed to be treated on-demand, with two doses of FVIII at the aforementioned cost of standard prophylaxis for a dose of 50 IU/kg of standard FVIII per bleed eventCitation25. Individuals in either treatment arm who underwent surgery incurred a cost of $40,560 for joint replacement surgeryCitation40,Citation41.
Utilities (health-related quality of life)
Utilities for states other than death (when utility equals 0) depend on the individual’s PS (Supplemental Table 1). HRQoL were assumed to be identical for the valoctocogene roxaparvovec arm and prophylactic FVIII arm. Bleed state utilities were constructed by taking a weighted average of two days of bleed state utility and five days of the average between the relevant bleed state and “no bleed” utilities. For individuals with a PS less than or equal to 12, the “no bleed” state was assigned a daily utility of 0.83Citation42, non-joint bleeds were assigned a daily utility of 0.66Citation43, and joint bleeds were assigned a daily utility of 0.54, reflecting a utility decrement relative to non-joint bleeds of 0.12Citation44. For individuals with a PS higher than 12, patients were assigned “no bleed” and bleed utilities based on three score categories: individuals with a PS greater than 12 and less than or equal to 21; individuals with PS greater than 21 and less than or equal to 39; and individuals with a PS greater than 39. These score categories have been shown to be empirically associated with progressively lower utilityCitation42. An additional utility decrement of 0.0004 per FVIII infusion was also applied in both valoctocogene roxaparvovec and prophylaxis comparator armsCitation45. Individuals who experience joint replacement surgery were subjected to a weekly utility decrement of 0.39 for four consecutive weeksCitation46.
Scenario analyses
A set of four scenario analyses were compared to the base case. As the simulation model captures much of the potential variation in the inputs and the resulting outputs, additional probabilistic sensitivity analyses, which are often performed for Markov cohort models, would not contribute meaningful insights. The four scenarios were: (1) limiting the model horizon to 10 years—to roughly coincide with some estimates of gene therapy’s potential; (2) increasing the utility for valoctocogene roxaparvovec across all health states to reflect the additional HRQoL benefits beyond what was captured in the health states; and incorporating (3) emicizumab-kxwh and (4) extended half-life prophylaxis (EHL) as comparator treatment arms.
Results
Base case
For the 10,000 simulated individuals who were administered valoctocogene roxaparvovec, of which 10% were assumed to have FVIII levels that did not prevent all spontaneous bleeds and who, subsequently, returned to prophylactic FVIII therapy, the average treatment durability was 11.13 years, with a median durability of 10.73 years (range 0.04–50.42 years). Among patients assumed to have FVIII levels shortly after treatment that prevented all spontaneous bleeds and survived the initial three years following treatment, average treatment durability was 12.43 years, with a median durability of 11.33 years (range 3.08–50.42 years). While patients experienced an average decline rate of 3.7 IU/dL per year after Year 3 based on clinical trial data, the modeled decline rate varied across patients with a minimum decline rate of 0.35 IU/dL per year (sustained response) and a maximum decline rate of 20.25 IU/dL per year (rapid loss of response).
The simulated population of 10,000 individuals who were administered valoctocogene roxaparvovec had an average ABR of 3.95 across all years modeled, with a median of 2.33 bleeds per year (range 0–22 bleeds per year); this includes bleeds that occurred after individuals lose response to treatment and transition to standard FVIII prophylaxis. The simulated population of 10,000 individuals who received prophylactic FVIII experienced an average ABR of 5.06, with a median of 2.88 bleeds per year (range 0–23.4 bleeds per year). On average, patients who received valoctocogene roxaparvovec had an 8.85 point change in PS, with a median of 3.65 point change in PS (range 0–69.4 point change in PS) compared with a 11.44 point change in PS, with a median of 5.24 point change in PS (range 0–72.36 point change in PS) among patients who received prophylactic FVIII. The model shows that both patients treated with valoctocogene roxaparvovec and those treated with prophylaxis experience on average six fewer QALYs than life-years (LYs) as a consequence of hemophilia A, equating to a 26% reduction in healthy life-years.
Table 5. Base case results.a
On average, patients treated with valoctocogene roxaparvovec incurred 43 fewer total bleed events over a lifetime, with a median of 10 fewer bleed events (range −754 to 243 total bleed events; ) compared with individuals on FVIII prophylaxis, of which an average of 33 were joint bleeds, with a median of nine fewer joint bleed events (range −61 to 585 fewer joint bleed events). Patients in the valoctocogene roxaparvovec arm also experienced 1,808 fewer average FVIII infusions over a lifetime, with a median of 1,741 fewer infusions (range −2 to 8,693 fewer infusions) compared with individuals on FVIII prophylaxis, who received on average 7,379 infusions over a lifetime.
Over a lifetime time horizon, the base case resulted in an average per-patient total cost of $16.7 M (range $823 K–$73.9 M) among those who were administered valoctocogene roxaparvovec and 18.07 QALYs gained (range 0.06–23.38 QALYs gained). The patients on prophylactic exogenous FVIII protein incurred an average per-patient total cost of $23.5 M (range $75 K–$69.6 M) and 17.32 QALYs gained (range 0.05–22.54 QALYs gained). Overall, treating patients with valoctocogene roxaparvovec is associated with an average of $6.8 M reduction in costs per patient (range −$4.3 M to $36.7 M reduction in costs) and an average of 0.75 increase in QALYs gained (range −0.75 to 4.13 QALYs gained) compared with standard FVIII prophylaxis. Patients treated with valoctocogene roxaparvovec experience on average a nine-month gain in healthy life years compared with patients treated with prophylaxis. As a result, the model identifies valoctocogene roxaparvovec as a “dominant” choice, being less costly and more effective on average than the comparator arm and current standard of care.
Scenario analyses
The model was used to simulate four alternative scenarios: their results are summarized in (Supplemental Tables 2-5). First, when the model time horizon is adjusted from lifetime to 10 years, patients treated with valoctocogene roxaparvovec can expect an average per-patient total cost of $3.5 M and 6.88 QALYs. The patients receiving prophylactic FVIII can expect an average per-patient total cost of $8.5 M and with 6.37 QALYs. Thus, the projected net result over a 10-year time horizon is that treatment with valoctocogene roxaparvovec would reduce costs by $5 M along with 0.5 QALYs gained compared with standard FVIII prophylaxis. Second, the scenario assuming increased utility (by 0.02) for valoctocogene roxaparvovec across all health states results in patients treated with valoctocogene roxaparvovec expecting 18.34 QALYs over a lifetime compared with 18.07 QALYs for this arm in the base case: this is greater than for patients treated with prophylactic FVIII, who can expect 17.32 QALYs as in the base case. Overall, the increase in utility for valoctocogene roxaparvovec patients results in a $6.8 M reduction in costs and a 1.02 gain in QALYs compared with standard prophylaxis. Third, in the scenario with emicizumab-kxwh as the comparator, patients were assumed to switch to emicizumab-kxwh once they met the threshold for loss of response to gene therapy. Valoctocogene roxaparvovec was found to be dominant compared with emicizumab-kxwh, resulting in a $4.8 M reduction in costs on average over a lifetime, and overall QALYs were similar between both arms—18.8 vs. 18.5 for valoctocogene roxaparvovec and emicizumab-kxwh, respectively, resulting in 0.3 QALYs gained, on average. Fourth, in the scenario where the comparator arm was assumed to receive prophylactic EHL instead of standard half-life FVIII, patients were assumed to switch to EHL once they met the threshold for loss of response to gene therapy. Again, valoctocogene roxaparvovec was found to be dominant compared with EHL, resulting in a $7.3 M reduction in costs on average over a lifetime. Projected overall QALYs were higher for valoctocogene roxaparvovec 18.36 vs. 17.79 for EHL, resulting in 0.57 QALYs gained, on average. Thus, in all four scenarios, treatment with valoctocogene roxaparvovec dominates the comparator—reducing total lifetime costs and improving quality-adjusted life expectancy.
Table 6. Summary of scenario analyses results.a
Discussion
Valoctocogene roxaparvovec is projected to be a dominant treatment choice—being both cost-saving and producing superior patient health outcomes—as an alternative to prophylactic exogenous FVIII protein utilization in men who are 30 years of age, with severe hemophilia A and no history of FVIII inhibitors. The durability of the response to valoctocogene roxaparvovec is a key driver of cost-effectiveness in our model, and under current durability assumptions the majority of clinical and health benefits occur in the first 10 years following administration. Patient HRQoL is expected to improve due to fewer infusions, bleeds, and surgeries.
The cost-effectiveness of gene therapy relative to prophylaxis in patients with severe hemophilia A has been explored in only one study to dateCitation25. While the analysis presented here is similar to this recently published study with regard to the patient population and overall conclusions, the cohort approach used in that model provided only limited opportunities to capture heterogeneity among individuals especially regarding duration of response and variation in cost of prophylactic FVIII. With our microsimulation model, we can consider patient-level metrics such as accumulated joint damage and number of annual and lifetime bleeds whereas a cohort model would be limited to presenting aggregate population output. Additionally, further evidence supporting durable gene expression is now available and is reflected in this model.
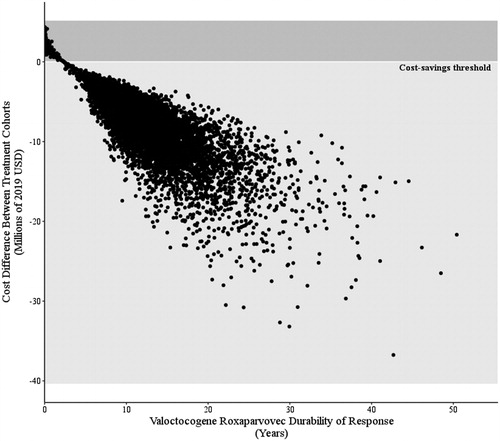
This is the first model in hemophilia A able to demonstrate the benefit that gene therapy may offer across a wide range of hemophilia A patients. Prophylactic treatment costs and bleed management costs vary substantially within the hemophilia A populationCitation47. To best capture this variability, this microsimulation incorporates patient-level heterogeneity through patient body weight, ABR (0 to 16), and valoctocogene roxaparvovec durability (3 to 50 years, average 11 years). Patients who experience the greatest cost-savings and QALY gains are those with higher weight and therefore higher prophylactic costs, longer survival, and higher ABRs while on prophylactic therapy. Still, valoctocogene roxaparvovec was projected to be cost saving for 89% of patients. The relationship between cost difference, QALYs gained, and durability indicate that durability of response plays a significant role in the potential cost-effectiveness of valoctocogene roxaparvovec (). Valoctocogene roxaparvovec is projected to be dominant relative to FVIII prophylaxis for all patients with at least 2.4 years of durable response, and the benefit of gene therapy can be realized relatively soon after administration.
Figure 3. Relationship between cost difference and valoctocogene roxaparvovec durability. Patients who experience longer periods of response show greater cost-savings. However, patients who respond to treatment for at least 2.4 years are projected to experience some cost-saving effects.
Furthermore, the 10-year scenario demonstrates that the bulk of cost offsets ($5.0 M of $6.8 M) occur within 10 years of valoctocogene roxaparvovec administration. When additional HRQoL benefits from gene therapy, such as overall reduced anxiety—from no longer managing a chronic illness with a long period of scheduled infusions and avoiding the uncertainties of potential bleeding events that require on-demand treatment—are incorporated into the model by increasing the utility for valoctocogene roxaparvovec patients across all health states, we see additional gains in QALYs. In a third scenario analysis, when compared to the most recently approved prophylactic therapy (emicizumab-kxwh), valoctocogene roxaparvovec was still found to be cost-saving and more effective despite this therapy requiring fewer injections relative to prophylactic FVIII. Finally, in a scenario where the comparator arm was assumed to receive prophylactic EHL instead of standard half-life FVIII, valoctocogene roxaparvovec was again found to be dominant.
While this model includes additional parameters to model hemophilia A disease progression and management compared with a cohort model, there are inherent limitations. There is uncertainty surrounding the valoctocogene roxaparvovec response, particularly concerning initial non-response, overall durability, and HRQoL. We have incorporated a conservative assumption for initial loss of response to align with previously published economic models and loss of response set to a factor level of 5 IU/dL, though patients may experience factor levels lower than this threshold and still not require prophylactic therapy. However, long-term follow-up results from the phase 3 trial are needed to validate these assumptions, but those data will be unavailable for years. In addition, the model only compares gene therapy with prophylactic FVIII and not with on-demand or episodic FVIII replacement therapy treatment regimens. This framing was based on prophylactic treatment being considered the “goal of therapy” by hemophilia treatment guidelines, and likely serves as a more appropriate comparator than on-demand treatmentCitation1. Related to treatment cost, the model assumes the cost of FVIII therapy remains constant over the time horizon. Research has shown that despite the amount of competition in the FVIII product market, cost of treatment has remained high; therefore, the model assumes a constant price of prophylactic FVIII treatment in future yearsCitation48. Furthermore, the model assumes no correlation between the ABR a patient experiences after treatment with gene therapy and that patient’s ABR after transitioning to FVIII prophylaxis. While this provides a more conservative approach by allowing patients to bleed more when receiving valoctocogene roxaparvovec compared with FVIII prophylaxis, it is not likely to be clinically realistic. The model includes extra costs for an invasive surgery tied to joint damage (measured by Pettersson score), but does not assume a difference in FVIII costs associated with these procedures. Due to the limited amount of information surrounding the difference in exogenous FVIII consumption between individuals on prophylactic therapy and those on gene therapy during invasive procedures, this assumption provides a conservative approximation of costs for patients who had received valoctocogene roxaparvovec. The microsimulation model generated a distribution of results under alternative scenarios; hence, no further probabilistic sensitivity analyses were conducted on the base-case assumptions. However, the patient-level simulations allowed for specific parameters (weight, starting PS, gene therapy durability) to vary as a method of capturing the levels of heterogeneity in the underlying population and offering a proxy for uncertainty around the mean values. While this exercise provides a critical advance in our understanding of therapeutic options and their costs, further research is needed to understand the effect of gene therapy on HRQoL for hemophilia A patients beyond reductions in bleeds and infusions.
Conclusion
Valoctocogene roxaparvovec for treatment of hemophilia A is projected to be cost-saving and producing improved patient HRQoL compared with prophylactic FVIII over a patient’s lifetime. These projections suggest substantial per-patient lifetime cost savings of about $6.8 M. Furthermore, even with FVIII prophylaxis, the clinical burden of hemophilia A is substantial and well established in terms of joint pain and excessive bleeding from injuries, as well as chronic bleeds that result in inflammation, joint degeneration, mobility limitations, and joint replacement surgery. These clinical impacts reduce estimated average patient quality of life on the order of 26%—equivalent to six healthy years of life. This new treatment may reduce this loss by 13%—equivalent to another nine healthy months of life. Furthermore, by using a microsimulation model, the benefit of gene therapy could be assessed across a wide range of patient characteristics, reflecting the real-world experience of patients with hemophilia A. Moreover, these assumptions and model outputs are robust and applicable to a wide range of durability scenarios.
Transparency
Declaration of funding
Analysis Group, Inc. received funding from BioMarin Pharmaceutical Inc. to conduct research.
Declaration of financial interests
KC, KA, JM, and AC are employees of Analysis Group. SF is a previous employee of Analysis Group. Analysis Group received consulting fees from BioMarin Pharmaceutical for the purposes of conducting this research. LG is an independent consultant under a subcontract with Analysis Group for the purposes of this research; he also serves as a health economic advisor on a product development strategic advisory board for BioMarin. JME peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
KC contributed to the design of the study, interpretation of the results, and helped draft and finalize the manuscript. SF assisted in study design, developed the microsimulation model, and contributed to finalizing the manuscript. KA assisted with the study design and drafted and finalized the manuscript. JM developed the microsimulation model and assisted in drafting and finalizing the manuscript. AC was involved in the design of the study, interpretation of results, and finalizing the manuscript. LG contributed to the study and its design, to the interpretation of the results, and to the drafting and finalization of the manuscript. All authors read and gave approval of the final manuscript.
Supplemental Material
Download MS Word (22.5 KB)Acknowledgements
The authors would like to acknowledge Sheridan Rea for her contributions as a research assistant.
References
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–47.
- Centers for Disease Control and Prevention. Data & statistics on hemophilia. [cited 2019 April 6]. Available from: https://www.cdc.gov/ncbddd/hemophilia/data.html.
- World Federation of Hemophilia.World Federation of Hemophilia report on the annual global survey 2017. Montreal, Quebec. [cited 2019 Apr 5]. Available from: http://www1.wfh.org/publications/files/pdf-1714.pdf.
- Centers for Disease Control and Prevention. What is Hemophilia? 2018 [updated 2018 Sep 6; cited 2019 July 23]. Available from: https://www.cdc.gov/ncbddd/hemophilia/facts.html.
- Melchiorre D, Manetti M, Matucci-Cerinic M. Pathophysiology of hemophilic arthropathy. J Clin Med. 2017;6(7):63.
- Knobe K, Berntorp E. Haemophilia and joint disease: pathophysiology, evaluation, and management. J Comorb. 2011;1(1):51–59.
- O’Hara J, Hughes D, Camp C, et al. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis. 2017; 12(1):106.
- Curtis R, Baker J, Riske B, et al. Young adults with hemophilia in the U.S.: demographics, comorbidities, and health status. Am J Hematol. 2015;90(Suppl 2):S11–S6.
- Croteau SE, Cheng D, Cohen AJ, et al. Regional variation and cost implications of prescribed extended half-life factor concentrates among U.S. Haemophilia Treatment Centres for patients with moderate and severe haemophilia. Haemophilia. 2019;25(4):668–675.
- Collins PW, Blanchette VS, Fischer K ,et al. Break-through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J Thromb Haemost. 2009;7(3):413–420.
- Berntorp E, Dolan G, Hay C, et al. European retrospective study of real-life haemophilia treatment. Haemophilia. 2017;23(1):105–114.
- Duncan N, Kronenberger WG, Krishnan S, et al. Adherence to prophylactic treatment in hemophilia A as measured using the Veritas-PRO and annual bleed rate (ABR). Value Health. 2014;17(3):A230.
- Krishnan S, Vietri J, Furlan R, et al. Adherence to prophylaxis is associated with better outcomes in moderate and severe haemophilia: results of a patient survey. Haemophilia. 2015 ;21(1):64–70.
- Shrestha A, Eldar-Lissai A, Hou N, et al. Real-world resource use and costs of haemophilia A-related bleeding. Haemophilia. 2017;23(4):e267–e275.
- Zhou ZY, Koerper MA, Johnson KA, et al. Burden of illness: direct and indirect costs among persons with hemophilia A in the United States. J Med Econ. 2015;18(6):457–465.
- Hemlibra (emicizumab-kxwh) injection. Prescribing information. South San Francisco, CA: Genentech, Inc; 2018.
- BioMarin eyes 2019 FDA filing for haemophilia A gene therapy [Internet]. 2018 [cited 2019 Mar 1]. Available from: http://www.pmlive.com/pharma_news/biomarin_eyes_2019_fda_filing_for_haemophilia_a_gene_therapy_1259266.
- Single-arm study to evaluate the efficacy and safety of valoctocogene roxaparvovec in hemophilia A patients (BMN 270-301). [cited 2019 Nov 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT03370913.2017.
- Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health. 2015;18(2):161–172.
- Pasi KJ, Rangarajan S, Mitchell N, et al. Multiyear Follow-up of AAV5-hFVIII-SQ Gene Therapy for Hemophilia A. N Engl J Med. 2020;382(1):29–40.
- Krijkamp EM, Alarid-Escudero F, Enns EA, et al. Microsimulation modeling for health decision sciences using R: a tutorial. Med Decis Making. 2018;38(3):400–422.
- Pettersson H, Ahlberg Å, Nilsson IM. A radiologic classification of hemophilic arthropathy. Clin Orthop Relat Res. 1980;(149):153–159.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103.
- Rangarajan S, Walsh L, Lester W, et al. AAV5–factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377(26):2519–2530.
- Machin N, Ragni MV, Smith KJ. Gene therapy in hemophilia A: a cost-effectiveness analysis. Blood Adv. 2018;2(14):1792–1798.
- Bienaime JH. Valactogene Roxaparvovec Phase 2 and Phase 3 Update 2019 [updated 2019 May 28; cited 2019 May 28]. Available from: https://investors.biomarin.com/events-presentations?item=58.
- Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without Inhibitors. N Engl J Med. 2018;379(9):811–822.
- den Uijl IE, Mauser Bunschoten EP, Roosendaal G, et al. Clinical severity of haemophilia A: does the classification of the 1950s still stand? Haemophilia. 2011;17(6):849–853.
- den Uijl IE, Fischer K, Van Der Bom JG, et al. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17(1):41–44.
- Arias E, Xu J, Kochanek KD. United States life tables, 2016. Natl Vital Stat Rep. 2019;68(4):1–66.
- Mejia ‐Carvajal C, Czapek E, Valentino L. Life expectancy in hemophilia outcome. J Thromb Haemost. 2006;4(3):507–509.
- Lofqvist T, Nilsson IM, Berntorp E, et al. Haemophilia prophylaxis in young patients–a long-term follow-up. J Intern Med. 1997;241(5):395–400.
- Fischer K, van Hout BA, van der Bom JG, et al. Association between joint bleeds and Pettersson scores in severe haemophilia. Acta Radiol. 2002;43(5):528–532.
- Earnshaw SR, Graham CN, McDade CL, et al. Factor VIII alloantibody inhibitors: cost analysis of immune tolerance induction vs. prophylaxis and on-demand with bypass treatment. Haemophilia. 2015;21(3):310–319.
- Fischer K, Pouw ME, Lewandowski D, et al. A modeling approach to evaluate long-term outcome of prophylactic and on demand treatment strategies for severe hemophilia A. Haematologica. 2011;96(5):738–743.
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544.
- Fryar CD, Gu Q, Ogden CL, et al. Anthropometric reference data for children and adults: United States, 2011–2014. Vital Health Stat. 2016;3:1–46.
- U.S. Department of Health Human Services. Using appropriate price indices for expenditure comparisons. Agency for Healthcare Research and Quality. 2014. [cited 2019 Jul 23]. Available from: https://meps.ahrq.gov/about_meps/Price_Index.shtml.
- U.S. Bureau of Economic Analysis. Personal Consumption Expenditures: Chain-type Price Index [PCEPI]. 2019. [cited 2019 Jul 23]. Available from: https://fred.stlouisfed.org/series/PCEPI.
- America’s Health Insurance Plans (AHIP). National Comparisons of Commercial and Medicare Fee-For-Service Payments to Hospitals. Data Brief. 2016.
- Rosas S, Buller LT, Plate J, et al. Total knee arthroplasty among medicare beneficiaries with hemophilia A and B is associated with increased complications and higher Costs. J Knee Surg. 2019.
- Fischer K, de Kleijn P, Negrier C, et al. The association of haemophilic arthropathy with health-related quality of life: a post hoc analysis. Haemophilia. 2016;22(6):833–840.
- Neufeld EJ, Sidonio RF, Jr., O’Day K, et al. Cost analysis of plasma-derived factor VIII/von Willebrand factor versus recombinant factor VIII for treatment of previously untreated patients with severe hemophilia A in the United States. J Med Econ. 2018;21(8):762–769.
- Mazza G, O’Hara J, Carroll L, et al. The impact of haemophilia complications on health-related quality of life for adults with severe haemophilia. Value Health. 2016;19:A347–A766.
- Matza LS, Sapra SJ, Dillon JF, et al. Health state utilities associated with attributes of treatments for hepatitis C. Eur J Health Econ. 2015;16(9):1005–1018.
- Ballal RD, Botteman MF, Foley I, et al. Economic evaluation of major knee surgery with recombinant activated factor VII in hemophilia patients with high titer inhibitors and advanced knee arthropathy: exploratory results via literature-based modeling. Curr Med Res Opin. 2008;24(3):753–768.
- Thorat T, Neumann PJ, Chambers JD. Hemophilia burden of disease: a systematic review of the cost-utility literature for hemophilia. J Manag Care Spec Pharm. 2018;24(7):632–642.
- Gold J. Miracle of hemophilia drugs comes at a steep price. 2018 [cited 2020 Jan 9]. Available from: https://www.npr.org/sections/health-shots/2018/03/05/589469361/miracle-of-hemophilia-drugs-comes-at-a-steep-price.
- Stephensen D, Tait RC, Brodie N, et al. Changing patterns of bleeding in patients with severe haemophilia A. Haemophilia. 2009;15(6):1210–1214.
- Eloctate (Antihemophilic factor (recombinant), FC fusion protein). Prescribing information. Cambridge, MA: Sanofi Genzyme; 2017.
- Centers for Medicare & Medicaid Services. Medicare physician fee schedule. 2019 [cited 2019 Jul]. Available from: https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx.
- IBM Micromedex®. Red Book® Online 2019. 2019. Available from: http://www.micromedexsolutions.com/micromedex2/librarian/
- Centers for Medicare & Medicaid Services. Clinical laboratory fee schedule. 2019 [cited 2019 Jul]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/index.html.
