Abstract
Background: Injectable botulinum neurotoxins are a mainstay of treatment for pediatric spasticity. AbobotulinumtoxinA and onabotulinumtoxinA are both injectable toxin therapies used to treat pediatric lower limb (PLL) spasticity in Canada. The objective of this study was to assess the cost-effectiveness of abobotulinumtoxinA vs. onabotulinumtoxinA in the treatment of PLL spasticity in Canada.
Methods: A probabilistic Markov cohort model with a 2-year time horizon was developed, with health states defined by response to therapy, as characterized by the goal attainment scale (GAS). Based on randomized controlled trial evidence, response to therapy was similar or higher for abobotulinumtoxinA relative to onabotulinumtoxinA; uncertainty was incorporated into model parameters, however, as the two therapies have not been compared head-to-head. Canadian resource use and cost data were incorporated.
Results: In the base case, abobotulinumtoxinA generated 1.48 quality-adjusted life years over the model time horizon, compared to 1.47 for onabotulinumtoxinA. AbobotulinumtoxinA was associated with cost savings of $123 CAD, reflecting lower costs in both medication acquisition and health services. The estimated improvement to quality of life and reduced costs result in an estimate of economic dominance for abobotulinumtoxinA over onabotulinumtoxinA. This dominant result persisted across probabilistic and scenario analyses.
Based on a review of available clinical evidence, abobotulinumtoxinA was found to have significant and/or numerical efficacy benefits to onabotulinumtoxinA on functional outcomes (Goal Attainment Scale) and tone (Modified Ashworth Scale) and in the treatment of pediatric lower limb spasticity
In this cost-effectiveness analysis, abobotulinumtoxinA was found to be associated with greater quality-adjusted life years and lower costs than onabotulinumtoxinA (economically dominant)
A limitation of this analysis was the uncertainty around key parameters. Specifically, the lack of head-to-head comparison data for the two therapies, and variable data regarding likely onabotulinumtoxinA dosing in PLL in clinical practice. However, across a range of plausible scenarios, the economic dominant result remained.
Key points for decision makers
Introduction
Cerebral palsy (CP) affects more than two children per 1,000 live birthsCitation1. Spastic CP occurs in 70–80% of CP patientsCitation2 and is characterized by a loss of muscle control leading to increased muscle tone and joint stiffness, compromising mobility.
Reduced functional capacity and resulting inactivity associated with lower limb spasticity can lead to clinical consequences such as osteoporosis, bladder and bowel problems, hip dislocation, and fixed contracturesCitation3. Spasticity is also associated with pain, discomfort, psychological disturbance, and social isolation, all of which affect patients’ quality of life (QoL)Citation4. CP also affects patients’ families, in particular, the main caregiver’s QoLCitation5. Families face challenges on a daily basis, and outcome is determined to a large extent by how well families manage to function and copeCitation5. Medical costs in children with CP have been reported as 10 times higher than non-CP childrenCitation6. Due to the impact this condition has on patients, their families, and the healthcare system, pediatrics with lower limb (PLL) spasticity is an important population from a research and treatment perspective.
Treatment for CP is multi-modal and involves regular physiotherapy, pharmacological interventions including botulinum neurotoxin type A (BoNT-A) therapy, and orthopedic and other surgeries. If not managed, spasticity may cause pain, result in complications that require surgery, and reduce QoLCitation7. Therefore, an individualized, goal focused management program should be developed in collaboration with patients and their families/caregiversCitation7,Citation8. Treatment goals in CP and spasticity management typically focus on functional improvement, rather than clinical manifestations of spasticity only. Setting short-(each injection cycle) and long-term goals is essential in the management, treatment and assessment of patients with spasticity in order to achieve functional improvement milestonesCitation9. With treatments such as BoNT-A, assessment of whether treatment goals have been achieved includes assessing both spasticity reduction as well as improvement in individualized goals that were set prior to treatmentCitation10. When assessing the effectiveness of treatments for spasticity, tone reduction by itself cannot be used to define effectiveness unless it is used in conjunction with an optimal clinical treatment outcome such as improvement in a specific functionCitation9. Spasticity is complex and high muscle tone can improve or impede function, therefore, assessment of general functional outcomes is more important when making decisions about treatments such as BoNT-A, than assessment of individual muscles by using measures such as the Ashworth ScaleCitation9.
The Modified Ashworth Scale (MAS) is a commonly used measure for assessing spasticityCitation11, but has limited validityCitation9,Citation10 and does not evaluate overall functionality. Spasticity is defined as “a velocity-dependent increase in muscle tone”Citation12, but because a single velocity is used to perform MAS, it cannot truly describe, measure, distinguish or rate spasticity and its use is no longer recommended to describe spasticityCitation10. The goal attainment scale (GAS) is a measure of patients’ success at meeting functional goals related to desired improvements in daily life that have been set along with their treatment teamCitation13, and is characterized as having a greater relevance to QoL in pediatric patients with lower limb spasticity, as reported by cliniciansCitation14. GAS is a valid, reliable and responsive tool that can be used in clinical practice and in research to (a) assess whether the intervention has achieved pre-set individualized goals, (b) recognize changes needed for clinical outcomes to be meaningful to patients and their families, and (c) helps patients and families to assess progress, make decisions and stay engaged in care and rehabilitationCitation10. The importance of goal-setting in assessing patient outcomes in pediatric patients with spasticity has been highlighted in European and United Kingdom disease management guidelinesCitation9,Citation10. Injectable BoNTs are widely administered in the Canadian setting to treat CP due to localized efficacy, favorable adverse events profile, and reversibility of treatment effectsCitation15. BoNT-A is approved for the treatment of spasticity in children in Canada; different products may differ in bioequivalence, efficacy and safetyCitation16. In December 2017, Health Canada approved abobotulinumtoxinA (Dysport, Ipsen Pharmaceuticals Paris, France.) for treatment of PLL spasticity in patients aged two and older. Prior to this, the only available BoNT in this patient population was onabotulinumtoxinA (Botox Allergan, Dublin, Ireland). The key clinical trial informing the clinical benefit of abobotulinumtoxinA was a prospective, multicenter, randomized, placebo-controlled study (clinicaltrials.gov identifier NCT01249417), and the subsequent open-label extension (clinicaltrials.gov identifier NCT01251380)Citation17,Citation18. Study NCT01249417 demonstrated a significant and sustained improvement achieved by abobotulinumtoxinA compared to placebo for numerous clinical outcomes, including the MAS, Physician’s Global Assessment (PGA), and GASCitation17,Citation18. GAS scores in the abobotulinumtoxinA group remained superior to placebo for 12 weeks following injection in both 10 and 15 U/kg/limb dosage groupsCitation19. With the availability of new treatments, health care decision makers are required to compare both the effectiveness and costs relative to current practice. To help inform the decision-making process, decision-analytic models such as the Markov cost-effectiveness model described here are developed and used to estimate cost-effectiveness of alternative treatments. Costs and benefits of competing treatment are estimated, and relative difference presented as a ratio of incremental costs to incremental benefits. This analytic tool is well-established to help inform health care decision makingCitation20–22.
A cost-effectiveness analysis was undertaken to compare costs and benefits of abobotulinumtoxinA to onabotulinumtoxinA for the treatment of PLL spasticity.
Methods
Model structure
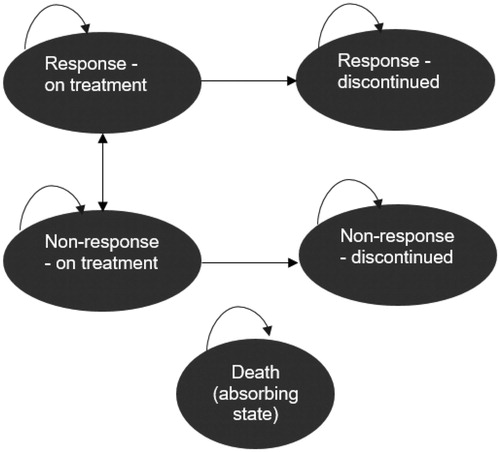
A probabilistic Markov cohort model with 12-week cycles was developed based on Canadian guidelines for economic evaluationsCitation23 to estimate the incremental cost–utility ratio (ICUR) for abobotulinumtoxinA vs. onabotulinumtoxinA in the treatment of PLL spasticity. Health states are characterized by response vs. non-response to therapy, and patients can transition from response to non-response, or non-response to response over time.
The patient population of interest was pediatric patients with lower limb spasticity with either unilateral or bilateral CP aged 2 years and older, although the base case starting age in the model (approximately 6 years) was based on the mean age observed in study NCT01249417. Study NCT01249417 was also used to estimate the proportion of patients with unilateral vs. bilateral disease, to inform the total dose received based on per-kg-per-limb dosing. OnabotulinumtoxinA was the comparator in this economic analysisCitation24. While abobotulinumtoxinA trials have only been conducted compared to placebo, onabotulinumtoxinA is considered the most relevant comparator given its position as standard of care in PLL spasticity in Canada. Treatment efficacy was estimated using GAS, which was adopted as the most relevant and meaningful patient-oriented scale for measuring outcomes in this population, after consultation with Canadian specialists experienced in treatment of PLL spasticityCitation25.
After an initial 12-week cycle of BoNT therapy, patients are classified as responders or non-responders, as defined by a GAS t-score <50 vs. ≥50, respectively. For each subsequent 12-week cycle, patients may transition from non-response to response, from response to non-response, or remain in their current state (). While an in-depth assessment of the underlying reason for response/non-response status and shifts over time is outside the scope of this economic analysis, the data to populate these health states were taken directly from the randomized trial data – in particular, initial response probabilities were based on abobotulinumtoxinA individual patient data and onabotulinumtoxinA trial data with the assumption of normality, while subsequent transitions between response categories were based on observed transitions in the initial abobotulinumtoxinA trial plus open label extension, assumed to be common across treatment arms. Patients may discontinue therapy (assumed 10% annually); at this point, they remain in the same response state as they were in at the time of discontinuation. Patients may transition to the health state associated with death from any response state. Mortality was calculated using Canadian age- and sex-specific life tablesCitation26, adjusted for increased mortality among children with CP (hazard ratio (HR)=3.68 in males and 4.99 in females)Citation27. The key difference between response-defined health states is change in QoL and healthcare resource use. The perspective adopted was a Canadian public payer. In the base case, costs and outcomes were evaluated over a 2-year time horizon, with costs and outcomes discounted at 1.5% per year based on Canadian guidelinesCitation23. A sensitivity analysis was conducted using a 12-year time horizon, derived from ages of patients in study NCT01249417 and time to adulthoodCitation17,Citation18. A limitation of the 12-year time horizon is the assumption that patients stay in their response or non-response states indefinitely. However, it is not expected to confer a systematic bias because it could equally relate to transitioning from response to non-response, as from non-response to response states.
Efficacy
In the base case, the efficacy for abobotulinumtoxinA was based on de novo analysis of patient-level data from NCT01249417, the 12-week GAS response rate (defined as a score ≥50) was 59.2%Citation18. The probability of transitioning between different health states in the model () was informed using the trial data (). No treatment effect was applied to these transitions, such that the only effect of treatment was the initial proportion responding to therapy at 12 weeks. Subsequent to this, every 12-weeks GAS response transitions from the prior period were calculated to inform the prior probabilities; the resulting transition probabilities are reported in .
Table 1. Transition probabilities.
Based on systematic literature reviewCitation28, the only two relevant studies for the GAS outcome were a phase III clinical trial reported by Bjornson et al. for onabotulinumtoxinACitation24, and the study conducted by Delgado et al. for abobotulinumtoxinACitation18. The methodology of the systematic literature review and network meta-analysis (NMA) is described in full in Guyot et al.Citation28 Ten randomized controlled trials met all the inclusion criteria, and a recent study by Kim et al.Citation29 was added when the search was updatedCitation28. Although four studies reported GAS scores, only Bjornson et al.Citation24 and Delgado et al.Citation18 reported GAS outcomes in sufficient detail to allow for inclusion in the NMACitation28. In the study conducted by Bjornson et al., no significant difference was found between onabotulinumtoxinA and placeboCitation24 in contrast to the significant abobotulinumtoxinA benefit reported by Delgado et al.Citation18 More recently, a randomized placebo-controlled trial conducted by Kim et al. (clinicaltrials.gov identifier NCT01603628) in 384 children with lower limb spasticity reported significant improvements in active and passive functioning at six weeksCitation29. However, notably, this study did not report sufficient numeric data to assess the total GAS score, and thus could not be included in either the Guyot analysisCitation28 or in the cost-effectiveness analysis described here.
While the 59.2% GAS response rate is directly available for abobotulinumtoxinA, the Bjornson et al. study of onabotulinumtoxinA only reports continuous scores (mean and standard deviation) which were subsequently converted to an estimated response rate based on the assumption of a normal distribution. The appropriateness of the normal distribution is based on a sample size (n = 33) sufficient to justify application of the central limit theorem; there is no evidence of a skewed distribution in either direction, such that no systemic bias is expected to result from this assumption. Two approaches were considered for estimating GAS response for onabotulinumtoxinA: (1) the change from baseline of onabotulinumtoxinA was used directly (48.18 baseline + 1.8 change = 49.98) without accounting for the fact that there was no differentiation observed relative to placebo and (2) a formal NMA was conducted using data from the Delgado et al. and Bjornson et al. studiesCitation28, accounting for the relative placebo effects observed in both studies. In analysis (2), accounting for the fact that onabotulinumtoxinA did not significantly differ from placebo for the GAS outcome, the final onabotulinumtoxinA response value was less favorable than in analysis (1), and was estimated to be 45.82, with a standard deviation of 7.42. Converting these to response rates via the normality assumption, analysis (1) was associated with a 49.9% response rate, while analysis (2) was associated with a 28.7% response rate. Although onabotulinumtoxinA is widely used in PLL, given the limited documented evidence of its differentiation from placebo, particularly for the GAS outcomeCitation24, a significant benefit for abobotulinumtoxinA vs. onabotulinumtoxinA is estimated in the NMA of published GAS evidenceCitation28. Therefore, as a conservative approach for abobotulinumtoxinA, the more favorable onabotulinumtoxinA results from analysis (1) are used in the base case, with analysis (2) considered in scenario analysis only. This strategy was used to ensure the most conservative estimation of benefit in the input parameters for abobotulinumtoxinA compared to that estimated in alternative scenarios in the NMA.
Resource utilization and cost
In the base case, an abobotulinumtoxinA dose of 15 U/kg/limb to an overall maximum dose of 1,000 U was used, consistent with the upper bound of the abobotulinumtoxinA dose in NCT01249417Citation17,Citation18, clinical practice, and the product monographCitation30. The dose for onabotulinumtoxinA was 12 U/kg to an overall maximum of 400 U, which is consistent with the Bjornson et al. trialCitation24. This dose is higher than the maximum cumulative dose of 8 U/kg or 300 U in a 3 month interval recommended in the product monograph for PLL spasticityCitation31, but similar or slightly lower than the 6–8 U/kg/limb that has been reported as most commonly used in Canada for the PLL indicationCitation15. For these doses, the cost per injection is lower for abobotulinumtoxinA relative to onabotulinumtoxinA. A survey of Canadian practice patterns in pediatric spasticity found that the majority of physicians (70%) used onabotulinumtoxinA at a dose of 12 U/kg or higher, which was considered in scenario analysis as described in further detail below.
Estimated changes in weight as patients in the model aged were applied to baseline weight to calculate dosage changes over time in both treatment armsCitation32. In the base case, it was assumed that vials would not be shared, and that any excess product would be wasted. An algorithm was used to select the most cost-efficient combination of vials. In an alternative scenario analysis, vial sharing was considered.
Resource utilization estimates were obtained from a survey of six Canadian clinicians, including pediatricians, pediatric neurologists, pediatric neurosurgeons, and orthopedic surgeons to capture the multidisciplinary treatment required for the patient populationCitation25. A main criterion for expert selection were clinicians who regularly treat PLL spasticity (at least 100 patients with at least 10 new patients per year), identified through employment at centers of excellence, scientific publications, or referralsCitation25. The aim of the survey was to identify best practices in Canada based on input from physicians with expertise and experience in a specialized patient population; this ensured the representativeness and relevance of results, noting that restriction led to a relatively small sampling from which to identify respondents. Clinicians responded to a questionnaire administered via structured interviews, with ongoing follow-up consultations regarding resource use for patients responding to treatment vs. non-responders. The resources considered included publicly funded inpatient and outpatient services and medications. In addition to BoNT therapy, these included oral pharmacotherapies, hospitalizations and ambulatory care, physiotherapy visits, and laboratory visits. Clinicians were also asked about the effect of BoNT therapy on resource use, which informed the relationship between response status and resource utilization ()Citation25.
Figure 2. Model treatment pathwayCitation22.
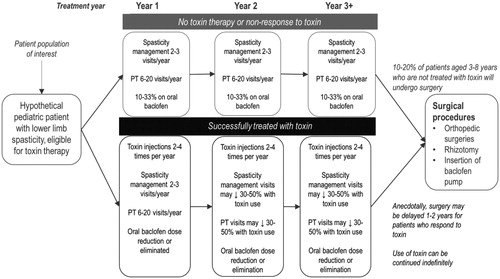
Consensus among clinicians was that hospitalizations in this population are typically not related to spasticity, and that sufficient data are not available to confirm whether surgeries are likely to differ by response status. Hospitalization and surgery costs were therefore not included in the model. Adverse events were not included in this economic model as low incidences have been reported for both abobotulinumtoxinA and onabotulinumtoxinACitation15,Citation18,Citation24. Adverse events that were reported in the abobotulinumtoxinA and onabotulinumtoxinA trials were mild in severity and often not treatment-relatedCitation18,Citation24.
Unit costs for medications and other elements of resource utilization were taken from an Ontario public payer perspective and reported in 2017 Canadian dollars (Table S1).
Health-related quality of life
Health state utilities were obtained by transforming Pediatric Quality of Life Inventory (PEDql) results to EQ-5D via a published algorithmCitation33. The PEDql data were obtained from clinical trial data for abobotulinumtoxinA vs. placebo, pooled across treatments, and stratified by GAS response. Using trial data from study NCT01249417Citation30, a mixed-effects regression model was fit to obtain utilities associated with GAS response states. The resulting utility values were 0.82 (standard error 0.0091) associated with response and 0.78 (standard error 0.0117) associated with non-response. The model assumes that the only difference in health outcomes by treatment arm is the utility implications of differential response rates.
Model outcomes
Model outcomes were captured in terms of quality adjusted life years (QALYs) gained and incremental cost per QALY gained. QALYs and cost per QALY are standard pharmaco-economic outcomes used in clinical drug reviews and health technology assessments, but can also be used to inform physicians and patients given the incorporation of both the quantity and QoL that can be achieved with a particular treatmentCitation34. An incremental cost per QALY gained indicates additional life-years spent in a better QoL on a treatment in question in comparison to a reference therapy.
Uncertainty
A 5,000-iteration probabilistic analysis (PA) was conducted in which uncertain parameters were varied stochastically according to statistical distributions. The variables varied in PA, and associated distributions are listed in .
Table 2. Input data parameters.
Base case and scenario analyses
The base case analysis was based on a two-year time horizon, with onabotulinumtoxinA response rate taken directly from the treatment arm of the Bjornson et al. study (the most favorable estimate for onabotulinumtoxinA), and dosing equivalent to that used in the Bjornson et al. study. Injections were assumed to be given every 12 weeks for both abobotulinumtoxinA and onabotulinumtoxinA.
Scenario analyses were performed to estimate ICURs under different model parameters. The effect of using the value of 28.7% response rate derived from the NMA (as described in analysis (2) above) to estimate onabotulinumtoxinA efficacy was explored. In further scenario analyses, values for onabotulinumtoxinA dosing were varied from 6 U/kg/limb to 8 U/kg/limb, with data sourced from a published survey of Canadian cliniciansCitation15, the effects of product sharing were assessed, a 12-year time horizon was explored based on the assumption that patients would remain in their response-based health state until adulthood, and, finally the duration of efficacy of abobotulinumtoxinA was varied based upon data from studies NCT01249417 and NCT01251380Citation17,Citation18. For this latter analysis (the 16-week duration analysis), while comparative data on efficacy duration were not available in PLL, there have been a number of real-world claims analyses, as well as an observational prospective study in upper limb spasticity that have found significantly longer treatment intervals in patients treated with abobotulinumtoxinA relative to onabotulinumtoxinA, motivating the potential for a differential effect duration in PLL to be explored in sensitivity analysisCitation35,Citation36.
Results
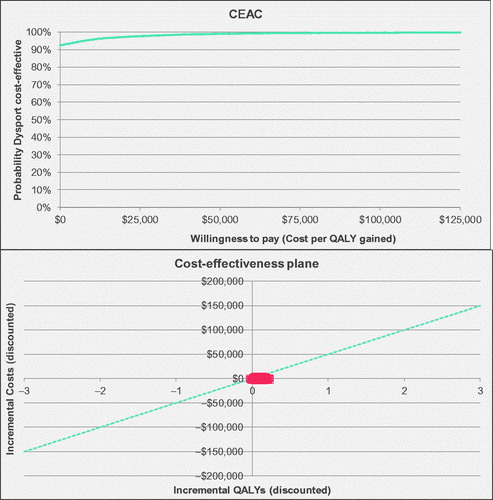
In the base case over a two-year time horizon, abobotulinumtoxinA generated 1.48 QALYs, compared to 1.47 for onabotulinumtoxinA. Survival was assumed consistent across treatment arms; therefore, the difference in health outcome was entirely due to improved QoL associated with greater GAS response. AbobotulinumtoxinA was associated with cost savings of $123 CAD, reflecting a decrease of $147 in health services costs associated with higher GAS response rate, and an increase of $24 in medication costs. Note that abobotulinumtoxinA is associated with a lower acquisition cost than onabotulinumtoxinA generally; the small increase in the acquisition cost observed in a two-year time horizon vs. cost-savings observed for a 12-year time horizon is related to vial size and wastage patterns across therapies for smaller bodyweight patients (in a starting cohort of age 6), compared to a longer time frame accounting for patient growing and therefore a higher average bodyweight, potentially associated with less wastage. The estimated improvement to QoL and reduced costs result in an estimate of economic dominance for abobotulinumtoxinA over onabotulinumtoxinA. Across probabilistic iterations, 70% were dominant, and the probability of cost-effectiveness was 93%, assuming a cost-effectiveness threshold of $50,000 CAD per QALY (, ).
Table 3. Base case results (discounted 1.5%) (2017 Canadian dollars).
AbobotulinumtoxinA remained economically dominant in all scenario analyses – consistently showing greater clinical effectiveness and lower costs (Table S2). As conservative values for input parameters were selected for the base case, scenario analyses were all associated with increased benefits to QALYs and cost savings relative to the base case. When the response rate of onabotulinumtoxinA was adjusted to 28.7% to reflect the results of the NMA, the QALY difference increased from 0.01 in the base case to 0.03. Across scenarios, the greatest cost savings (approximately $2,295 CAD) was associated with the scenario in which the time horizon was increased from two to 12 years. For a 12-year time horizon, treatment costs are lower for abobotulinumtoxinA, as vial wastage no longer induces increased utilization as for the two-year time horizon. For the two-year time horizon, scenario analyses regarding increased onabotulinumtoxinA dose and abobotulinumtoxinA duration of injections both resulted in substantial increases in cost-savings relative to the base case. Thus, while uncertainty remains in some specific numeric values, the overall outcome of economic dominance for abobotulinumtoxinA is supported by the available data, with uncertainty reflected primarily in the total magnitude of cost and health benefits (as explored in scenario analyses).
Discussion
Despite the widespread use of botulinum toxins in Canadian clinical practice in pediatric spasticity, to the best of the authors’ knowledge, this study is the first economic evaluation of these therapies in PLL spasticity in the Canadian context. These findings suggest that abobotulinumtoxinA is likely to be economically dominant, relative to onabotulinumtoxinA for the treatment of PLL spasticity in Canada, based on both a lower acquisition cost and greater efficacy leading to health benefits and lower disease management costs. In Canada, abobotulinumtoxinA is priced lower than onabotulinumtoxinA for clinically similar doses.
A scenario analysis was also conducted to consider the impact of longer duration between injections for abobotulinumtoxinA on model outcomes. This was based on evidence from an open-label extension study in PLL, reporting that the majority (74%) of patients receiving abobotulinumtoxinA did not require retreatment for at least 16 weeks, with 17.7% not requiring retreatment for 28 weeksCitation17. In the Canadian SmPC of abobotulinumtoxinA, it was acknowledged that “a majority of patients in the clinical study were re-treated between 16‒22 weeks; however, some patients had a longer duration of response, i.e. 28 weeks”Citation37. Further, an international prospective observational study has shown significantly longer time between injections for abobotulinumtoxinA compared to onabotulinumtoxinA in adult upper limb spasticity, another major indication for botulinum toxinsCitation36. An exploration of potential economic implications from this duration of effect with abobotulinumtoxinA suggested cost savings greater than that reported in the base case.
The main conclusions above are consistent with findings from other economic studies in PLL. In real-world longitudinal studies, abobotulinumtoxinA has been reported to be similarly or more effective and less costly than onabotulinumtoxinACitation35,Citation38–41. In a Russian study, abobotulinumtoxinA was found to be the most cost-effective option compared to either onabotulinumtoxinA or standard of care therapies aloneCitation42. In the two-year time horizon of the analysis presented in this paper, the cost savings was driven by a reduction in health-services costs associated with greater response rates. This is consistent with a UK survey of 221 physicians that reported costs of care to be associated with severity of spasticityCitation43.
The model presented in this work was based on the GAS outcome, measuring a patient’s success at meeting the goals they have set as a part of receiving therapy, in which patients are involved in defining the criteria for success so as to ensure that the goals are realistic and achievableCitation44. Hence GAS is a patient-centered outcome related to QoL, and has been confirmed by Canadian physicians as the more informative measure in Canadian clinical practiceCitation25. Conversely, MAS is a more objective clinical measure but with limited validity for describing spasticityCitation9,Citation10, and while it may not be as directly related to a patient’s functional status as GAS, it is more commonly reported in clinical studiesCitation28. In NMA, abobotulinumtoxinA was also found to have superior outcomes to onabotulinumtoxinA on the MAS scale.
There are several limitations to the study presented. This includes lack of data regarding the most relevant dose for onabotulinumtoxinA. A wide variety of prescription patterns have been observed in Canada. In a survey of Canadian pediatric physicians, doses of 12–16 U/kg (equivalent to 6–8 U/kg/limb) were routinely usedCitation15 for onabotulinumtoxinA, equivalent to or higher than the dose of 12 U/kg used in the Bjornson et al. trialCitation24, justifying the assumption of the trial dose for the base case as a relatively conservative assumption. In a recent phase III trial, 4 U/kg was not found to have significant benefits relative to placeboCitation29, and thus this lower dose was not considered a relevant comparator.
A systematic literature review and related NMA was considered as a basis for the efficacy inputs in the model. This existing NMA was conducted to estimate the difference in treatment effect between abobotulinumtoxinA and onabotulinumtoxinACitation28. As pointed out by authors of the NMA, the underlying evidence base was limited. The sole included GAS study by Bjornson et al. reported no differences on the GAS scale between onabotulinumtoxinA and placeboCitation24. More recently, a new large phase III trial on onabotulinumtoxinA was presentedCitation29. In this study, and in contrast to other onabotulinumtoxinA and abobotulinumtoxinA studies included in the NMA, the GAS score was reported as active and passive functional goal attainment rather than an overall score, and therefore this study could not be directly synthesized in the GAS total score NMACitation29. However, in the new onabotulinumtoxinA study in questionCitation29, there was no separation from placebo on the key efficacy endpoint MAS at 12 weeks (in contrast to abobotulinumtoxinA study by Delgado et al.Citation18). This suggests that, had the overall efficacy of onabotulinumtoxinA from the study by Kim et al.Citation29 been considered for inclusion in the NMA, it would not change the direction of the conclusions. Therefore, at this time, the Bjornson et al. and Delgado et al. studies remain the only two studies available for quantitative analysis, and all currently available data have been incorporated in this analysis. Considering the above limitation of the NMA, in the base case, the absolute GAS improvements from Bjornson et al. were used in the economic model presented rather than relative values from the NMA, reflecting the greatest plausible treatment benefit for onabotulinumtoxinA. Of note, the recent phase III study for onabotulinumtoxinA did include sufficient data to support a quantitative comparison for MAS, and was incorporated along with five other studies into an NMA for MAS, along with other published dataCitation28. In that analysis, a statistically significant benefit in 12-week MAS scores was estimated for abobotulinumtoxinA relative to onabotulinumtoxinA, highlighting that the observed benefits of abobotulinumtoxinA are consistent across multiple measures. This study took a conservative approach by considering outcomes within a two-year time horizon in the base case, supported by empirical trial data. In a scenario analysis, a common and standard practice for economic evaluations was employed by extrapolating the outcomes to a longer-term time horizon (12 years), reflective of the chronic treatment throughout the childhood (mean 5.9 ± 3.3 years of age in the open-label extension trial NCT01251380 until 18 years of age). The extrapolation of abobotulinumtoxinA of shorter-term response data to longer-term outcomes (beyond 2 years) is consistent with examples of other chronic disease modelsCitation45–48. In order to limit assumptions or variability while modeling long-term response from available trial data, it was assumed that patients would remain in their 24-month state for the duration of the 12-year time horizon. While this does assume that patients stay in their response state and do not worsen over time, it also prevents any spontaneous response or improvement over the remainder of the time horizon. This approach was applied equally to both abobotulinumtoxinA and onabotulinumtoxinA treatment arms, to minimize a risk of bias. Thus, while the magnitude of cost savings and health benefits are smaller for a shorter time horizon, given the observed clinical benefit and lower acquisition cost of abobotulinumtoxinA, the overall trend of economic dominance remains consistent across time horizon differences. Therefore, although the cost and clinical differences between the two treatments are modest in the base case, and the exact numerical results presented for an extrapolated economic analysis is uncertain, confidence can be placed in the overall interpretation of findings. Based on the totality of available evidence, the following overall relationships have consistently been reported: (1) all available clinical data have reported better clinical outcomes for abobotulinumtoxinA than onabotulinumtoxinA on the MAS and GAS scales, and this has been formally quantitatively assessed with NMA; (2) better response to toxin therapy has been consistently reported by Canadian clinicians to be associated with lower health resource utilization and costs; and (3) the acquisition cost of abobotulinumtoxinA is lower than that for an analogous dose of onabotulinumtoxinA.
In the population of children with lower limb spasticity QoL of both patients and their families are affectedCitation5, requiring a management program that take the impact on patients and families into accountCitation7. Evaluation of optimal treatment strategies is thus critical, although a limited evidence base is a notable data limitation. While the analysis reported here comprises the best currently available evidence, future economic evaluations in BoNT therapy would benefit from expanded real-world evidence regarding treatment patterns in PLL spasticity. Such data may accrue over time as both abobotulinumtoxinA and onabotulinumtoxinA are used in real-world settings. Furthermore, if additional GAS results from phase III clinical trials are published, then the differences in efficacy between abobotulinumtoxinA and onabotulinumtoxinA will be clearly defined and can be incorporated.
Conclusions
In conclusion, abobotulinumtoxinA was found to be associated with cost-savings and increased QALYs compared to onabotulinumtoxinA (i.e. economically dominant). Thus, adoption of abobotulinumtoxinA in Canadian patients with PLL spasticity has the potential to improve quality-of-life for patients, while reducing health care expenditure.
Transparency
Declaration of funding
This study was sponsored by Ipsen.
Declaration of financial/other relationships
Karissa Johnston: No conflicts of interest to declare. Ryan Hansen: Reports receiving consulting fees from Ipsen. Natalya Danchenko: An employee of Ipsen. Jerome Dinet: Exempt status of former Ipsen Pharma employee has no other conflicts of interest. Anna Liovas: Actively employed with Ipsen and owns stocks in the company. Ava Armstrong: Actively employed with Ipsen and owns stocks in the company. Savreet Bains: Previously an employee of Ipsen Biopharmaceuticals Inc. Sean Sullivan: Received funding for the conduct of this work. A peer reviewer on this manuscript has disclosed that they have previously received grants and consulting fees from Ipsen and consulting fees from Allergan. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
KJ contributed to the model development and analysis, and wrote the first draft of the manuscript. RH led model development and analysis and contributed to manuscript drafting and review. ND provided feedback on the model development process and contributed to manuscript drafting and review. JD provided feedback on the model development process and contributed to manuscript drafting and review. AL provided feedback on the model development process and contributed to manuscript drafting and review. AA provided feedback on the model development process and contributed to manuscript drafting and review. SB provided feedback on the model development process and contributed to manuscript drafting and review. SS oversaw model development and analysis and contributed to manuscript drafting and review.
Supplemental Material
Download MS Word (36.9 KB)Acknowledgements
The authors thank all patients involved in the abobotulinumtoxinA trials, as well as their caregivers, care team, investigators and research staff in participating institutions. The authors thank Antoinette Cheung and Lauren Stewart of Broadstreet HEOR (Vancouver, Canada) for providing medical writing support, which was sponsored by Ipsen (Paris, France) in accordance with Good Publication Practice guidelines.
References
- Blair E, Watson L. Epidemiology of cerebral palsy. Semin Fetal Neonat Med. 2006;11(2):117–125.
- Abdel-Hamid HZ, Bazzano ATF, Ratanawongsa B. Cerebral Palsy. Medscape. 2018. [cited 2020 Jan 31]. Available from https://emedicine.medscape.com/article/1179555-overview
- Awaad Y, Rizk T. Spasticity in children. J Taibah Univ Med Sci. 2012;7(2):53–60.
- Santamato A. Safety and efficacy of incobotulinumtoxinA as a potential treatment for poststroke spasticity. Neuropsychiatr Dis Treat. 2016;12:251–263.
- Wood E. The child with cerebral palsy: diagnosis and beyond. Semin Pediatr Neurol. 2006;13(4):286–296.
- Kancherla V, Amendah DD, Grosse SD, et al. Medical expenditures attributable to cerebral palsy and intellectual disability among Medicaid-enrolled children. Res Dev Disabil. 2012;33(3):832–840.
- Mugglestone M, Eunson P, Murphy M, et al. Spasticity in children and young people with non-progressive brain disorders: summary of NICE guidance. BMJ. 2012;345(2):e4845.
- NICE. Clinical guideline [CG145] spasticity in under 19s: management. National Institute for Health and Care Excellence; 2012; [updated Nov 2016; cited 2019 Oct 22]. Available from: https://www.nice.org.uk/guidance/CG145
- Strobl W, Theologis T, Brunner R, et al. Best clinical practice in botulinum toxin treatment for children with cerebral palsy. Toxins. 2015;7(5):1629–1648.
- Love S, Novak I, Kentish M, et al. Botulinum toxin assessment, intervention and after-care for lower limb spasticity in children with cerebral palsy: international consensus statement. Eur J Neurol. 2010;17:9–37.
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–207.
- Scholtes V, Becher J, Beelen A, et al. Clinical assessment of spasticity in children with cerebral palsy: a critical review of available instruments. Dev Med Child Neurol. 2005;48(01):64–73.
- Kiresuk TJ, Sherman RE. Goal attainment scaling: a general method for evaluating comprehensive community mental health programs. Commun Ment Health J. 1968;4(6):443–453.
- Gaasterland CMW, Jansen-van der Weide MC, Weinreich SS, et al. A systematic review to investigate the measurement properties of goal attainment scaling, towards use in drug trials. BMC Med Res Methodol. 2016;16(1):99–99.
- Fehlings D, Narayanan U, Andersen J, et al. Botulinum toxin-A use in paediatric hypertonia: Canadian practice patterns. Can J Neurol Sci. 2012;39(4):508–515.
- Delgado MR, Hirtz D, Aisen M, et al. Practice parameter: pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2010;74(4):336–343.
- Delgado MR, Bonikowski M, Carranza J, et al. Safety and efficacy of repeat open-label abobotulinumtoxinA treatment in pediatric cerebral palsy. J Child Neurol. 2017;32(13):1058–1064.
- Delgado MR, Tilton A, Russman B, et al. AbobotulinumtoxinA for equinus foot deformity in cerebral palsy: a randomized controlled trial. Pediatrics. 2016;137(2):e20152830.
- Tilton A, Russman B, Aydin R, et al. AbobotulinumtoxinA (Dysport((R))) improves function according to goal attainment in children with dynamic equinus due to cerebral palsy. J Child Neurol. 2017;32(5):482–487.
- Sanders GD, Maciejewski ML, Basu A. Overview of cost-effectiveness analysis. JAMA. 2019;321(14):1400.
- Inotai A, Kalo Z, Meszaros A. Decision analytic modeling and their impact on health care decision making. Acta Pharm Hung. 2009;79(2):63–69.
- Andy Briggs KC, Schulpher M. Decision modelling for health economic evaluation. Oxford, UK: Oxford University Press; 2006.
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies. 4th ed. Canada: Canadian Agency for Drugs and Technologies in Health; 2017.
- Bjornson K, Hays R, Graubert C, et al. Botulinum toxin for spasticity in children with cerebral palsy: a comprehensive evaluation. Pediatrics. 2007;120(1):49–58.
- Powell L, Johnston K, Liovas A, et al. Resource use related to pediatric lower limb spasticity in Canada: physician questionnaire. Paper presented at: International Society for Pharmacoeconomics and Outcomes Research; 2018 May 19–23; Baltimore, MD. [cited 2018 Oct 24]. Available from: https://www.broadstreetheor.com/wp-content/uploads/sites/5/2018/05/Resource-use-related-to-pediatric-lower-limb-spasticity-in-Canada-Physician-questionnaire.pdf
- Statistics Canada. Life Tables, Canada, Provinces and Territories (84-537-X); 2017. [cited 2018 Oct 24]. Available from: http://www5.statcan.gc.ca/olc-cel/olc.action?objId=84-537-X&objType=2&lang=en&limit=0
- Himmelmann K, Sundh V. Survival with cerebral palsy over five decades in western Sweden. Dev Med Child Neurol. 2015;57(8):762–767.
- Guyot P, Kalyvas C, Mamane C, et al. Botulinum toxins type A (Bont-A) in the management of lower limb spasticity in children: a systematic literature review and Bayesian network meta-analysis. J Child Neurol. 2019;34(7):371–381.
- Kim H, Meilahn J, Liu C, et al. Efficacy and safety of OnabotulinumtoxinA for the treatment of pediatric lower limb spasticity: primary results (S29.007). Neurology. 2018;90(15 Suppl.):S29.007.
- Ipsen Biopharmaceuticals. Product Monograph. PrDYSPORT THERAPEUTIC™ abobotulinumtoxinA for injection Ph. Eur. Ipsen Biopharm Limited; 2018; [cited 2018 Oct 24]. Available from: https://ipsen.com/websites/IPSENCOM-PROD/wp-content/uploads/sites/18/2018/11/08151628/dysport-therapeutic-pm-Oct.24.2018_EN.pdf
- Allergen Pharmaceuticals. BOTOX (onabotulinumtoxinA) prescribing information. Madison (NJ): Allergen Pharmaceuticals; 2019.
- National Center for Health Statistics. US Centers for Disease Control and Prevention. Weight for age charts; 2000. [cited 2018 Oct 24]. Available from: https://www.cdc.gov/growthcharts/percentile_data_files.htm
- Khan KA, Petrou S, Rivero-Arias O, et al. Mapping EQ-5D utility scores from the PedsQL generic core scales. PharmacoEconomics. 2014;32(7):693–706.
- NICE. Glossary. National Institute for Health and Care Excellence; 2013; [cited 2019 Nov 5]. Available from: https://www.nice.org.uk/glossary?letter=q
- Eckwright D, Burke J, Gleason P. Real-world botulinum toxin (BT) utilization and treatment cost for cervical dystonia (CD) and limb spasticity (LS). Academy of Managed Care Pharmacy’s (AMCP) Managed Care & Specialty Pharmacy Nexus Meeting; Oct 29 to Nov 1; 2019.
- Turner-Stokes L, Ashford S, Fheodoroff K, et al. Time to retreatment with botulinum toxin A in upper limb spasticity management: upper limb international spasticity (ULIS)-III study interim analysis. Paper presented at: Toxins; 2019 Jan 16–19; Copenhagen, Denmark; 2019.
- Ipsen Biopharmaceuticals. DYSPORT THERAPEUTIC™ Product Monograph. AbobotulinumtoxinA for injection Ph. Eur. Mississauga, ON; 2018.
- Tedroff K, Befrits G, Tedroff CJ, et al. To switch from Botox to Dysport in children with CP, a real world, dose conversion, cost-effectiveness study. Eur J Paediatr Neurol. 2018;22(3):412–418.
- Tapias G, Garcia-Romero M, Crespo C, et al. Cost-minimization analysis in the treatment of spasticity in children with cerebral palsy with botulinum toxin type A: an observational, longitudinal, retrospective study. Farm Hosp. 2016;40(5):412–426.
- Dursun N, Akarsu M, Gokbel T, et al. Switching from onabotulinumtoxin-A to abobotulinumtoxin-A in children with cerebral palsy treated for spasticity: a retrospective safety and efficacy evaluation. J Rehabil Med. 2019;51(5):390–394.
- Al-Mazraawy B. Potential cost savings were identified across the Yale Health System when primarily using Dysport® vs. Botox®. 53rd Annual ASHP Midyear Clinical Meeting; 2018 Dec 2–6; Anaheim, CA.
- Ugrekhelidze DT, Kulikov AY. Pharmacoeconomic analysis of different types of treatment for spastic forms of cerebral palsy. Pharmacoeconom Theory Pract. 2015;3(3):71–78.
- Stevenson VL, Gras A, Bardos JI, et al. The high cost of spasticity in multiple sclerosis to individuals and society. Mult Scler. 2015;21(12):1583–1592.
- Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil. 2009;23(4):362–370.
- Woolacott N, Hawkins N, Mason A, et al. Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technol Assess. 2006;10(46):1–233, i–iv.
- Sizto S, Bansback N, Feldman S, et al. Economic evaluation of systemic therapies for moderate to severe psoriasis. Br J Dermatol. 2009;160(6):1264–1272.
- Domínguez-Ortega J, Phillips-Anglés E, Barranco P, et al. Cost-effectiveness of asthma therapy, a comprehensive review. J Asthma. 2015;52(6):529–537.
- Bodger K, Kikuchi T, Hughes D. Cost-effectiveness of biological therapy for Crohn’s disease: Markov cohort analyses incorporating United Kingdom patient-level cost data. Aliment Pharmacol Ther. 2009;30(3):265–274.