Abstract
Aims: This study estimated the total costs associated with tisagenlecleucel treatment in adult patients with relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL) based on the JULIET trial from a United States hospital’s perspective.
Methods: An economic model was developed to assess the total costs associated with tisagenlecleucel treatment (from leukapheresis to two months post-infusion) in adults (aged ≥18 years) with r/r DLBCL using a fee-for-service approach. Costs were considered during the pre-treatment, tisagenlecleucel infusion, and follow-up periods, and were estimated based on the health resource utilization and safety data from the JULIET trial. Cost components included leukapheresis, lymphodepleting chemotherapy, tisagenlecleucel infusion/administration, inpatient and intensive care unit (ICU) admission, medical professional visits, lab tests/procedures, and management of adverse events (AEs). The base-case model estimated the total costs using observed hospitalization, ICU, and AE data from JULIET, while scenario analyses varied key assumptions related to AEs and hospitalization.
Results: The estimated overall cost associated with tisagenlecleucel treatment from leukapheresis to two months post-infusion was $437,927/patient, of which $64,784 (14.8%) was additional to tisagenlecleucel’s list price ($373,000) and the associated administration cost ($143). The top three key drivers of the additional cost were AE management ($30,594; 47.2%), inpatient/ICU not attributed to AEs ($24,285; 37.5%), and lab tests/procedures ($5,443; 8.4%). In the scenario analyses, total costs ranged from $382,702 (no AEs, no hospitalization) to $469,006 (cytokine release syndrome and B-cell aplasia, hospitalization).
Limitations: This analysis was limited to two months of follow-up after tisagenlecleucel infusion, which cannot capture long-term safety outcomes associated with the treatment and may underestimate AE costs.
Conclusions: The total cost of tisagenlecleucel administration from leukapheresis to two months was estimated at $437,927. In addition to tisagenlecleucel’s price, the main drivers were AE management costs and inpatient/ICU costs. Future studies based on real-world, long-term use of tisagenlecleucel are warranted.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a lymphoid malignancy characterized by the proliferation of mature, larger than normal size B-cellsCitation1. In 2016, it was estimated to affect approximately 5 per 1000 people in the United States (US)Citation2. DLBCL is the most common form of non-Hodgkin’s lymphoma (NHL), comprising 25% to 40% of all NHL cases in the USCitation1,Citation3,Citation4. Among patients with DLBCL, about a third will develop relapsing or refractory (r/r) disease after initial treatment (typically a combination of chemotherapy and the monoclonal antibody rituximab)Citation5. Only 50% of patients with r/r DLBCL are eligible for autologous hematopoietic stem cell transplant (ASCT), while the remaining 50% are ineligible due to age, comorbidity profile, advanced disease, etc.Citation5 These eligible patients will receive second-line chemotherapy, and only half of them will achieve remission and proceed to ASCTCitation6. Among the patients who undergo ASCT, approximate one-third will relapseCitation7. Limited treatment options were available before 2018 for patients who were ineligible for ASCT or patients who failed two lines of therapy with or without ASCT, and these include salvage chemotherapy, allogeneic hematopoietic stem cell transplant (allo-SCT) when feasible, palliative radiotherapy, and supportive careCitation8–10. However, the median overall survival for these patients is very low, ranging from 4.4 to 10.0 monthsCitation7,Citation10,Citation11.
A new approach to the treatment of r/r DLBCL is the chimeric antigen receptor T-cell (CAR-T) therapy tisagenlecleucel, which is created by engineering a patient’s own T cells to identify the cancer cell antigen CD19 and destroy cancer cellsCitation12,Citation13. This cell-based gene therapy was approved by the US Food and Drug Administration in May 2018 for adult patients with r/r DLBCL after two or more lines of systemic therapyCitation14,Citation15. The safety and efficacy of tisagenlecleucel among patients with r/r DLBCL was evaluated in the single-arm, open-label Phase II trial JULIETCitation16. In that trial, tisagenlecleucel was associated with a 54% response rate (95% confidence interval [CI]: 43–64%), with 40% of patients achieving complete response and 13% achieving partial response. The median duration of response among patients with response was not reached. The median overall survival for all infused patients was 11.1 months and not reached for patients with complete responseCitation16.
With the novel biologic mechanism and clinical benefits demonstrated in the JULIET trial, the wholesale acquisition price (WAC) of tisagenlecleucel was set at $373,000 in the US. Unlike conventional chemotherapy, which requires long-term treatment regimens, tisagenlecleucel is a one-time therapy with effects that may endure over years or a lifetime. However, because tisagenlecleucel is a first in-class CAR-T immunotherapy that is individually manufactured for each patient using their own blood cells, the associated healthcare resource use (HRU) is also likely to differ from other cancer treatments. For example, before tisagenlecleucel is manufactured and ready for infusion, apheresis is required to cultivate patients’ cells for use in the process. Afterwards, bridging chemotherapy treatments and lymphodepleting regimens may also be used to stabilize patients’ condition and prepare them for infusion, respectively. Thus, there are currently unmet needs to understand the HRU associated with tisagenlecleucel treatment and to estimate the cost of care in addition to tisagenlecleucel infusion for patients with r/r DLBCL.
Because tisagenlecleucel is designed to use a patient’s own immune system to eliminate cancer cells, it is associated with unique adverse events (AEs): cytokine release syndrome (CRS) and B-cell aplasiaCitation17. Both of these AEs may require management with expensive therapies (i.e. tocilizumab and immunoglobulin), and patients who develop severe CRS may require intensive care unit (ICU) admission. In addition, as CAR-T therapies are relatively new to the market, there is limited real-world evidence to inform analyses of HRU. However, comprehensive HRU data were collected in the pivotal JULIET trialCitation16 and included information related to hospitalization, ICU stay, and treatment utilization for bridging chemotherapy, lymphodepleting regimens, CRS, and B-cell aplasia. The data from this trial present an up-to-date resource for estimating HRU among patients with r/r DLBCL treated with tisagenlecleucel.
To address the lack of real-world evidence on HRU associated with CAR-T therapy, this study used the clinical trial data from JULIET and publically available data to comprehensively evaluate the total costs of tisagenlecleucel treatment among patients who received the infusion, including costs in addition to the procedure and encompassing multiple treatment periods. Scenario analyses were also performed based on different hospital treatment settings, the presence of AEs, and HRU as observed in a real-world study.
Methods
Model overview
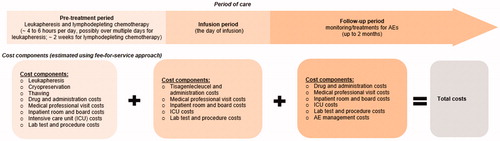
An economic model was developed to assess the total costs associated with adult patients with r/r DLBCL receiving tisagenlecleucel infusions from a US hospital’s perspective. The total costs were estimated from the time of leukapheresis to two months post-infusion, which was consistent with the timeframe of collecting HRU related to the tisagenlecleucel infusion per the trial protocol. The model was developed using a fee-for-service approach, which estimated costs based on the HRU and safety data from the pivotal Phase II JULIET trial (N = 115 patients who received the infusion; data cut-off: 11 December 2018)Citation18. Of these patients, 86% were based in the US and 14% were outside the US.
The model considered costs of leukapheresis, lymphodepleting chemotherapy, tisagenlecleucel infusion and hospital administration, inpatient and ICU admission, medical professional visits, lab tests and procedures, and additional medication and HRU for the management of major AEs (e.g. grade 3/4 CRS).
Different cost components were estimated by tisagenlecleucel treatment periods (pre-treatment, infusion, and follow-up periods). The detailed model framework is presented in . All costs were inflation-adjusted to 2019 US dollars (USD). The base-case model estimated the total costs using the observed hospitalization, ICU, and AE data from all patients receiving tisagenlecleucel infusion in the JULIET trial. Scenario analyses were conducted varying key assumptions related to AEs and hospitalization.
Model assumptions
This model used the observed hospitalization data from the JULIET trialCitation18 and considered the period of care to begin from cell collection to two months following tisagenlecleucel infusion, which was assumed to capture the major AEs related to the infusion. JULIET reported that 14% of patients received their infusion in an outpatient setting. The current study assumed these locations were hospital affiliated outpatient facilities and were considered in the current hospital model for cost estimates. In addition, the model separately estimated costs associated with AEs (including drugs, procedures, and hospitalizations) and costs associated with inpatient and ICU admissions. Only grade 3 or 4 AEs with a ≥ 5% incidence rate were considered in this model. To avoid double counting, inpatient admissions due to AEs were excluded from the hospitalization estimates. The model did not consider patients who did not receive the tisagenlecleucel infusion or who did not incur costs related to the infusion due to various reasons.
Model inputs
Lymphodepleting drug and administration costs
Costs associated with lymphodepleting chemotherapies were considered during the pre-treatment period. As estimated from the JULIET trial dataCitation18, 93% of the infused population received lymphodepleting therapies (74% received regimen 1 [combination fludarabine–cyclophosphamide] and 19% received regimen 2 [bendamustine]) during the pre-treatment period. The remaining 7% of patients did not receive lymphodepleting therapies, as observed in JULIET. The default cost inputs for medication and administration were obtained from the public domain and were based on payment from the payers (i.e. costs reimbursed by payers). Specifically, medication costs were estimated using the WAC from IBM Micromedex RED BOOKCitation19, and unit costs for drug administration were from the Centers for Medicare & Medicaid Services (CMS) Physician Fee ScheduleCitation20. Detailed information regarding the dosing schedules and unit drug costs are presented in .
Table 1. Estimated drug and administration cost inputs (2019 USD).
Tisagenlecleucel drug and administration costs
Tisagenlecleucel drug and administration costs were considered during the infusion period; infusion procedure cost was obtained from the WAC in the RED BOOK and the administration cost was derived from the CMS Physician Fee ScheduleCitation19,Citation20.
Inpatient costs not due to AEs and ICU costs not due to CRS
Inpatient and ICU costs were estimated during the entire treatment period. The proportions of patients with inpatient and ICU admissions and the lengths of stay (LOS) were estimated from the JULIET trial dataCitation18 separately for the pre-treatment, infusion, and follow-up periods. For inpatient costs, to avoid double counting with the AE management costs, hospitalizations caused by AEs as reported in the JULIET trial were excluded and only hospitalizations not due to AEs were considered. For ICU admissions, the model considered ICU costs due to CRS and those not due to CRS separately. ICU costs due to CRS were considered as part of the AE management costs, while the ICU costs not due to CRS were reported together with inpatient costs.
The Premier Healthcare Database was used to estimate the real-world inpatient and ICU room and board daily costs incurred by the hospitals for DLBCL-related servicesCitation21. Premier is a large US hospital-based, service-level database that provides detailed resource utilization data along with patients’ primary and secondary diagnosis and procedure codes in both inpatient and outpatient facility settings. Data from the most recent 3 years of the Premier database (2015 Q4–2018 Q2) related to 37,932 unique patients diagnosed with DLBCL were included in this analysis. Daily costs of inpatient and ICU room and board services were estimated based on all inpatient hospital encounters with an admitting or primary diagnosis of DLBCL in the Premier database. The median costs were used. The detailed inpatient and ICU rate, LOS, and unit costs are presented in . The Supplemental Methods provide further details regarding the Premier database analysis, including the sample selection and statistical methods.
Table 2. Hospitalization and ICU cost inputs by tisagenlecleucel treatment period (2019 USD).
Medical professional visit costs
Medical professional visit costs are relevant to the entire treatment period and were considered in the model. The resource utilization frequencies were acquired from the JULIET trial protocolCitation18, and the unit costs of medical professional visits were obtained from the CMS Physician Fee ScheduleCitation20. Supplemental Table 1 summarizes the detailed frequencies and unit costs of medical professional visit.
Lab and procedure costs
Laboratory tests and procedures associated with DLBCL included bone marrow biopsy and/or aspirate, chemistry panel, coagulation, electrocardiogram, flow cytometry, hematology panel, hepatitis B and C test, human immunodeficiency virus test, imaging (positron emission tomography-computed tomography), influenza A and B test, pause oximetry, serum immunoglobulin levels, and urine pregnancy test. The frequencies and unit costs of labs and procedures are presented in Supplemental Table 1.
Leukapheresis cost was considered during pre-treatment period and other lab and procedure costs were considered throughout the tisagenlecleucel treatment period. The frequency inputs of labs and procedures during each treatment period were based on the JULIET trial protocolCitation18. The physician cost associated with leukapheresis was calculated using the 2019 CMS Physician Fee Schedule ratesCitation22, and the real-world lab and procedure costs were based on the Premier Healthcare DatabaseCitation21. Unit costs of lab services and procedures were estimated based on inpatient or outpatient encounters with an admitting, primary, or secondary diagnosis of DLBCL. The Supplemental Methods provide further details for the Premier database analysis.
AE costs
AE rates during the follow-up period were obtained from the JULIET trial data, and only grade 3 or 4 AEs with ≥5% rates were considered in the modelCitation18. The two most important tisagenlecleucel-related AEs are grade 3 or 4 CRS and B-cell aplasia, with observed rates in JULIET of 23% and 18%, respectively. The HRU related to these two AEs were estimated separately and the detailed cost calculation for grade 3 or 4 CRS and B-cell aplasia can be found in . For patients with grade 3 or 4 CRS, the proportion with ICU admission, the proportion receiving tocilizumab treatment, and the dosage used for CRS management were estimated based on JULIET trial data. Similarly, the intravenous immunoglobulin dosage among patients with B-cell aplasia was also estimated. The other grade 3 or 4 AEs rates observed in JULIET are summarized in Supplemental Table 2. Unit costs for these AEs were derived from the Healthcare Cost and Utilization Project (HCUP) inpatient databaseCitation23.
Table 3. Associated adverse event cost for B-cell aplasia and grade 3 or 4 CRS (2019 USD).
Model calculations
The model aimed to represent the average cost of DLBCL-related treatment for a patient over different treatment periods using data from the JULIET trial. Costs of relevant HRU were calculated separately for each treatment period and then summed to evaluate the total cost over the entire episode of care using a micro-costing approach.
The base-case model estimated the total costs using the observed hospitalization, ICU, and AE data from all patients receiving tisagenlecleucel infusion in the JULIET trial. Scenario analyses were conducted to assess the sensitivity of the model results to variations in model assumptions and inputs. Specifically, the scenario analyses 1) assumed all patients received tisagenlecleucel in an outpatient setting and experienced no AEs during the follow-up; 2) assumed all patients received tisagenlecleucel in an inpatient setting and experienced grade 3 or 4 CRS and B-cell aplasia; 3) varied hospitalization and AE costs by ±25%; and 4) used Riedell et al. (a real-world study published in 2020) to inform HRU, grade 3/4 CRS, and tocilizumab use (Supplemental Table 3)Citation24. The study by Riedell et al. retrospectively analyzed data from patients with r/r B-cell NHL who underwent apheresis for commercial tisagenlecleucel or axicabtagene ciloleucel treatment from eight US academic centers. The data collection started after 1 May 2018 with data cut off on 1 November 2019. Because the CRS grading system as well as the management guideline have been updated in real-world practice since the launch of tisagenlecleucel, the resource use and AE rates of tisagenlecleucel could be different in a real-world study compared with the JULIET trial. Therefore, we additionally conducted a scenario analysis using Riedell et al. to inform HRU, grade 3/4 CRS, and tocilizumab use for tisagenlecleucel (inputs listed in Supplemental Table 3).
Results
Base-case results
The overall cost associated with the tisagenlecleucel treatment from leukapheresis to two months post-infusion for patients with r/r DLBCL was estimated at $437,927/patient (2019 USD) (). Considering the list price of tisagenlecleucel to be $373,000 with an associated administration cost of $143, the model calculated the additional cost of care to be $64,784. This total included $30,594 (47.2%) for AE management, $24,285 (37.5%) for inpatient (unrelated to AEs) and ICU admissions (unrelated to CRS), $5,443 (8.4%) for lab tests and procedures, $3,052 (4.7%) for lymphodepleting drugs and administration, and $1,410 (2.2%) for medical professional visits (). The costs incurred during the pre-treatment, infusion, and follow-up periods were $12,363, $374,395, and $51,169, respectively. The primary cost drivers per period were inpatient and ICU admissions ($6,734; 54.5%) in pre-treatment period, the tisagenlecleucel procedure and administration ($373,143; 99.7%) in the infusion period, and AE management ($30,594; 9.8%) during the follow-up period.
Table 4. Base-case results of tisagenlecleucel associated costs by treatment period (2019 USD).
Scenario analyses
In the scenario analyses, the total costs ranged from $382,702 (outpatient setting without AEs) to $469,006 (inpatient setting with both grade 3 or 4 CRS and B-cell aplasia) (). Under the scenario that a patient received tisagenlecleucel treatment in an outpatient setting and did not experience any AEs, the model calculated a cost of $9,559 in addition to the tisagenlecleucel procedure and administration, which included $5,443 (56.9%) for lab tests and procedures, $3,052 (31.9%) for lymphodepleting drugs and administration, and $1,064 (11.1%) for medical professional visits.
Table 5. Sensitivity analyses of tisagenlecleucel-associated costs by treatment period (2019 USD).
Under the scenario that a patient received tisagenlecleucel treatment in an inpatient setting and experienced both grade 3 or 4 CRS and B-cell aplasia, the model calculated a cost of $95,862 in addition to the tisagenlecleucel procedure and administration, which included $51,767 (54.0%) for AE management, $34,007 (35.5%) for inpatient (unrelated to AEs) and ICU admissions (unrelated to CRS), $5,443 (5.7%) for lab tests and procedures, $3,052 (3.2%) for lymphodepleting drugs and administration, and $1,593 (1.7%) for medical professional visits ().
Under the scenario that hospitalization and AE costs varied by ±25%, the total costs in addition to the tisagenlecleucel procedure and administration ranged from $51,488 to $78,080. Of this amount, 45.4% to 48.4% of the costs were for AE management, 35.4% to 38.9% were for inpatient (unrelated to AEs) and ICU admissions (unrelated to CRS), 7.0% to 10.6%% were for lab tests and procedures, 3.9% to 5.9% were for lymphodepleting drugs and administration, and 1.8% to 2.7% were for medical professional visits.
Under the scenario using the real-world study by Riedell et al. to inform rates of HRU, grade 3/4 CRS, and tocilizumab use, the model calculated a cost of $37,270 in addition to the tisagenlecleucel procedure and administration, which included $26,565 (71.3%) for AE management, $5,443 (14.6%) for lab tests and procedures, $3,052 (8.2%) for lymphodepleting drugs and administration, $1,211 (3.2%) for inpatient and ICU admissions, and $999 (2.7%) for medical professional visits ().
Discussion
To our knowledge, this is the first study to use comprehensive HRU data collected in a clinical trial to evaluate the costs associated with tisagenlecleucel treatment among patients with r/r DLBCL who received the tisagenlecleucel infusion from a US hospital’s perspective. Additionally, the economic model explored different scenarios to provide the plausible range of the cost estimates based on different hospitalization and AE assumptions. The model assessed the total costs from leukapheresis to two months post-infusion of tisagenlecleucel among adult patients with r/r DLBCL to be $437,927/patient, from a US hospital’s perspective. Of this total, $64,784 (14.8%) was in addition to the list price of tisagenlecleucel ($373,000) and the associated administration cost ($143). The top three key cost drivers were AE management (47.2%), inpatient and ICU costs unrelated to AEs (37.5%), and lab tests and procedures (8.4%).
The current study contributes valuable information on the total treatment costs associated with CAR-T therapy in r/r DLBCL and benefits from several strengths. First, this study used comprehensive HRU data collected from the pivotal trial of tisagenlecleucel (JULIET) to estimate the total costs related to tisagenlecleucel. Secondly, the model used a fee-for-service approach with detailed breakdowns by treatment periods (i.e. pre-treatment, infusion, and post-infusion periods of care) and cost components. With the granularity and flexibility of considering different treatment scenarios, the model was able to provide a plausible estimate of the total costs of tisagenlecleucel. Finally, real-world hospital data (i.e. the Premier Healthcare Database) were used to inform the unit costs associated with hospitalization and ICU stays and lab tests and procedures. Because the cost of hospitalization and ICU costs was one of the key cost drivers, using real-world hospital data enables a better approximation of the true costs incurred in a US hospital by this patient population; other studies which have used literature or public databases for the inputs and the estimates might not be accurate.
The costs estimated in this model are largely consistent with prior published studies. A budget impact model (BIM) was previously developed for tisagenlecleucel in r/r DLBCL from a US third-party perspective and considered similar cost components as the current studyCitation25. In that BIM, the total costs of tisagenlecleucel over one year were estimated to be around $517,000/patient, higher than the estimate in the present study (approximately $438,000 from the time of cell collection until two months after the infusion). This can be attributed to the different timeframes considered; the BIM estimated the total annual cost of tisagenlecleucel, therefore additional HRU beyond two months post-infusion was included. If the time horizon of the BIM was limited to 2 months post-infusion, the estimated total cost is expected to be similar to the present study. Similar findings were reported in a cost-effectiveness analysis (CEA) of patients with r/r DLBCL from a US payer’s perspectiveCitation26. Lin et al. estimated the total cost of tisagenlecleucel to range from $521,000 to $529,000 with the majority of the costs arising from the drug procedure (around 71%). The remaining costs included both one-time costs (e.g. AE costs, lymphodepleting regimen costs) and medical costs that would be incurred over time. Again, the CEA evaluated tisagenlecleucel costs over different time horizons and considered slightly different cost components and assumptions compared to the current study, although the total costs of tisagenlecleucel when the time horizon was limited to 2 months are expected to be similar to the present study. In the scenario analysis using the real-world study by Riedell et al. to inform HRU, grade 3/4 CRS, and tocilizumab use for tisagenlecleucel, the estimated total costs based on the real-world study with a median follow-up time of 5.8 months were similar to those based on the JULIET trial with a 2-month follow up. In fact, the costs based on the real world data decreased by $27,514 due to lower rates of HRU and AEs compared to the JULIET trial, which could be partially due to the fact that follow-up care in clinical trials is typically more intense than that in real world practice. In addition, this difference might reflect improvements in clinical practice as the knowledge around effective management of safety events for CAR-T therapy matures.
Another study by Hernandez et al. evaluated the total costs of CAR-T immunotherapy and reported a mean expected cost of $402,647/patient for patients with r/r DLBCL who received axicabtagene ciloleucelCitation27. That study considered key cost components including physician and facility cost for leukapheresis, lymphodepleting therapy, CAR-T drug cost, and the facility cost of CRS-related hospitalization. However, significant costs such as those related to the management of B-cell aplasia and hospitalization associated with CAR-T infusion and subsequent monitoring were omitted. Conversely, the additional costs incurred during AE management and detailed hospitalization costs during each period of care were included in the present study. In addition, while the total cost was estimated via a weighted average based on selected patient-treatment pathways (e.g. with or without CRS), similar to the present study, their selected pathways were less comprehensive and did not capture all possible treatment scenarios. Due to the difference in cost components and narrower treatment pathways excluding B-cell aplasia and hospitalization, the estimated cost in that study was $35,000 lower compared to the current study. When using the same cost categories and a more comprehensive treatment pathway, the estimates would be similar.
The total cost of care associated with tisagenlecleucel treatment, $437,927 in this HRU cost model, is lower than the cost of allo-SCT, the only potentially curative option for adult patients with r/r DLBCL before tisagenlecleucel became available. For example, Maziarz et al. calculated the total costs for allo-SCT to be $455,741 for adults with DLBCL in the first year, with costs of $92,720 during the second year and $72,957 during the third yearCitation28. Resource use and costs are especially high in patients who receive SCT due to the high rate of extended complications associated with the procedure such as chronic graft versus host disease (GvHD), which requires long-term treatmentCitation29,Citation30. Fenske et al. reported that the rates of acute and chronic GvHD at day 100+ after HSCT to be 28%–44% and 40%, respectively, in patients with DLBCLCitation30. Acute GvHD is associated with high mortality risk and chronic GvHD requires long-term HRU to manage, thus incurring high additional costs. In contrast, the majority of AEs associated with tisagenlecleucel occur within two months post-infusion and are manageableCitation16.
As estimated in our HRU cost model, tisagenlecleucel treatment contributed to the majority of the cost of care for treated patients with r/r DLBCL, and only 14.8% of the total costs were unrelated to the infusion procedure. In absolute terms, the total costs aside from the tisagenlecleucel procedure were estimated to be approximately $64,784 for the average patient, but could range from $9,559 to $95,862 depending on the hospitalization and AE assumptions. The estimated costs in addition to the tisagenlecleucel procedure are less than the non-pharmaceutical costs associated with salvage chemotherapy for r/r DLBCL. For example, Lin et al. reported the total cost of salvage chemotherapy ranged from $145,000 to $195,000 over a lifetime, with the non-pharmaceutical costs comprising 85%–89% of the total costsCitation26. Although the tisagenlecleucel procedure is associated with a high price, it can provide medical cost offsets over a lifetime compared to other treatments due to being potentially curative.
An important consideration for any new therapy is its expected economic burden to patients and society. Upon the entry of CAR-T therapy (including tisagenlecleucel), it is expected the total treatment costs of DLBCL would increase due to the higher costs of CAR-T therapy compared to conventional treatments, although the medical costs might decrease. This assumption is validated in a study by Kilgore et al., which reported that for Medicare patients with B-cell lymphoma, the median healthcare cost per patient per month, not including treatment costs, decreased by 27% after receiving CAR T-cell therapyCitation31. Second, the entry of CAR-T therapy could impact indirect costs, which are not assessed in the current study. As tisagenlecleucel is a one-time infusion with curative potential, there are expected benefits of less travel time, less time spent in the hospital over the long-term, and reduced burden on caregivers. In addition, treated patients might return to work, which would be associated with productivity gains. Future studies are needed to further assess the economic impact of CAR-T therapy over the long term, considering both direct and indirect costs to the society, as well as the value of the therapy to patients as a curative option.
The cost proportion of the tisagenlecleucel procedure is generally consistent with the trend of other innovative cell and gene therapies, where the drug/procedure cost is the major component of the overall costs. The CAR-T procedure cost for axicabtagene ciloleucel is 71% of the total treatment cost for patients with B-cell lymphomaCitation32, similar to the proportion of the treatment costs for AVXS-101 ($2.4–4.9 million [58%–73%] of $4.2–6.6 million total cost) and voretigene neparvovec ($850,000 [82%–88%] of 0.96–1.04 million total cost)Citation33,Citation34. Although the up-front costs of the tisagenlecleucel procedure are high, this can be potentially offset by the lower non-procedure costs. Indeed, a recent study by Kilgore et al. that used real-world data from 100% Medicare Fee-For-Service Part A and B claims reported that the HRU and total costs incurred by older patients with DLBCL (mean age: 70 years) decreased after CAR-T infusionCitation31.
This study is subject to several limitations and the results should be interpreted in light of these. First, the study used clinical trial data to estimate the HRU among patients who received tisagenlecleucel treatment, which may subject to the following limitations related to data itself: 1) the study reflected HRU collected from the trial data to the extent possible, but may differ from real-world HRU with a more heterogeneous patient population and practice pattern; and 2) the trial data only collected HRU limited to two months of follow-up after tisagenlecleucel infusion, per trial protocol, which cannot capture the long-term safety outcomes (e.g. intravenous immunoglobulin for B-cell aplasia) associated with the treatment. A scenario analysis was conducted using HRU, grade 3/4 CRS, and tocilizumab information reported in a real-world study by Riedell et al. with a longer median follow-up of 5.8 months and similar results were observed as those based on the clinical trial. Second, this model may not capture cost components such as non-protocol based HRU, management of grade 1/2 AEs, supportive care medications, and other additional costs for managing r/r disease. However, as evaluated by previous economic models in oncologyCitation35–37, the HRU and costs associated with grade 1/2 AEs are minimal and the costs of other components are expected to be small compared to the total costs. Thus, this current model should capture the majority of the costs associated with tisagenlecleucel infusion. Third, the study collected cost information from public sources and each data source was subject to its own limitations. The drug costs were estimated using the WAC in RED BOOK, and administration and medical visit costs were estimated using the 2019 CMS Physician Fee Schedule. The costs reported in these databases may not represent the true costs incurred by hospitals. For example, based on the CMS, the reimbursement to a hospital will be only $242,450 in 2020 for CAR-T therapy, which is much lower than the list price of CAR-T therapy, although this amount may not be the price the hospital originally paid for tisagenlecleucelCitation38. In addition, the unit costs for AEs were derived from the HCUP inpatient database, which may overestimate the total AE management cost. Particularly, the model assumed that each AE would be treated in the hospital and the AE management costs were additive. However, in the real world, patients might have multiple AEs treated within one hospitalization, thus the simple sum of AE costs might be an overestimation. Finally, the model did not consider patients who did not receive tisagenlecleucel infusion or who did not incur costs related to tisagenlecleucel infusion due to various reasons (i.e. failure in manufacturing, patients progressed to terminal disease before infusion, etc.). Future research with long-term follow-up data and focusing on a real-world setting will be important to provide alternative perspectives on the HRU and costs associated with tisagenlecleucel treatment.
Conclusions
Using the clinical trial data from the JULIET trial of patients with r/r DLBCL, this HRU model estimated the total costs of tisagenlecleucel treatment from leukapheresis to two months post-infusion to be $437,927/patient from a US hospital’s perspective. The largest cost component was the list price and associated administration cost of tisagenlecleucel ($373,143), with an additional $64,784 in costs related to AE management (47.2%), inpatient and ICU costs unrelated to AEs (37.5%), and lab tests and procedures (8.4%). The cost of care in addition to the list price and administration cost of tisagenlecleucel infusion only accounted for a small proportion (14.8%) of the total estimated cost of tisagenlecleucel treatment. This study contributes valuable data that may help inform decision-making by patients, health care professionals, hospitals, and other stakeholders.
Transparency
Declaration of funding
This study was supported by Novartis.
Declaration of financial/other interests
YH is employee of Novartis and owns stock/stock options. HY, XC, CZQ, and EQW are employees of Analysis Group, Inc., which has received consulting fees from Novartis. A peer reviewer on this manuscript has disclosed that they have received support for invited speech, consultation, meetings and clinical studies from Janssen, Celgene, Gilead, Amgen, Abbvie and Takeda. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental_Material_revised_clean_041020.docx
Download MS Word (25.4 KB)Acknowledgements
Medical writing assistance was provided by Qing Liu and Shelley Batts, employees of Analysis Group, Inc. Support for this assistance was provided by the sponsor.
References
- Rodriguez-Abreu D, Bordoni A, Zucca E. Epidemiology of hematological malignancies. Ann Oncol. 2007;18(Suppl 1):i3–i8.
- National Cancer Insitute- Surveillance E, and End Results Program (SEER). Cancer stat facts: NHL – diffuse large B-cell lymphoma (DLBCL); 2016 [cited 2019 Oct 22]. Available from: https://seer.cancer.gov/statfacts/html/dlbcl.html
- Al-Hamadani M, Habermann TM, Cerhan JR, et al. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90(9):790–795.
- Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–459.
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011(1):498–505.
- Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. JCO. 2014;32(31):3490–3496.
- Van Den Neste E, Schmitz N, Mounier N, et al. Outcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL study. Bone Marrow Transplant. 2017;52(2):216–221.
- Chaganti S, Illidge T, Barrington S, et al. Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol. 2016;174(1):43–56.
- Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116–v125.
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808.
- Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51(1):51–57.
- Gorovits B, Koren E. Immunogenicity of chimeric antigen receptor T-cell therapeutics. BioDrugs. 2019;33(3):275–284.
- Liu Y, Chen X, Han W, et al. Tisagenlecleucel, an approved anti-CD19 chimeric antigen receptor T-cell therapy for the treatment of leukemia. Drugs Today. 2017;53(11):597–608.
- United States Food and Drug Administration (FDA). FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma; 2018 [cited 2019 Oct 22]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-adults-relapsed-or-refractory-large-b-cell-lymphoma
- United States Food and Drug Administration (FDA). Highlights of prescribing information: KYMRIAH™ (tisagenlecleucel); 2018 [cited 2019 Oct 28]. Available from: https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert. —KYMRIAH.pdf
- Schuster SJ, Bishop MR, Tam CS, et al.; JULIET Investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56.
- O'Leary MC, Lu X, Huang Y, et al. FDA approval summary: Tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Res. 2019;25(4):1142–1146.
- NCT02445248: Study of efficacy and safety of CTL019 in adult DLBCL patients (JULIET) [cited 2020 Apr 9]. Available from: https://clinicaltrials.gov/ct2/show/NCT02445248
- IBM Micromedex. Wholesale acquisition cost (WAC), REDBOOK; 2019 [cited 2019 Jun 23]. Available from: https://www.micromedexsolutions.com/micromedex2/librarian
- Centers for Medicare & Medicaid Services (CMS). CMS Physician Fee Schedule; [cited 2019 Jun 23]. Available from: https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx
- Premier Applied Sciences. Premier Healthcare Database; 2019 [cited 2019 Oct 24]. Available from: https://products.premierinc.com/downloads/PremierHealthcareDatabaseWhitepaper.pdf
- Centers for Medicare & Medicaid Services (CMS). CMS Outpatient Prospective Payment System (PPS); 2019 [cited 2019 Jun 23]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices-Items/CMS-1695-FC.html
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Inpatient database [cited 2019 Jun 23]. Available from: https://www.ahrq.gov/data/hcup/index.html
- Riedell PA, Walling C, Nastoupil LJ, et al. A multicenter retrospective analysis of clinical outcomes, toxicities, and patterns of use in institutions utilizing commercial axicabtagene ciloleucel and tisagenlecleucel for relapsed/refractory aggressive B-cell lymphomas. Biol Blood Marrow Transplant. 2020;26(3):S41–S42.
- Yang H, Hao Y, Chai X, et al. Budget impact associated with the introduction of tisagenlecleucel for the treatment of relapsed or refractory diffuse large B-cell lymphoma. Meeting Abstracts - Academy of Managed Care Pharmacy Nexus 2018. J Manag Care Spec Pharm. 2018;24(10-a Suppl):S29. DOI:10.18553/jmcp.2018.24.10-a.s1
- Lin JK, Muffly LS, Spinner MA, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J Clin Oncol. 2019;37(24):2105–2119.
- Hernandez I, Prasad V, Gellad WF. Total costs of chimeric antigen receptor T-cell immunotherapy. JAMA Oncol. 2018;4(7):994–996.
- Maziarz RT, Hao Y, Guerin A, et al. Economic burden following allogeneic hematopoietic stem cell transplant in patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2018;59(5):1133–1142.
- Huntington S, Keshishian A, McGuire M, et al. Costs of relapsed diffuse large B-cell lymphoma among Medicare patients. Leuk Lymphoma. 2018;59(12):2880–2887.
- Fenske TS, Ahn KW, Graff TM, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol. 2016;174(2):235–248.
- Kilgore K, Mohammadi I, Schroeder A, et al. Medicare patients receiving chimeric antigen receptor T-cell therapy for non-Hodgkin lymphoma: A first real-world look at patient characteristics, healthcare utilization and costs. 61st ASH Annual Meeting and Exposition; December 7-10, 2019. Orlando, FL, US, 2019.
- Institute for Clinical and Economic Review (ICER). ICER final evidence report: Chimeric antigen receptor T-cell therapy for B-cell cancers: Effectiveness and value; 2018 [cited 2019 Oct 30]. Available from: https://icer-review.org/wp-content/uploads/2017/07/ICER_CAR_T_Final_Evidence_Report_032318.pdf
- Malone DC, Dean R, Arjunji R, et al. Cost-effectiveness analysis of using onasemnogene abeparvocec (AVXS-101) in spinal muscular atrophy type 1 patients. J Mark Access Health Policy. 2019;7(1):1601484.
- Institute for Clinical and Economic Review (ICER). ICER final evidence report: Voretigene neparvovec for biallelic RPE65-mediated retinal disease: Effectiveness and value; 2018 [cited 2019 Nov 26]. Available from: https://icer-review.org/wp-content/uploads/2017/06/MWCEPAC_VORETIGENE_FINAL_EVIDENCE_REPORT_02142018.pdf
- Mickisch G, Gore M, Escudier B, et al. Costs of managing adverse events in the treatment of first-line metastatic renal cell carcinoma: bevacizumab in combination with interferon-alpha2a compared with sunitinib. Br J Cancer. 2010;102(1):80–86.
- Wong W, Yim YM, Kim A, et al. Assessment of costs associated with adverse events in patients with cancer. PLos One. 2018;13(4):e0196007.
- Copley-Merriman C, Stevinson K, Liu FX, et al. Direct costs associated with adverse events of systemic therapies for advanced melanoma: Systematic literature review. Medicine (Baltimore). 2018;97(31):e11736.
- Centers for Medicare & Medicaid Services (CMS). Fiscal Year (FY) 2020 Medicare Hospital Inpatient Prospective Payment System (IPPS) and Long Term Acute Care Hospital (LTCH) prospective payment system proposed rule and request for information 2020 [cited 2019 Nov 17]. Available from: https://www.cms.gov/newsroom/fact-sheets/fiscal-year-fy-2020-medicare-hospital-inpatient-prospective-payment-system-ipps-and-long-term-acute