Abstract
Objective: To assess the cost-effectiveness of pembrolizumab monotherapy compared with standard chemotherapy for the treatment of advanced non-small cell lung cancer (NSCLC) in previously untreated adults who have a high programmed death ligand 1 (PD-L1) tumor proportion score of 50% or greater in Singapore.
Materials and methods: A partitioned-survival analysis model was developed from a healthcare system’s perspective that extrapolated clinical and economic outcomes of first-line pembrolizumab (maximum treatment duration of 2 years) versus platinum doublet chemotherapy over a 10-year time horizon for patients with advanced NSCLC. The model consisted of three health states: alive with no progression, alive with progression, and dead. Key clinical inputs were based on Kaplan-Meier survival curves from the interim (median follow-up = 11.2 months) and updated analysis (median follow-up = 25.2 months) of the KEYNOTE-024 randomized controlled trial. Local cost data were applied. Utilities were derived from published international estimates. Both one-way and multivariate probabilistic sensitivity analyses (PSA) were conducted to identify key drivers of the results.
Results: Using the results from the updated analysis of KEYNOTE-024, patients treated with pembrolizumab experienced more quality adjusted life-years (QALYs), but incurred higher costs compared to chemotherapy over a 10-year time horizon (pembrolizumab: 1.9983 QALYs, SGD215,761; chemotherapy: 1.1317 QALYs, SGD70,444). The base-case incremental cost-effectiveness ratio (ICER) was SGD167,692 per QALY gained. One-way sensitivity analysis showed the ICER was most sensitive to the cost of pembrolizumab, followed by the time horizon. Multivariate PSA indicated that pembrolizumab had 0% probability of being cost-effective at a hypothetical willingness-to-pay threshold of SGD100,000 per QALY gained.
Conclusion: While pembrolizumab is superior to standard chemotherapy in improving overall survival and progression-free survival, results suggest that it is unlikely to be cost-effective at its current price in Singapore. Factors including clinical effectiveness, safety, and budget impact should also be considered when making national funding decisions.
Introduction
Lung cancer is the most common cancer globally, and the leading cause of cancer deathsCitation1. In Singapore, up to 85% of all lung cancers are classified as non-small cell lung cancer (NSCLC; comprising squamous cell carcinoma, adenocarcinoma, and large cell carcinoma), and the majority of patients are diagnosed at advanced stages (more than 60% at stage IV)Citation2.
Programmed death ligand 1 (PD-L1) protein expression is used as a predictive biomarker for tumor response to anti-PD-L1/PD-1 immunotherapy. Clinical studies have shown that higher tumor PD-L1 expression is associated with higher objective response rates or overall survivalCitation3,Citation4. Multiple anti-PD-L1/PD-1 immunotherapy agents have regulatory approval in Singapore, but only pembrolizumab is currently approved for use as monotherapy for previously untreated metastatic NSCLC. Pembrolizumab, a humanized monoclonal antibody, binds to the PD-1 receptor on T-cells and blocks their interaction with ligands PD-L1 and PD-L2, potentiating T-cell anti-tumor responses.
The efficacy of pembrolizumab monotherapy versus platinum-based chemotherapy in adults with previously untreated advanced NSCLC with a PD-L1 tumor proportion score (TPS) of 50% or greater and without EGFR mutation or ALK translocation, was initially reported by Reck et al.Citation5 following planned interim analyses of the phase III KEYNOTE-024 study (ClinicalTrials.gov identifier: NCT02142738). At a median follow-up of 11.2 months, pembrolizumab monotherapy was associated with significantly improved progression-free survival (PFS; hazard ratio [HR] = 0.50; 95% CI = 0.37–0.68; p < 0.001) and overall survival (OS; HR = 0.60; 95% CI = 0.41–0.89, p = 0.005) compared to platinum doublet chemotherapy. An updated analysis of the OS curves with a longer median follow-up of 25.2 months showed similar findingsCitation6.
Though several cost-effectiveness analyses have been published based on the interim resultsCitation7,Citation8, there is a high level of uncertainty associated with these analyses due to the short duration of follow-up in the study and long period of extrapolation required. Furthermore, none of these analyses were performed in Asian healthcare settings. To address this gap, a cost-effectiveness analysis was conducted using results from the updated analysis of the KEYNOTE-024 study, from the perspective of the Singapore healthcare system, to inform local drug subsidy decisions.
Methods
Model structure
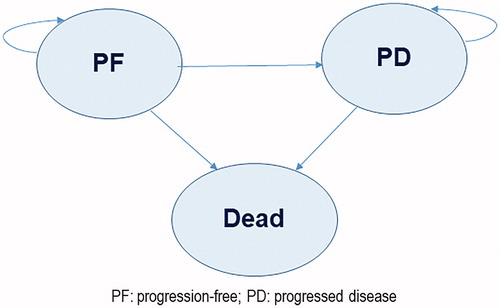
A partitioned-survival (area under the curve) model was developed in Microsoft Excel 2013 (Microsoft Corp, Redmond, WA) from the perspective of the Singapore healthcare system (comprising government subsidies, insurance, and patient co-payments) to compare pembrolizumab 200 mg every 3 weeks (maximum of 2 year treatment) with platinum doublet chemotherapyCitation9. The model was used to project the outcomes and costs for patients with NSCLC in three mutually exclusive health states: alive with no progression [“Progression free” (PF)], alive with progression [“progressed disease” (PD)], and dead (). All patients entered the model in the PF health state. At the beginning of each weekly cycle, patients could either remain in the PF health state or transition to the PD health state or die. After the first cycle, patients could also move from the PD health state to death. The proportion of patients in the PF health state over time was calculated based on estimated PFS distributions. The proportion of patients in the PD health state was derived from the difference between the OS and PFS curves. A time horizon of 10 years was used in the base case, which was deemed to be sufficiently long enough to capture all survival benefits and costs accrued in the comparator arm. A discount rate of 3% was applied to both health outcomes and costs.
Data and sources
Clinical data
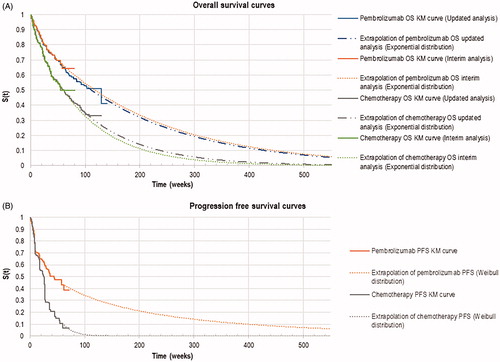
Clinical inputs for the model were predominantly derived from the pivotal KEYNOTE-024 study which compared pembrolizumab monotherapy (n = 154) versus platinum doublet chemotherapy (n = 151) in patients with previously untreated advanced NSCLC with PD-L1 expressionCitation5,Citation6. Platinum doublet chemotherapy consisted of carboplatin plus pemetrexed (44%), cisplatin plus pemetrexed (24%), carboplatin plus gemcitabine (13%), cisplatin plus gemcitabine (8%), or carboplatin plus paclitaxel (11%). The area under the curve (AUC) from the KEYNOTE-024 study was used to determine the mean time that patients remained in each health state. By the updated analysis cutoff date in this study, 64.2% of the patients in the chemotherapy arm had crossed over to pembrolizumab or another anti-PD-1 treatment. As pembrolizumab also has regulatory approval as a second-line treatment, the intention-to-treat Kaplan-Meier (KM) PFS and OS curves (unadjusted for the crossover effect) were used in the model. In order to simulate a 10 year time horizon, extrapolation of the survival curves beyond the study duration was warranted. As individual patient-level data from the KEYNOTE-024 study was not available, the Guyot methodCitation10 was adopted to extrapolate the survival curves.
In the base-case analysis, OS KM curves from the updated KEYNOTE-024 analysis reported by Reck et al.Citation6 were used. The OS curves from the interim analysis were used in scenario analyses to investigate the extent of uncertainty in the cost-effectiveness results if the analysis was conducted with immature survival curves. Candidate functions for the parametric extrapolations of the survival curves were the Weibull, exponential, log-logistic, log-normal, Gompertz, and generalized gamma distributions. Diagnostic plots of ln(-ln S(t)) over ln(t) indicated that the proportional hazards assumption was violated and hence parametric functions were derived via the “independent model” approach in which the curves were obtained by fitting distribution functions onto the pembrolizumab and standard chemotherapy KM curves separately. The goodness-of-fit of these functions was assessed using the Akaike Information Criterion (AIC) and a visual inspection of the parametric curves against the actual data. As the curves did not provide a good visual fit to the observed KM data, a two-piece model fitting of the parametric curves was chosen as the base-case.
For the OS curves, week 33 was chosen as the time point for the two-piece fitting based on the changes to the cumulative hazards. Two additional possible time points were identified from the cumulative hazards curve (week 23 and week 43) and were explored in scenario analyses (Supplementary Figure S1). The exponential distribution was chosen for the base-case analysis based on the AIC value and clinical plausibility (). The Weibull distribution was tested in scenario analyses (Supplementary Figure S2). Other parametric forms were deemed clinically implausible by clinicians consulted as they were overly optimistic with 5 year OS rates of more than 30% and 15% in the pembrolizumab and standard chemotherapy arms, respectively. The PFS curves from the updated analysis of KEYNOTE-024 were not published, therefore, only the curves from the interim analysis could be used in the model. Week 9 was chosen for the two-piece fitting because of a significant drop in the curve at this time point as protocol-driven clinical assessments to determine disease progression took place during that week. The Weibull distribution with the lowest AIC value was chosen for the base-case analysis. When consulted, clinical experts agreed that the Weibull distribution was the most likely candidate function for the chemotherapy arm. However, for the pembrolizumab arm, generalized gamma distribution was considered more clinically plausible. Uncertainty arising from the fitting of different survival distributions was tested in scenario analyses. All parameter values are summarized in .
Table 1. Key input data for base-case analysis.
In the base-case analysis, the treatment effect of pembrolizumab was assumed to be maintained over the entire 10 year time horizon, even though the maximum duration of treatment was only 2 years in the KEYNOTE-024 study and longer-term treatment effects remain uncertain. Thus, scenario analyses limiting the treatment effect of pembrolizumab to 2 years and 5 years were explored, by assuming the same mortality hazard rates as the chemotherapy treatment arm after 2 and 5 years, respectively.
Utility data
In the absence of local data, utility values for PF and PD health states for each treatment arm were extracted from a prospective, international quality-of-life survey of patients (mean age 64.7 years) with advanced NSCLC receiving first-, second-, or subsequent-line pharmacotherapy or best supportive care (BSC) by Chouaid et al.Citation11. These values, derived using an EQ-5D questionnaire, were consistent with an earlier cost-effectiveness analysis evaluating tyrosine kinase inhibitors for metastatic NSCLC in SingaporeCitation12.
The KEYNOTE-024 study also reported utility values using the EQ-5D questionnaire. Two approaches were used in the application of utility weights in the model: (1) by health state; and (2) by time-to-deathCitation7,Citation13,Citation14. In the time-to-death analyses, the utility values were reported in four time periods based on the patient’s time to death after the EQ-5D questionnaires were completed – more than 360 days, 180–360 days, 30–180 days, and less than 30 days. These values were tested in the scenario analyses (see Supplementary Table S1).
As the utility values attached to the PF and PD health states were not treatment-specific, utility decrements for adverse events were added to model the differences between pembrolizumab and platinum-based chemotherapy. Pembrolizumab was associated with more immune-related adverse events such as pneumonitis while chemotherapy was associated with more general adverse events such as neutropenia. These utility decrements were obtained from the KEYNOTE-024 study and were consistent with decrements previously accepted by NICE (UK) to inform their health technology assessmentCitation15,Citation16.
Resource use and cost data
Only direct medical costs were included in the model, in accordance with the perspective of the analysis. Cost of treatment was calculated based on the prices charged to patients at local public tertiary cancer centers. In the KEYNOTE-024 study, patients in either treatment arm who were in a clinically stable condition and were considered by the investigator to be deriving clinical benefit could continue therapy after disease progression. Hence, costs for first-line treatment were accumulated in the model cycles based on a time-on-treatment (ToT) KM curve published by Huang et al.Citation7 The types and duration of treatment used first- and second-line were based on the KEYNOTE-024 study, while third-line treatment was derived from local clinical expert opinion (see Supplementary Table S2).
Disease management costs for both treatment arms included medical consultation visits, computerized tomography scans, liver function tests, full blood counts, and renal panel tests. An additional cost for thyroid function tests was added in the pembrolizumab arm as thyroid disorders have been reported in patients receiving pembrolizumab. Only the costs for adverse events that were grade 3 and above which led to inpatient hospitalization (i.e. neutropenia, pneumonitis, and severe skin reactions) were included in line with local expert opinion. Costs were obtained from the Ministry of Health case-mix dataset (2017). It was anticipated that for patients nearing death, additional costs were incurred for palliative treatment such as inpatient hospice or home-care hospice visits. Costs were obtained from local public hospice centers which are the main provider of end-of-life care. It was assumed that, on average, the costs for hospice care accrued for a duration of 28 days per patient, and were reflected in the model based on the proportion of patients who died in each cycle.
Outcomes
The main outcomes of interest were overall life-years (LY), quality-adjusted life-years (QALYs), the total costs in both treatment arms, and the incremental cost-effectiveness ratio (ICER).
A comparison of the outcomes using the interim and updated analyses of the survival data from KEYNOTE-024 was also conducted.
Sensitivity analyses
To identify key drivers of the model, a one-way sensitivity analysis (OWSA) was conducted over the range of values of the point estimate (i.e. 95% confidence interval) for each model parameter tested. Results were plotted as a Tornado diagram according to the impact of the parameter on the ICER. Probabilistic sensitivity analysis (PSA) using 15,000 Monte Carlo simulations was also performed to assess the robustness of the model results and the simultaneous impact of the variables on the ICER.
Results
Base-case results
Using the KM curves from the KEYNOTE-024 updated analysisCitation6, the base-case results showed that patients treated with pembrolizumab experienced more QALYs, but incurred higher costs compared to standard chemotherapy over a 10-year time horizon (). The ICER for pembrolizumab versus standard chemotherapy was SGD167,692 per QALY gained (incremental costs SGD145,317; incremental QALYs 0.8666). A large proportion (95%) of the incremental costs was due to the large difference in the costs between pembrolizumab and standard chemotherapy.
Table 2. Results of base-case and scenario analyses.
Sensitivity analyses
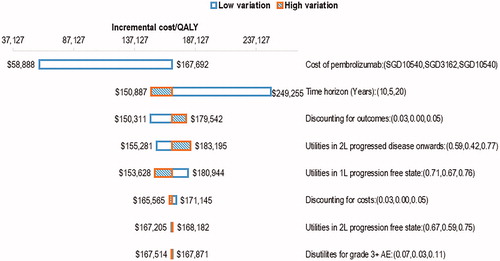
One-way sensitivity analysis (OWSA) showed that the model was most sensitive to the cost of pembrolizumab (). The time horizon modelled also influenced the ICER; when it was reduced to 5 years, the ICER increased to SGD249,255 per QALY gained. It was evident from the OWSA results that there was no instance in which the ICER fell below SGD100,000 per QALY gained except when the cost of pembrolizumab was reduced substantially. The base-case ICER remained unfavorably high across the range of possible values assumed for the model parameters.
Figure 3. One-way sensitivity analysis tornado diagram for pembrolizumab versus chemotherapy (base-case ICER: SGD167,692 per QALY). Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, Quality-adjusted life-years; SGD, Singapore dollars; 1L, first-line; 2L, second line; AE, adverse events.
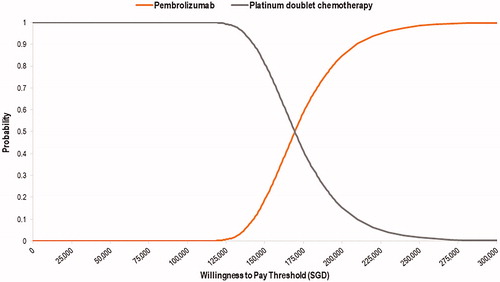
Results from the PSA simulations were similar to the base-case results (ICER: SGD169,561 per QALY). Pembrolizumab had 0% probability of being cost-effective at a hypothetical willingness-to-pay (WTP) of SGD100,000 per QALY. The cost-effectiveness acceptability curve () showed that pembrolizumab may only be more cost effective than standard chemotherapy at a hypothetical WTP of more than SGD170,000 per QALY, which is not considered to be an acceptable use of healthcare resources in the local context.
Scenario analyses
Results for the scenario analyses performed to test the assumptions of the model are in . When the OS curves from the KEYNOTE-024 interim analysis with a shorter median follow-up time were used, the ICER reduced by SGD22,000 to SGD145,774 per QALY gained. Limiting the treatment effect of pembrolizumab to 2 years based on the maximum duration of pembrolizumab treatment in the KEYNOTE-024 study increased the ICER substantially to SGD341,073 per QALY gained. A hypothetical reduction of the cost of pembrolizumab by 20% to 70% resulted in ICERs of SGD136,608 to SGD58,888 per QALY gained, respectively.
Discussion
Pembrolizumab is increasingly used by oncologists in Singapore for previously untreated patients with advanced and/or metastatic wild type NSCLC with PD-L1 TPS more than 50%, due to its superior efficacy in improving overall survival compared to standard chemotherapy. However, its high cost currently limits treatment access for most patients. Results of this study show that, at current costs, the ICER for pembrolizumab compared to chemotherapy is high at SGD167,692 per QALY gained from the perspective of the Singapore healthcare system. Even though there is no fixed WTP threshold in Singapore, it is unlikely that pembrolizumab would be considered cost-effective for NSCLC without a substantial price reduction.
Our model was validated by comparing the 5-year OS rates that were simulated with other sources of evidence. Data from Singapore’s cancer registryCitation2 suggest 5-year survival rates of 3.3% for patients with advanced NSCLC, which is lower than the rate (8.2%) estimated in the model for the chemotherapy arm. The slightly higher OS rate in our model is considered plausible as the rates from the local registry include all patients, including those with more severe disease than the population represented in the KEYNOTE-024 study, as well as those who are not actively treated. The trial population in the chemotherapy arm, however, consisted of patients who were all treated with chemotherapy as first-line therapy, and some received pembrolizumab as second-line treatment. The trial population also had a good ECOG status (0 or 1) at baseline. An alternative source for validation came from long-term follow-up data from the KEYNOTE-001 study (Phase I dose ranging trial) which showed a 5-year OS of 29.6% among patients treated with pembrolizumab with PD-L1 TPS of 50% or moreCitation17. While this is slightly higher than our modelled estimate of 23.6% in the pembrolizumab arm, the results of the KEYNOTE-001 study are highly uncertain due to the small number of patients (n = 27) in the trial.
Another pembrolizumab study (KEYNOTE-042)Citation18 in patients with metastatic NSCLC and PD-L1 TPS 1% or more was published after KEYNOTE-024. While KEYNOTE-042 included patients with Chinese ethnicity, we did not use the results from this study in our model as pembrolizumab monotherapy is only used in patients with PD-L1 TPS 50% or more in local clinical practice, in line with the licensed indication in Singapore. Moreover, there is no evidence so far to suggest any difference in efficacy of pembrolizumab based on ethnicity. Hence, KEYNOTE-024, which had a much longer follow-up period than KEYNOTE-042 (25.2 months versus 12.8 months) was considered to be the most appropriate study to inform this cost-effectiveness evaluation.
Most published economic evaluations of pembrolizumab in this population to date use OS curves from the interim analysis of KEYNOTE-024, which had a fairly short follow-up periodCitation7,Citation8,Citation19–22. In this analysis, the median OS for patients receiving pembrolizumab was not reached, therefore, extrapolating the survival benefit with such limited data is associated with significant uncertainty. When we used the OS curves from the updated analysis of KEYNOTE-024 to extrapolate beyond the trial period, the 5-year OS was 23.6% and 8.2% for the pembrolizumab and chemotherapy arms, respectively. If the OS curves from the interim analysis of KEYNOTE-024Citation5 were used, the 5-year OS was higher for the pembrolizumab arm (24.9%) and lower for the chemotherapy arm (5.9%). Hence, using the interim analysis data is likely to have overestimated the incremental benefits of pembrolizumab and underestimated the ICER (SGD22,000 lower) in the published economic evaluations.
Tan et al.Citation12 published a conference abstract of a cost-effectiveness analysis of pembrolizumab monotherapy versus standard chemotherapy in patients with metastatic NSCLC in Singapore. A longer time horizon of 20 years was used in their analysis, compared to 10 years in our model. As pembrolizumab improves overall survival, a longer time horizon would allow the pembrolizumab arm to accumulate more QALYs, and thus lower the resulting ICER. When a 20-year time horizon was applied in the OWSA in our model (), the ICER dropped to SGD150,887 per QALY gained, which was similar to the ICER reported by Tan et al. (SGD155,630 per QALY gained). However, we considered a 20-year time horizon to be overly optimistic given the poor long-term survival observed in advanced non-small cell lung cancer.
There are a number of limitations of our economic model. First, the key clinical and utility inputs were obtained from studies conducted predominantly in Western countries. Asian representation in the studies was low (∼13% in the KEYNOTE-024 studyCitation5 and ∼9% in Chouaid et al.’sCitation11 utility study). However, there have been no clinical data published to suggest that Asian or Caucasian patients have differential responses to anti-PD1/PD-L1 inhibitors when used in the setting of wild type NSCLC with high PD-L1 TPS score. Undoubtedly, there is a higher proportion of EGFR mutant NSCLC in Asian populations and limited efficacy of anti-PD1/PD-L1 inhibitors in this group of patients, but this is not within the scope of our study. Similarly, while country-specific utility values were preferred, such data were not available in Singapore.
Secondly, we extrapolated the survival curves from a median follow-up of 25.2 months to a 10-year time horizon and assumed that the treatment effect of pembrolizumab would continue beyond the treatment period of 2 years used in the KEYNOTE-024 study. This assumption is supported by indirect evidence from other anti-PD1 inhibitor trials such as KEYNOTE-006 in melanoma where a high proportion (up to 86%) of patients who completed 2 years of treatment with pembrolizumab remained in remission after a further 2 years of follow-up. However, if the treatment effect of pembrolizumab reduces over time, our base case ICER would be substantially higher.
Thirdly, the current model assumed a fixed 2-year treatment duration of pembrolizumab. However, the cost-effectiveness of pembrolizumab may worsen in the real world if its use exceeds 2 years as a result of incurring ongoing costs of treatment without a proportional increase in survival benefits beyond 2 years.
Last, a large proportion of patients in the chemotherapy arm in KEYNOTE-024 crossed over to pembrolizumab as second-line therapy upon progression. As patient level data were not available to inform our analysis, crossover adjustment was not performed. However, the results remain generalizable to the local setting given that pembrolizumab is used as a second-line treatment locally, and the proportion of patients who switch to pembrolizumab after chemotherapy in the local setting was similar to that in the KEYNOTE-024 study.
Conclusion
Our study reports the cost-effectiveness of pembrolizumab compared to standard chemotherapy in previously untreated patients with advanced NSCLC and a PD-L1 TPS of 50% or more in Singapore. Due to the high cost of pembrolizumab in the local context, the base case ICER is high at SGD167,692 per QALY gained, suggesting that pembrolizumab may not represent good value for limited healthcare dollars compared with platinum-based chemotherapy in Singapore. These findings will be useful to inform local clinical decision-making and national funding decisions about the use of pembrolizumab for advanced NSCLC.
Transparency
Declaration of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of financial/other relationships
All authors have no relevant financial or other relationships to disclose.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Table_S2.docx
Download MS Word (15.7 KB)Table_S1.docx
Download MS Word (20.6 KB)Figure_S.2.tif
Download TIFF Image (89.9 KB)Figure_S.1.tif
Download TIFF Image (90.2 KB)Acknowledgements
The authors would like to acknowledge Dr Niti Matthew for providing the national case-mix data to inform our analyses.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953.
- Cancer survival in Singapore 1973. 2012. Singapore Cancer Registry. National Registry of Diseases Office; [cited 2020 Feb 29]. Available from: https://www.nrdo.gov.sg/publications/cancer.
- Baas P, Garon EB, Herbst RS, et al. Relationship between level of PD-L1 expression and outcomes in the KEYNOTE-010 study of pembrolizumab vs docetaxel for previously treated, PD-Ll-Positive NSCLC. J Clin Oncol. 2016;34(15_suppl):9015–9015.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639.
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833.
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer With PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–546.
- Huang M, Lou Y, Pellissier J, et al. Cost effectiveness of pembrolizumab vs. standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. Pharmacoeconomics. 2017;35(8):831–844.
- Chouaid C, Bensimon L, Clay E, et al. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer. 2019;127:44–52.
- Woods B, Sideris E, Palmer S, et al. NICE DSU Technical support document 19: partitioned survival analysis for decision modelling in health care: a critical review. 2017; [cited 2020 Feb 29]. Available from: www.nicedsu.org.uk/wp-content/uploads/2017/06/Partitioned-Survival-Analysis-final-report.pdf.
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9
- Chouaid C, Agulnik J, Goker E, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8(8):997–1003.
- Tan PT, Aziz MIA, Pearce F, et al. Cost effectiveness analysis of afatinib versus pemetrexed-cisplatin for first-line treatment of locally advanced or metastatic EGFR mutation positive non-small-cell lung cancer from the Singapore healthcare payer’s perspective. BMC Cancer. 2018;18(1):352
- Huang M, Pellissier J, Liao J. A trial-based euroqol EQ-5D health utility analysis in patients with previously treated advanced NSCLC. Value in Health. 2016;19(7):A744.
- Huang M, Pellissier J, Kong F. A trial-based euroqol EQ-5D health utility analysis in patients with previously untreated metastatic NSCLC. Value in Health. 2017;20(5):A115.
- Pembrolizumab for untreated PD-L1-positive metastatic non-small-cell lung cancer. NICE TA 447. 2017; [cited 2020 Feb 29]. Available from: https://www.nice.org.uk/guidance/ta447.
- Pembrolizumab for untreated PD-L1-positive metastatic non-small-cell lung cancer. NICE TA531; [cited 2020 Feb 29]. Available from: https://www.nice.org.uk/guidance/ta531.
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with pembrolizumab: results from the Phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–2527.
- Mok T, Wu Y, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830.
- Liao W, Huang J, Hutton D, et al. Cost-effectiveness analysis of first-line pembrolizumab treatment for PD-L1 positive, non-small cell lung cancer in China. J Med Econ. 2019;22(4):344–349.
- Hu X, Goldman DP. First-line pembrolizumab in PD-l1 positive non-small cell lung cancer: a cost-effectiveness analysis from a UK healthcare perspective. Value in Health. 2017;20(9):A399.
- Bhadhuri A, Insinga R, Guggisberg P, et al. Cost effectiveness of pembrolizumab vs chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in Switzerland. Swiss Med Wkly. 2019;149:w20170.
- Huang M, Lopes G, Insinga R, et al. Cost-effectiveness of pembrolizumab versus chemotherapy as first-line treatment in PD-L1-positive advanced non-small-cell lung cancer in the USA. Immunotherapy. 2019;11(17):1463–1478.