?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims
reSET-O is a Food and Drug Administration-cleared prescription digital therapeutic (PDT) indicated to improve outpatient-treatment retention of patients with opioid use disorder (OUD). This study examined the cost-effectiveness and budget impact of reSET-O in conjunction with treatment as usual (reSET-O + TAU) relative to TAU.
Materials and methods
Adult patients with ≥1 OUD diagnosis, treated with buprenorphine from 1 January 2015 to 30 March 2018, were identified from Truven Health MarketScan Commercial and Medicare Supplemental Research Databases. Twelve-week healthcare resource utilization (HCRU) costs for patients categorized as adherent and nonadherent to buprenorphine treatment were estimated. Total 12-week costs included OUD treatment and other HCRU costs. The cost-effectiveness of reSET-O + TAU was modeled in accordance with prior clinical trial outcomes. The 12-week budget impact of reSET-O was modeled for a 1 million-member healthcare plan.
Results
Higher buprenorphine adherence was associated with lower HCRU costs in claims data. Twelve-week per-patient total costs were $305 more for those receiving reSET-O + TAU than those receiving TAU. The incremental cost-effectiveness ratio was $18.70 per 1 percentage-point increase in the treatment retention rate. The probability that reSET-O + TAU would be considered cost-effective was over 92% for willingness-to-pay thresholds of $6,000 or more. The 12-week budget impact of reSET-O was $8,908, translating to $0.003 per member per month.
Limitations
The findings of the cost-effectiveness and budget impact modeling are limited by the assumptions of the models due to uncertainty around some inputs. While no model is free of bias, the inputs for this model were carefully selected to reflect contemporary treatment patterns.
Conclusions
Depending on the payer’s willingness to pay, reSET-O may be cost-effective in increasing buprenorphine treatment retention rates. reSET-O results in an approximate budget impact of $0.003 per member per month, depending on market share and the prevalence of the population receiving treatment for OUD.
Introduction
Opioid use disorder (OUD) is a large public health problem, resulting in a substantial economic burdenCitation1–3. The National Survey of Drug Use and Health estimated that 10.3 million individuals in the United States misused opioids in 2018 and 2.0 million had an OUDCitation4. The economic burden of OUD arises from various personal and public costs, including excess healthcare resource utilization (HCRU), lost workplace productivity, criminal activity, and premature mortalityCitation3,Citation5. Altogether, the annual economic cost of OUD has been estimated to be $504 billion (2015 US dollars), the majority of which (∼85%) is due to premature mortalityCitation5.
Pharmacotherapy (buprenorphine, methadone, and naltrexone) is the recommended first-line treatment for OUDCitation6,Citation7. Treatment with pharmacotherapy and counseling services can reduce overall healthcare costs for patients with OUDCitation8–10. For example, Lynch et al. found that patients who received buprenorphine maintenance therapy and addiction counseling had lower annual healthcare costs ($17,462 adjusted to 2019 US dollars) than patients who received little to no additional treatment ($39,912 adjusted to 2019 US dollars)Citation10,Citation11. In addition, several studies of patients undergoing treatment with buprenorphine have shown that greater treatment adherence is associated with lower healthcare costsCitation12–14. The lower overall healthcare costs associated with treatment have been found to result from reduced use of high-cost medical services, such as inpatient care, emergency departments, and certain other outpatient services, which often more than offset the cost of pharmacy services and outpatient office visits associated with OUD treatmentCitation8,Citation12–14 .Research indicates that most individuals need at least 3 months in treatment to significantly reduce or stop their drug use and that the best outcomes occur with longer durations of treatmentCitation6.
A relatively new approach to the treatment of OUD is the use of prescription digital therapeutics (PDTs) to deliver cognitive-behavioral interventions such as the community reinforcement approach (CRA) and contingency management (CM) on mobile devices. With PDTs, behavioral interventions may be delivered via learning modules consisting of text, audio, and graphics designed to teach and reinforce skills and behaviors important for managing OUD.
The reSET-O therapeutic is an FDA-cleared, 84-day PDTCitation15 in use since January 2019 and indicated for improving retention in outpatient therapy for patients with OUDCitation16,Citation17. reSET-O delivers neurobehavioral therapy based on the CRA, an intensive form of cognitive-behavioral therapy (CBT) validated for OUD. CRA is delivered in reSET-O as a series of 67 on-demand audio and video lessons, each lasting between 10 and 20 min, which are sequentially unlocked as patients progress through the programCitation16. Fluency training immediately follows lessons in the form of a simple quiz designed to reinforce retention and understanding of the specific coping skills and positive adaptive behaviors covered during each lesson. CM follows each successful completion of a lesson/fluency training session dyad to immediately recognize and reward patients’ engagement with therapy. Patients earn rewards (merit badges or gift cards of modest value) by spinning a virtual rewards wheel within reSET and reSET-O for up to four lessons completed within a week, and for each negative drug urine screen loggedCitation17.
We modeled the cost-effectiveness and budget impact of reSET-O in conjunction with treatment as usual (reSET-O + TAU), relative to TAU (buprenorphine treatment, counseling services, and urinalysis) alone, from a third-party payer perspective. The model combined “real-world” HCRU costs of patients with OUD who were treated with buprenorphine, and efficacy data from Christensen et al., which found that patients treated with the digital precursor for reSET-O, the Therapeutic Education System (TES), in conjunction with buprenorphine had higher treatment retention than patients treated with buprenorphine and CM without TES (80.4% versus 64.1%; hazard ratio of treatment dropout: 2.21, p = .013)Citation18.
Methods
Real-world HCRU and costs
A retrospective study using administrative claims data from 1 January 2015 to 30 March 2018, in Truven Health MarketScan Commercial and Medicare Supplemental Research Databases was conducted. Data included enrollment information, demographics, inpatient and outpatient medical claims, and outpatient pharmacy claims. Commercial data represented individuals <65 years of age (primary insured, spouse, or dependent). Medicare data represented the healthcare experience of retirees with Medicare supplemental insurance paid by employers. This study does not meet the definition of human subjects research, because the data were pre-existing, obtained without intervention or interaction with the individuals, and contained no individually identifying information.
Adult patients with ≥1 OUD diagnosis (International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification diagnosis codes 304.X [ICD-9] and F11.X [ICD-10]) and treated with buprenorphine (buprenorphine mono tablet or buprenorphine/naloxone combination sublingual tablet [4:1 ratio]) were identified. Patients were required to have initiated buprenorphine, measured as having filled at least one prescription of buprenorphine, to be included in the study sample. The first diagnosis of OUD was defined as the index date, and the follow-up period was defined as the 12 weeks post-index date. Patients were required to have continuous enrollment during the follow-up period. The 12-week time horizon was selected because it aligns with both the reSET-O treatment period from the Christensen et al.Citation18 clinical trial, and the finding that the minimum duration of treatment needed to significantly reduce or stop drug use in most individuals with OUD is 3 monthsCitation6.
Adherence to treatment was defined as proportion of days covered by buprenorphine across the 12 weeks. Patients with a proportion of days covered ≥0.8 and <0.8 were categorized as adherent and nonadherent, respectivelyCitation13,Citation19. All-cause medical (e.g. inpatient and outpatient) and pharmacy costs were captured from patients between the ages of 18 and 64, and ≥65 years. Costs were measured as the combined expenditures of the health plan and patient, based on administrative claims. All costs were adjusted to 2019 US dollars using the annual medical care service component of the Consumer Price IndexCitation11. Average per-patient HCRU costs for the adherent and nonadherent patients were estimated for use in the model.
All study variables were analyzed descriptively. Wilcoxon rank sum tests were used to determine differences in costs among adherence groups, and 95% confidence intervals (95% CIs) were reported. A two-tailed a priori alpha level of 0.05 was used. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Economic modeling inputs
Effectiveness
The effectiveness of reSET-O + TAU and TAU was defined by the rates of treatment retention observed in Christensen et al.Citation18 Participants in the clinical trial met the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, criteria for opioid dependence and met the FDA qualification criteria for buprenorphine treatment. Treatment retention rate was defined as the proportion of participants completing the 12-week intervention. As noted above, 80.4% of participants in the reSET-O + TAU arm and 64.1% of those in the TAU arm were retained at the end of the trialCitation18. In the Christensen et al. studyCitation18, TES was delivered in the clinic, using a clinic computer, and counselors were aware of participation. We assumed that reSET-O would be as effective as the clinic-based PDT, given that reSET-O includes a case manager who engages directly with the patient to support onboarding with the PDT and provides assistance to the patient throughout therapy.
Treatment costs
The 12-week per-patient treatment cost inputs for reSET-O + TAU and TAU were calculated by multiplying the number of healthcare resource units associated with a patient who fully adheres to the recommended treatment protocol over 12 weeksCitation16,Citation18, by the respective unit costs adjusted to 2019 US dollarsCitation11,Citation20,Citation21. Thus, the estimated 12-week treatment cost of TAU = 6 office visits×$33/visit + 36 urinalyses×$70/urinalysis + 3 months of buprenorphine×$123/month=$3,086. Treatment costs for reSET-O + TAU included the costs of TAU and an additional one-time $1,500 for the reSET-O license, which comes to $4,586 for 12 weeks.
Cost-effectiveness model
The incremental cost-effectiveness ratio (ICER) of reSET-O + TAU versus TAU was calculated as:
where
and
are 12-week, per-patient treatment costs calculated above, and
and
are weighted averages of adherent and nonadherent per-patient HCRU costs from the claims analysis above, calculated as:
Probabilistic sensitivity analysis was performed to estimate the probability that reSET-O + TAU would be considered cost-effective at various willingness-to-pay thresholds. The probabilistic values for each input parameter to the cost-effectiveness model were represented as gamma or beta distributions. The low and high values associated with the point estimate of each input parameter were ±10% of the point estimates, except in the case of the HCRU costs, for which the 95% CI of the point estimates reported from the MarketScan database analysis were used. A Monte Carlo simulation was performed with 1,000 iterations by sampling the input parameters from the distributions and calculating an ICER for each iteration. Based on the simulation, cost-effectiveness acceptability curves were created to show the percentage of ICERs that fell below the selected willingness-to-pay thresholds.
Budget impact model
The 12-week incremental total cost of including reSET-O in the market was also modeled. Incremental total costs were calculated as the difference in costs between a hypothetical “current market” in which TAU has 100% market share and a hypothetical “new market” in which reSET-O + TAU has a range of market share uptake from TAU. Costs within each market were determined by the treatment costs and HCRU costs, the number of individuals in the target population, and the proportion of the market held by each treatment.
The target population was defined as members in the healthcare plan with an OUD diagnosis treated with buprenorphine in an outpatient setting, which would be the population eligible for reSET-O treatment. A hypothetical healthcare plan with 1,000,000 members was assumed; the prevalence of OUD and the proportion of patients receiving buprenorphine treatment at the plan level were derived from the MarketScan analysis.
For each hypothetical market, per-patient treatment costs and HCRU costs were summed for reSET-O + TAU and TAU, separately. The costs associated with each treatment were then multiplied by the number of members in the target population and proportion of the market share held by the treatment. A range from 10% to 50% market share uptake was used for reSET-O + TAU in the budget impact model. The incremental total cost over 3 months (12 weeks), incremental cost per treated member per month, and incremental cost per member per month of reSET-O + TAU were reported as the budget impact. A one-way sensitivity analysis was conducted to test the impact of model inputs and study assumptions. Inputs such as cost of reSET-O, rate of hospitalization, and rate of emergency department visits were varied over a range of estimates (±25%) to test model robustness.
The cost of CM was not included in the comparator arm in this analysis. Although CM is a powerful component of substance and OUD treatment, with the highest effect size compared to other neurobehavioral treatmentsCitation22–24, it remains underutilized in real-world treatment-as-usual, and is wholly contained and algorithmically managed within reSET-O. A scenario analysis including the cost of CM in the TAU arm was conducted to determine the amount that could be spent on CM before reSET-O would become cost-saving compared to pharmacotherapy + CM alone.
Results
Real-world HCRU and costs
The number of adult patients with an OUD diagnosis identified in MarketScan from 1 January 2015 to 30 March 2018 was 81,539, of whom 76,350 remained after applying the 12-week continuous enrollment requirement. The final requirement of evidence of treatment with buprenorphine resulted in a final cohort of 9,850 patients for inclusion in the analysis.
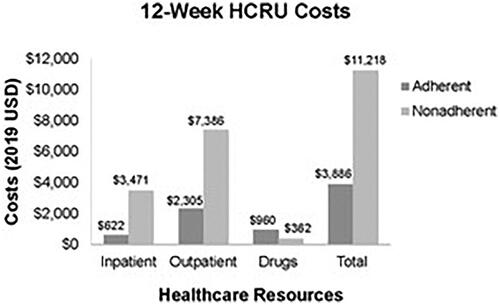
Overall, 65.31% of patients treated with buprenorphine were identified as adherent according to the proportion-of-days-covered threshold of 0.8Citation13,Citation19, 34.69% were identified as nonadherent. Average 12-week HCRU costs for adherent and nonadherent patients are shown in . The largest difference was seen in outpatient costs, which were $7,386 (95% CI: $7,176, $7,803) for nonadherent patients versus $2,305 (95% CI: $1,921, $2,573) for adherent patients (p< .001). The inpatient costs for nonadherent patients ($3,471, 95% CI: $2,942, $3,878) were also higher than for those adherent to treatment ($622, 95% CI: $617, $675, p< .001). On average, HCRU costs were $7,332 more for nonadherent patients than for adherent patients (p< .001).
Model treatment and HCRU costs
The weighted-average 12-week per-patient HCRUreSET-O + TAU costs and HCRUTAU costs were $5,323 and $6,519, respectively. The combined 12-week per-patient treatment and HCRU costs for reSET-O + TAU and TAU were $9,910 and $9,605, respectively.
Cost-effectiveness model
The ICER point estimate of reSET-O + TAU versus TAU was $18.70 per 1 percentage-point increase in the treatment retention rate. The probabilistic sensitivity analysis demonstrated the certainty that reSET-O + TAU would be considered cost-effective versus TAU across a range of willingness-to-pay thresholds (, ). At a willingness-to-pay threshold of $2,000 per 1 percentage-point increase in buprenorphine treatment retention, the probability that reSET-O + TAU would be considered cost-effective was 49.4%; it increased substantially to 92.2% at a willingness-to-pay threshold of $6,000.
Figure 2. Cost-effectiveness acceptability. Lines labeled reSET-O + TAU and TAU indicate the probability of a payer considering the incremental cost-effectiveness ratio of reSET-O in conjunction with treatment as usual (reSET-O + TAU) acceptable or unacceptable, respectively, as a function of the willingness-to-pay (WTP) threshold per 1 percentage-point increase in the buprenorphine treatment retention rate.
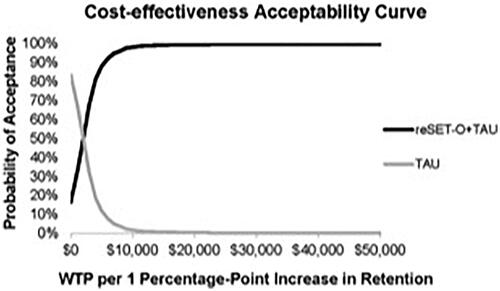
Table 1. Specifics of the input parameters used for the probabilistic sensitivity analysis.
Budget impact model
The population cohort built for the budget impact model is shown in . Of the 1 million members, 69.8% were 18–64 years of age and 7.3% were ≥65 years of age. Among these two groups, 0.15% and 0.03% were diagnosed with OUD, respectively. Of those diagnosed with OUD, the percentage seeking outpatient treatment was 36.1% in those 18–64 years of age compared to 56.2% in those ≥65 years of age. Among these two groups, the percentage of those treated with buprenorphine was 28.5% and 10.39%, respectively. Based on these values, the target population consisted of 109 members.
Table 2. Percentage of patients in the MarketScan databases meeting specified criteria.
shows the 12-week budget impact of reSET-O with a range of market-share uptake. Total costs for the current market (reSET-O not available) were $1,047,811 while total costs for the new market (with reSET-O available) ranged from $1,051,374 at 10% market share to $1,065,626 at 50% market share. The incremental total cost at 25% market share was $8,908, indicating that including reSET-O + TAU in the formulary would result in an additional $8,908 over 12 weeks. The incremental cost per treated member per month at 25% market share was $27.24 and the incremental cost per member per month was $0.003. The incremental cost per member per month ranged from $0.0012 at 10% market share to $0.006 at 50% market share. According to the one-way sensitivity analysis, the most influential variables affecting the incremental total cost were the cost of reSET-O and hospitalization costs.
Table 3. Healthcare costs (in 2019 US dollars) associated with reSET-O + TAU and TAU for hypothetical markets in which reSET-O + TAU takes 0%, 10%, 30%, and 50% of the market share of TAU.
Scenario analysis of inclusion of CM cost in TAU alone arm
The scenario analysis of the 12-week budget impact of including a cost of CM in the TAU arm indicated reSET-O would become cost-saving upon the cost of CM reaching $305 per-patient per 12 weeks, compared to pharmacotherapy + CM alone.
Discussion
Previous studies have shown that TES, which is the precursor of reSET-O, improves abstinence and treatment retention for patients with OUDCitation18,Citation25. This study modeled the cost-effectiveness and budget impact of reSET-O + TAU versus TAU over 12 weeks, from a third-party payer perspective. The results demonstrate a minimal increase in average total costs per percentage-point increase in buprenorphine retention, and in the overall budget associated with including reSET-O in treatment of patients with OUD.
After weighting the HCRU costs based on retention rates found by Christensen et al.Citation18, per-patient HCRU costs were lower with reSET-O + TAU ($5,323) than with TAU ($6,519), which partially offset the reSET-O fee. The ICER of reSET-O + TAU versus TAU was $18.70 per 1 percentage-point increase in retention, or $187 per 10 percentage-point increase in retention. Probabilistic sensitivity analysis indicated that reSET-O + TAU would be considered cost-effective with a probability of 92.2% or higher for payers with willingness-to-pay thresholds of $6,000 or more per 1 percentage-point increase in the buprenorphine treatment retention rate (). The budget impact model indicated that reSET-O would result in a 12-week budget impact of less than one-tenth of a penny to slightly more than half of a penny per-member per-month (), depending on the market share of reSET-O, the higher cost occurring at a market share as high as 50%. The economic models of the present study included only direct costs of third-party payers, and it is likely that other public costs such as criminal activity and lost productivity would also be impacted by increasing treatment adherence through PDTs.
The scenario analysis of the 12-week budget impact of including a cost of CM in the TAU arm indicated that reSET-O would become cost-saving compared to pharmacotherapy + CM alone at a CM cost of $305 per-patient per 12 weeks. This tipping point in the budget impact is of relevance to healthcare providers and payers, as the literature shows that the cost of implementing and maintaining a CM program over 12 weeks ranges between $300 and $1,200 per patientCitation22.
The findings suggest that, compared to OUD pharmacotherapy alone, the provision of reSET-O in conjunction with pharmacotherapy may be cost-effective for third-party payers, and would have a minimal budget impact, or could even be cost-saving. Generally accepted willingness-to-pay thresholds for increases in buprenorphine treatment retention do not exist, thereby limiting our interpretation of the cost-effectiveness of reSET-O. However, when the measure of effectiveness is quality-adjusted life year, the willingness-to-pay threshold has been suggested to be $100,000–$200,000 in the United StatesCitation26. Studies have shown that treatment of OUD is associated with higher quality of lifeCitation27–29, although the relationship between treatment adherence and quality-adjusted life years has not been fully defined.
The real-world analysis based on MarketScan claims showed that 65.3% of patients with OUD treated with buprenorphine were adherent to buprenorphine treatment. This value is similar to the 64.1% treatment retention found by Christensen et al. for patients with opioid dependence receiving treatment without TES, and supports the assumption of 64.1% adherence to buprenorphine for patients receiving TAU in the modelingCitation18. The real-world analysis also showed that patients who were adherent to buprenorphine had lower HCRU costs than patients who were nonadherent due to lower inpatient and outpatient service costs (), which is consistent with findings of previous studiesCitation12–14.
The findings of the cost-effectiveness and budget impact modeling are limited by the assumptions of the models. One assumption was that treatment retention as defined in the trial by Christensen et al. is comparable to our measure of treatment adherenceCitation18. Adherence in this study was determined by proportion of days covered by buprenorphine, while treatment retention was defined by Christensen et al. as whether patients completed all clinic visits. An additional assumption was that the treatment retention rate observed by Christensen et al. for patients treated with the precursor of reSET-O (80.4%) would translate to the sample of patients observed in the real-world analysis of this studyCitation18. It should be noted that the population studied by Christensen et al. included long-term, active opioid users with an average of approximately 5 years of opioid use and a 40–50% average rate of prior OUD treatment experienceCitation18. With regard to OUD treatment costs, an assumption was that all reSET-O + TAU and TAU patients remained in treatment for the full 12 weeks.
The budget impact model included assumptions about the proportion of market share held by reSET-O + TAU and TAU. One specific assumption was that reSET-O + TAU would replace a portion of the market share held by TAU and would not increase the proportion of patients treated for OUD. One consideration for this assumption is that it has been suggested that PDTs may increase the reach of patient careCitation30,Citation31. Increased reach of patient care may be due to the convenience of PDTs and the fact that PDTs increase the variety of treatment options available to patients. Possibly, reSET-O could influence the number of patients treated for OUD by adding a novel treatment option to the market, which could affect the budget impact of reSET-O. However, increased access to treatment has the potential for greater cost savings, because more patients would be impacted by a potentially beneficial intervention, as has been shown in recent population-based models for New EnglandCitation32. Additionally, downstream and long-term economic benefits to payers are expected from reSET-O, which may more than offset the additional cost found in this short-term analysisCitation5. These potential additional economic benefits should be addressed in future research.
The 12-week time horizon of the model is another limitation. Research indicates that most individuals with OUD need at least 3 months in treatment to significantly reduce or stop their drug use, however, the best outcomes occur with even longer durations of treatmentCitation6. Additional comparative effectiveness data evaluating the longer-term impact of reSET-O will be important to confirm its impact. Current long-term follow-up studies are underway to collect real-world utilization and outcomes of patients with an initial prescription of reSET-O as the treatment is taken up in clinical settings of care. Another limitation of this study is the use of data from administrative claims. Administrative claims data are collected for healthcare goods and services reimbursement and may have clerical inaccuracies, recording bias due to financial incentives, changes in billing codes, and a lack of clinically-relevant variablesCitation33,Citation34. Also, as this dataset is for patients in US health plans, the findings may not be generalizable to patients in other countries, or US patients with other types of healthcare coverage.
The incremental effectiveness data for reSET-O + TAU versus TAU in this study were derived from findings from a clinical trial, whereas the healthcare resource use and cost data were derived from an observational “real-world” database. RCTs are the gold standard for assessing the efficacy of therapeutic interventions, as they provide a high degree of internal validityCitation35,Citation36. However, RCTs can be inadequate for addressing questions about real-world resource utilization and costsCitation35,Citation36. The use of high-quality “real-world” observational data has advantages in economic evaluations as it better reflects real-world conditions, a central concept of comparative effectiveness researchCitation37.
Finally, in the Christensen et al.Citation18 study, TES was delivered in the clinic using a clinic computer and involved counselors being aware of participation. We assume that reSET-O would be similarly effective, given that it includes a case manager who engages directly with the patient to support onboarding with the PDT, and provide assistance throughout therapy.
Conclusions
Based on the economic modeling of this study, reSET-O is expected to increase buprenorphine treatment retention rates among persons with OUD at a small cost to third-party payers. Compared to the provision of pharmacotherapy alone for patients with OUD, reSET-O + pharmacotherapy may be cost-effective, and potentially cost-saving, in increasing buprenorphine treatment retention rates.
Transparency
Declaration of funding
This study was funded by Sandoz, a Novartis Division.
Declaration of financial/other relationships
WW and NGL are employees of Sandoz, a Novartis Division, and were compensated to perform the research. SMM and AJ were not compensated to perform the research or for their roles as authors. SMM consulted for Sandoz on the development of the economic models. No author received an honorarium related to the development of this manuscript.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
All authors were involved with conception, study design, interpretation of the data, writing the manuscript, and the final approval of the version to be published.
Previous presentations
This study was presented as a poster presentation at the Academy of Managed Care Pharmacy (AMCP) 2020 eMeeting from 21–24 April 2020. The abstract is titled “Budget Impact Analysis of reSET-O, an FDA-Cleared Prescription Digital Therapeutic for Patients with Opioid Use Disorder.”
Acknowledgements
Editorial assistance was provided by IMPRINT Science, New York, NY, and was supported by Sandoz, a Novartis Division. The authors were fully responsible for the content, editorial decisions, and opinions expressed in the current article.
Data availability statement
The data that support the findings of this study are available from Truven Health MarketScan but restrictions apply to the availability of these data, which were used under license for this study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of IBM Truven Health MarketScan.
References
- Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999–2015. NCHS data brief, no 273. Hyattsville (MD): National Center for Health Statistics; 2017.
- Han B, Compton WM, Jones CM, et al. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA. 2015;14(14):1468–1478.
- Florence CS, Zhou C, Luo F, et al. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901–906.
- Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 national survey on drug use an health. 2019. [2019 Nov 20]. Available from: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
- The Council of Economic Advisers. The underestimated cost of the opioid crisis. 2017. Available from: https://www.whitehouse.gov/briefings-statements/cea-report-underestimated-cost-opioid-crisis/
- U.S. Department of Health & Human Services. Facing addiction in America - the surgeon general’s spotlight on opioids. In: Services USDoHH, editor. Washington (DC): U.S. Department of Health & Human Services; 2018.
- Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
- Murphy SM, Polsky D. Economic evaluations of opioid use disorder interventions. Pharmacoeconomics. 2016;34(9):863–887.
- Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(8):S235–S248.
- Lynch FL, McCarty D, Mertens J, et al. Costs of care for persons with opioid dependence in commercial integrated health systems. Addict Sci Clin Pract. 2014;9(1):16.
- Bureau of Labor Statistics. CPI - all urban consumers - U.S. medical care services. 2019. [2019 Oct 22]. Available from: http://data.bls.gov/cgi-bin/surveymost?su
- Ronquest NA, Willson TM, Montejano LB, et al. Relationship between buprenorphine adherence and relapse, health care utilization and costs in privately and publicly insured patients with opioid use disorder. Subst Abuse Rehabil. 2018;9:59–78.
- Ruetsch C, Tkacz J, Nadipelli VR, et al. Heterogeneity of nonadherent buprenorphine patients: subgroup characteristics and outcomes. Am J Manag Care. 2017;23(6):e172–e179.
- Tkacz J, Volpicelli J, Un H, et al. Relationship between buprenorphine adherence and health service utilization and costs among opioid dependent patients. J Subst Abuse Treat. 2014;46(4):456–462.
- AMCP Partnership Forum. Digital therapeutics-what are they and where do they fit in pharmacy and medical benefits? J Manag Care Spec Pharm. 2020;26(5):674–681.
- Pear Therapeutics I. reSET-O [clinician directions for use]. 2019.
- FDA clears mobile medical app to help those with opioid use disorder stay in recovery programs [Internet]. 2018. [2018 Sep 10]. Available from: https://www.fda.gov/news-events/press-announcements/fda-clears-mobile-medical-app-help-those-opioid-use-disorder-stay-recovery-programs
- Christensen DR, Landes RD, Jackson L, et al. Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 2014;82(6):964–972.
- Karve S, Cleves MA, Helm M, et al. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303–2310.
- McCollister K, Yang X, Sayed B, et al. Monetary conversion factors for economic evaluations of substance use disorders. J Subst Abuse Treat. 2017;81:25–34.
- Red Book Product Details. [Internet]. 2019. [cited 2019 Oct 2]. Available from: https://www.micromedexsolutions.com/
- Petry NM, Alessi SM, Olmstead TA, et al. Contingency management treatment for substance use disorders: how far has it come, and where does it need to go? Psychol Addict Behav. 2017;31(8):897–906.
- Ainscough TS, McNeill A, Strang J, et al. Contingency Management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318–339.
- Rash CJ, DePhilippis D. Considerations for implementing contingency management in substance abuse treatment clinics: the Veterans affairs initiative as a model. Perspect Behav Sci. 2019;42(3):479–499.
- Bickel WK, Marsch LA, Buchhalter AR, et al. Computerized behavior therapy for opioid-dependent outpatients: a randomized controlled trial. Exp Clin Psychopharmacol. 2008;16(2):132–143.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness-the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797.
- Chang KC, Lee KY, Lu TH, et al. Opioid agonist treatment reduces losses in quality of life and quality-adjusted life expectancy in heroin users: evidence from real world data. Drug Alcohol Depend. 2019;201:197–204.
- Teesson M, Havard A, Ross J, et al. Outcomes after detoxification for heroin dependence: findings from the Australian Treatment Outcome Study (ATOS). Drug Alcohol Rev. 2006;25(3):241–247.
- Williamson A, Darke S, Ross J, et al. Changes and predictors of change in the physical health status of heroin users over 24 months. Addiction. 2009;104(3):465–470.
- Marsch LA, Guarino H, Acosta M, et al. Web-based behavioral treatment for substance use disorders as a partial replacement of standard methadone maintenance treatment. J Subst Abuse Treat. 2014;46(1):43–51.
- Kim SJ, Marsch LA, Guarino H, et al. Predictors of outcome from computer-based treatment for substance use disorders: results from a randomized clinical trial. Drug Alcohol Depend. 2015;157:174–178.
- Institute for Clinical and Economic Review. Management of patients with opioid dependence: a review of clinical, delivery system, and policy options. 2014. Available from: https://icer-review.org/wp-content/uploads/2016/01/CEPAC-Opioid-Dependence-Final-Report-For-Posting-July-211.pdf
- Patel AA, Singh K, Nunley RM, et al. Administrative databases in orthopaedic research: pearls and pitfalls of big data. J Am Acad Orthop Surg. 2016;24(3):172–179.
- Bohl DD, Singh K, Grauer JN. Nationwide databases in orthopaedic surgery research. J Am Acad Orthop Surg. 2016;24(10):673–682.
- Dreyer NA, Schneeweiss S, McNeil BJ, et al. GRACE principles: recognizing high-quality observational studies of comparative effectiveness. Am J Manag Care. 2010;16(6):467–471.
- Schneeweiss S, Gagne JJ, Glynn RJ, et al. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther. 2011;90(6):777–790.
- Chang TI, Winkelmayer WC. Comparative effectiveness research: what is it and why do we need it in nephrology? Nephrol Dial Transplant. 2012;27(6):2156–2161.