Abstract
Aims
To estimate the budget impact of adding capmatinib, the first FDA approved MET inhibitor, to a US commercial or Medicare health plan for patients with metastatic non-small cell lung cancer (mNSCLC) whose tumors have a mutation that leads to MET exon 14 (METex14) skipping.
Methods
Target population size was estimated using published epidemiology data. Clinical data were obtained from the GEOMETRY mono-1 capmatinib trial and published trials. Treatments in the market mix included crizotinib, pembrolizumab, ramucirumab, and chemotherapy. Uptake of capmatinib and testing rates were based on market research. All costs (drug acquisition and administration, pre-progression, progression, terminal care, adverse event, and testing) were estimated based on public sources (2020 USD).
Results
The number of patients eligible for capmatinib in the first three years was estimated to be 2–3 in a hypothetical 1 million member commercial plan and 34–44 in a hypothetical 1 million member Medicare plan each year. The estimated total budget impact ranged from $9,695 to $67,725 for a commercial plan and $141,350 to $985,695 for Medicare. With capmatinib included, a marginal per member per month budget impact was estimated (commercial: $0.0008 to $0.0056; Medicare: $0.0118 to $0.0821). Capmatinib inclusion resulted in lower medical costs (commercial: –$0.0003 to –$0.0007; Medicare: –$0.0037 to –$0.0106), partially offsetting increased drug costs ($0.0011 to $0.0064; $0.0154 to $0.0928, respectively), and were primarily driven by reductions in progression and terminal care costs (–$0.0003 to –$0.0009; –$0.0037 to –$0.0125, respectively). The results were most sensitive to capmatinib market share, capmatinib price, and treatment duration.
Limitations
Certain assumptions were applied to the model to account for inputs with limited evidence.
Conclusions
The estimated budget impact of including capmatinib for mNSCLC with a METex14 skipping mutation is minimal, and the increased drug costs were partially offset by savings in AEs, and progression-related and terminal care costs.
Introduction
Lung cancer is the leading cause of cancer deaths worldwide and is estimated to have caused over 135,000 deaths in the United States (US) in 2020Citation1. Lung cancer is most commonly diagnosed in people aged 65 years or older, with the average age of diagnosis approximately 70 yearsCitation2. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 85% of all casesCitation3. Mutations that lead to mesenchymal–epithelial transition exon 14 (METex14) skipping occur in 3–4% of NSCLC adenocarcinomas, 2% of squamous cell carcinomas, and 1–8% of other subtypes of lung cancerCitation4–7. METex14 skipping mutations occur more often in older patients (median: 73 years) and are usually mutually exclusive with other frequently occurring driver mutations in lung cancerCitation6.
Approximately, 4,000–5,000 people per year in the US are diagnosed with metastatic NSCLC (mNSCLC) with METex14 skipping mutationsCitation8,Citation9, and these patients face a poor prognosis due to the high rates of brain, bone, and liver metastases which contribute to worse outcomes compared to patients without the mutationCitation10,Citation11. Patients also tend to be older, making the use of standard treatments with chemotherapy and immunotherapies challenging. Thus, there is an urgent need for innovative and effective new therapies for this patient population. Until recently, there were no approved targeted therapies for patients with METex14 mutated mNSCLC. Capmatinib, an oral kinase inhibitor, was the first MET inhibitor approved by the US Food and Drug Administration (FDA) in May 2020 for the treatment of adults with mNSCLC whose tumors have a mutation that leads to METex14 skippingCitation12. The approval was supported by data from the Phase II GEOMETRY mono-1 trial, which evaluated the safety and efficacy of capmatinib in adult patients with mNSCLC with a METex14 skipping mutationCitation12,Citation13. In GEOMETRY mono-1, the objective response rate (ORR) determined by a blinded independent review committee (BIRC) was reported as 68% (95% confidence interval (CI): 48–84%) in treatment-naïve patients and 41% (95% CI: 29–53%) in previously treated patientsCitation12,Citation13.
Prior to the entry of capmatinib in the US market, there was no MET inhibitor approved for patients with mNSCLC with METex14 skipping mutations. These patients could receive crizotinib (off-label use) or standard of care treatments similar to patients without a driver mutation, such as pembrolizumab monotherapy, pembrolizumab in combination with chemotherapy, or platinum doublet chemotherapyCitation14. The most recent National Comprehensive Cancer Network (NCCN) guideline for mNSCLC noted patients with METex14 skipping mutations has a modest response to immunotherapy, even those with high PD-L1 levels; after the FDA approval, capmatinib has been recommended as a category 2A therapy option (preferred) for patients with METex14 skipping mutations, while crizotinib and other systemic therapy options are designated as useful in certain circumstancesCitation15. Therefore, information regarding the expected budget impact of capmatinib is needed to guide payer decisions regarding coverage and formulary placement in light of resource constraints. To address this question, the objective of this study was to estimate the budget impact of adding capmatinib for adults with mNSCLC whose tumors have a mutation that leads to METex14 skipping to a US commercial or Medicare health plan.
Methods
Model overview
An economic model was developed to estimate the budget impact over the first 3 years (from 2020 to 2022) with capmatinib market entry from commercial and Medicare payer perspectives (). The annual numbers of new patients eligible to receive capmatinib were estimated based on published epidemiological studies, market research, and assumptions. Eligible patients entered the model with progression-free disease and could experience treatment discontinuation, disease progression, or death over the 3-year time horizon. The total budget impact and per member per month (PMPM) budget impact were estimated as the cost difference between the scenarios with and without capmatinib market entry. All costs were in 2020 US dollars based on the US Census Bureau Consumer Price IndexCitation16.
The conduct and reporting of this budget impact analysis (BIA) is consistent with the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) best practice methods standardCitation17,Citation18.
Model inputs and calculations
Target population
The population of interest, as indicated in the FDA label, composed of treatment-naïve and previously treated adult patients with mNSCLC with METex14 skipping mutations. The model started with a hypothetical plan population of 1 million enrollees and used published epidemiological data to define the population eligible for capmatinib (). Other than distinguishing insurance type based on age and proportion of patients with lung cancer, the published epidemiological data used to estimate the target population (i.e. proportion receiving testing for METex14 skipping mutation and proportion with the mutation) were assumed to be the same in both the commercial and Medicare populations. All epidemiological data used to estimate the patient population were assumed to remain constant across the 3-year time horizon except for the proportion tested for METex14 skipping mutations and the proportion split between treatment-naïve and previously treated patients.
Table 1. Target population inputs.
Market share
The treatment options included in the model and market shares considered for both scenarios are presented in. The market share inputs were estimated based on market research data (data on file, Novartis) along with expert input from clinical practice experience of treating NSCLC with METex14 skipping mutation. Capmatinib market share uptake in the first three years, regardless of insurance status, was expected to be 11.1% (2020), 37.5% (2021), and 41.8% (2022) in the treatment-naïve population, and 9.7% (2020), 33.8% (2021), and 37.4% (2022) in the previously treated population. The proportions of patients receiving each treatment were estimated in each year in both scenarios, with and without capmatinib, and in the treatment-naïve and previously treated populations separately due to different treatment options in each group.
Table 2. Market share assumptions used in the model.
Drug and drug administration costs
The unit costs for drugs were obtained from national reference sources including the IBM Micromedex RedBookCitation23 and 2020 data from the Centers for Medicare & Medicaid Services (CMS)Citation24. Drug administration unit costs were obtained from the 2020 CMS Physician Fee ScheduleCitation25 (Supplementary Table 1).
The drug cost for oral capmatinib was $17,950 per 28-day supplyCitation23. All drug costs were based on the RedBook wholesale acquisition cost (WAC)Citation23, except for intravenous therapies in the Medicare setting, which were obtained from the 2020 CMS Average Sales Price Drug Pricing fileCitation24. Daily dosing schedules were based on the FDA prescribing information for each treatment. Drug units per week were determined using a body surface area based on adults with mNSCLC in the US Flatiron database (1.84 m2)Citation26 and an estimated glomerular filtration rate of 87.4 mL/min/1.73 m3 from a general population of US adultsCitation27.
Where there were optional treatment components within combination therapies, the proportion of patients using each component was obtained from clinical trials and, where unavailable, an equal split was assumed. Relative dose intensities were incorporated to account for the possibility that patients may not take the full planned doses due to dose interruptions, reductions associated with adverse events (AEs), or non-compliance, and were based on relevant clinical trials, the FDA labels, and assumptions. Relative dose intensities were applied to all drugs except for capmatinib and crizotinib because both drugs have a fixed price for different pill strengths. While the GEOMETRY trial reported the primary reason for discontinuing treatment (disease progression), the other comparators did not report this level of detail in the literature. Therefore, we assumed a constant rate of treatment discontinuation to ensure consistency across the assumptions made for different treatments.
An area under the curve approach was employed to estimate restricted mean time on treatment in the first three years using median treatment duration. The model assumed a constant treatment discontinuation rate. When the median treatment duration was not available in the published literature, median progression-free survival (PFS) was used as a proxy. Monthly drug acquisition costs and time on treatment for each treatment are presented in Supplementary Table 2.
Medical costs
The medical costs associated with pre-progression, AE management, progression, and terminal care were included in the model. Services and frequencies for pre-progression monitoring (inpatient bed days, outpatient visits, complete blood counts, liver and renal function tests, and computed tomography and magnetic resonance imaging scans) were determined or assumed based on the 2019 NCCN NSCLC guidelinesCitation14, published literature, and the FDA prescribing information for each treatment (full details are listed in Supplementary Table 3). Unit costs for monitoring services were obtained from the CMS Clinical Laboratory Fee ScheduleCitation28 and the CMS Physician Fee ScheduleCitation25.
Grade 3/4 AEs reported in at least 5% of patients for at least one of the comparator treatments were assumed to incur one-time hospitalization costs. The rates of AEs were obtained from clinical trials, and the unit costs for individual AEs were obtained from the Healthcare Cost and Utilization Project national inpatient sampleCitation29.
Patients were assumed to incur monthly progression-related costs following disease progression. Monthly costs associated with progression (hospitalizations, subsequent treatment costs after progression, procedures, infused supportive care, and physician office visits) were estimated to be $19,274 (2016 USD) based on Skinner et al., and were inflated to $21,289 (2020 USD)Citation30. Annual progression-related costs for each treatment were calculated using the difference between restricted mean overall survival (OS) and restricted mean PFS in each year to determine the time spent in a post-progression state. The mean survival times were estimated from median estimates for PFS and OS reported in trials (Supplementary Table 2)Citation13,Citation31.
Terminal care costs were assumed to be incurred as one-time costs of $8,805 (2013 USD) before death, based on Bittoni et al., and inflated to $10,607 (2020 USD)Citation32. The proportion of patients who survived in each year was estimated from the median OS, assuming a constant rate of death.
Testing costs
The model assumed that next-generation sequencing (NGS) is used for all METex14 mutation testing. The total annual testing costs were calculated using the unit costs of testing and the proportion of treatment-naïve and previously treated patients tested in each year in the model. Medicare testing costs were obtained from the CMS Clinical Laboratory Fee ScheduleCitation28 and commercial testing costs were assumed from published literatureCitation33,Citation34. The NGS testing cost was assumed to be $6,068.79 (average cost of commercially available NGS testing of >50 genes) for the commercial perspectiveCitation34 and $1,758.76 for the Medicare perspectiveCitation28. The model also assumed that testing for PD-L1 was performed in 75% of patientsCitation35, and the unit costs of PD-L1 testing were assumed to be $267.23 (commercial) and $127.40 (Medicare)Citation25. Other mutation testing costs were not explicitly considered in the model.
Sensitivity analysis
A deterministic sensitivity analysis (DSA) was employed to test the robustness of the model results by varying one parameter at a time while holding other inputs at base-case values. The parameters varied in the DSA included the market share uptake for capmatinib, unit price of capmatinib, treatment duration for capmatinib, monthly pre-progression medical costs, AE unit costs, monthly progression-related costs, terminal care costs, and METex14 mutation testing costs. The DSA also tested the sensitivity of the results by using the median PFS to estimate treatment duration for all comparators. All parameters included in the DSA were varied by 10% above and below the base case values.
Results
Commercial payer perspective
In a hypothetical commercial health plan with 1 million members, the target population (adults with mNSCLC and a METex14 skipping mutation) was estimated to be two patients in the first year, three patients in the second year, and three patients in the third year after capmatinib market entry. The estimated plan total budget impact from a commercial perspective was $9,695 in the first year, $38,783 in the second year, and $67,725 in the third year after capmatinib market entry compared to the scenario without capmatinib (Supplementary Table 4).
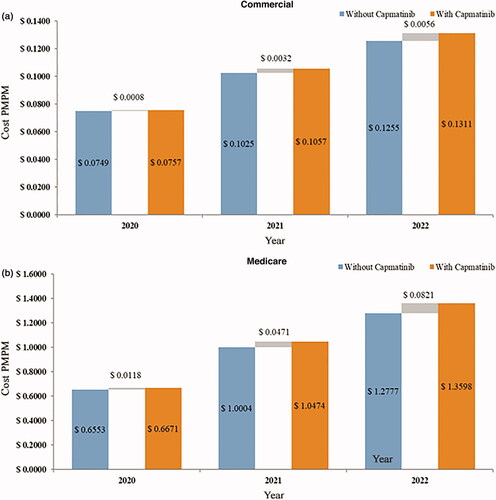
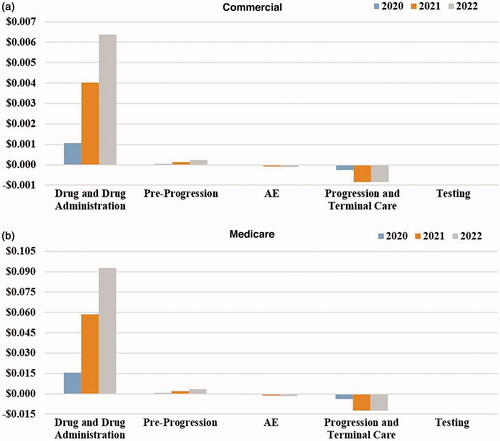
At the PMPM level, the budget impact ranged from $0.0008 to $0.0056 per health plan member in the first three years (). The increasing budget impact over the three years, in terms of plan total and PMPM, was a result of increased drug costs, drug administration costs, and pre-progression medical costs due to an increase in capmatinib market share (). PMPM increases in drug and drug administration costs were $0.0011 in the first year, $0.0040 in the second year and $0.0064 in the third year. These increased costs were partially offset by reductions in costs related to AE management, progression, and terminal care, including PMPM cost savings of –$0.0003 in the first year, –$0.0008 in the second year, and –$0.0009 in the third year for progression and terminal care costs.
Medicare payer perspective
The target population for a Medicare health plan with 1 million members was estimated to be 34 in the first year, 39 in the second year, and 44 in the third year. The estimated plan total budget impact of capmatinib market entry was $141,350 in the first year, $564,706 in the second year, and $985,695 in the third year after capmatinib market entry (Supplementary Table 5).
At the PMPM level, the budget impact ranged from $0.0118 to $0.0821 in the first three years (). Similar to the commercial payer perspective, the increasing budget impact over the three years resulted from increased drug costs and pre-progression monitoring costs, which were offset by reductions in AE costs, progression-related costs, and terminal care costs (). Increased PMPM drug costs ranged from $0.0154 to $0.0928 per year in the first three years, while savings in AE costs, progression and terminal care costs ranged from –$0.0042 to –$0.0141 per year.
Sensitivity analysis
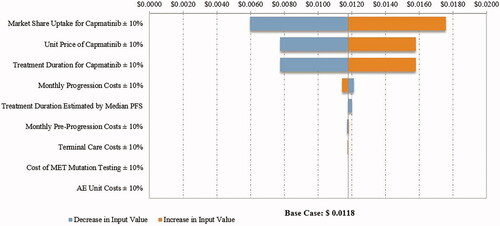
The PMPM budget impact in the DSA ranged from $0.0004 to $0.0012 in the first year, $0.0014 to $0.0050 in the second year, and $0.0026 to $0.0087 in the third year after capmatinib market entry from a commercial payer perspective (first year costs are illustrated in ). The results were most sensitive to the market share uptake for capmatinib, followed by the price of capmatinib and the estimates of treatment duration. Similar results were observed in the DSA from a Medicare perspective (first year costs are illustrated in ).
Figure 4. Tornado diagram for PMPM budget impact in the first year from a commercial perspective. Abbreviations. AE, Adverse event; MET, Mesenchymal–epithelial transition exon 14 skipping mutation; PFS, Progression-free survival; PMPM, Per member per month. Costs estimated in 2020 United States dollars.
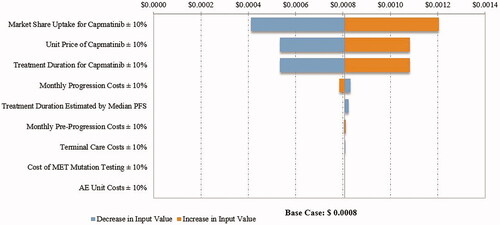
Figure 5. Tornado diagram for PMPM budget impact in the first year from a Medicare perspective. Abbreviations. AE, Adverse event; MET, Mesenchymal–epithelial transition exon 14 skipping mutation; PFS, Progression-free survival; PMPM, Per member per month. Costs estimated in 2020 United States dollars.
Discussion
Capmatinib is the first FDA-approved targeted therapy for adult patients with mNSCLC and METex14 skipping mutations, providing a new treatment option for this rare type of lung cancer. Prior to capmatinib, patients in the US commonly received crizotinib (off-label use) or the same standard of care as those who have NSCLC without any driver mutation. Following the FDA approval, capmatinib is expected to partially displace chemotherapy, immunotherapy, and off-label use of crizotinib in the next three years. However, the uptake of capmatinib may be influenced by other MET inhibitors, such as tepotinib, as the treatment landscape continues to evolve. The present analysis is based on the current assumed treatment landscape and is subject to change in the future.
As with the introduction of any new therapy to the market, it is essential for commercial and public health plan payers to understand the budget impact of including capmatinib in their formulary. Patients with lung cancer harboring a METex14 skipping mutation are often diagnosed at older ages (median: 73 years) and thus the introduction of capmatinib is expected to have more impact on MedicareCitation6. The present BIA contributes valuable new information among a crowded market for NSCLC on the budget impact of capmatinib, the first FDA approved MET inhibitor for patients with METex14 skipping mutation.
The estimated numbers of treatment-naïve and previously treated patients eligible for capmatinib were small (2–3 in a commercial plan and 34–44 patients in a Medicare plan), as only approximately 3–4% of patients with mNSCLC have a METex14 skipping mutation. During the first three years of capmatinib entry, the total annual budget impact was estimated to range from $9,695 to $67,725 for commercial plans and $141,350 to $985,695 for Medicare. The inclusion of capmatinib in commercial and Medicare health plans was estimated to have a marginal increase in PMPM budget impact (ranging from $0.0008 to $0.0056 for commercial and $0.0118 to $0.0821 for Medicare) as a result of concurrently lower medical costs, offsetting the increased drug costs. Lower medical costs were driven by reductions in AE management costs and progression and terminal care costs with capmatinib market entry. A caveat of these estimates is that the model analysis was based on the WAC price for therapies without applying any potential discounts.
This study is subject to certain limitations. First, as with most economic models, the results of this BIA are contingent upon the assumptions applied and the available data. There was a lack of clinical data for comparators in the study population. For example, the PFS and OS data for the pembrolizumab and chemotherapy arms were derived from patients who did not necessarily harbor a METex14 skipping mutation, as data for patients with the mutation were not available. Studies have suggested that MET mutation is associated with a poor prognosis in patients with NSCLCCitation36,Citation37. Therefore, the model analysis may have underestimated the benefit and budget impact of capmatinib relative to comparators. Second, patients were assumed to incur a one-time AE cost if they experienced a grade 3/4 AE present in at least 5% of patients on any treatment, but costs for lower grade or less common AEs were not included in the model. However, these costs are expected to be low due to these AEs being less severe, and are likely to have a limited impact on the budgets of health plans. Third, the estimates for relative dose intensity and treatment duration were based on data reported in clinical trials and may differ from those observed in a real-world treatment setting. Fourth, wastage for intravenous drugs was not considered in the model, providing a conservative estimate of the budget impact. Fifth, capmatinib is an oral medication and patients may bear additional out-of-pocket expenses, including co-payments, which were not accounted for in the calculation of budget impact to health plans.
Conclusions
The additional cost of adding capmatinib to the commercial or Medicare formulary for the treatment of adults with mNSCLC with a METex14 skipping mutation should be minimal mainly due to the small number of patients eligible for treatment. The differences in plan total and PMPM costs between a setting with and without capmatinib market entry are expected to grow over the three years following capmatinib market entry among commercially-insured and Medicare-insured individuals. The predicted increases in PMPM budget impact, attributable to increases in capmatinib market share leading to increased drug acquisition, drug administration, and pre-progression costs, are partially offset by cost reductions in progression-related, terminal care, and AE costs. Future analyses considering the budget impact of emerging therapies on the current model’s results should be performed when sufficient data become available following their market entry.
Transparency
Declaration of funding
This study and any associated publication fees were funded by Novartis Pharmaceuticals Corporation.
Declaration of financial/other relationships
BC, MS, and DM are employees of Novartis Pharmaceuticals Corporation. Z-YZ, WX, and NCH are employees of Analysis Group, Inc., which has received consulting fees from Novartis Pharmaceuticals Corporation. GO, DB, and JB are principals of Millcreek Outcomes Group and received consulting fees from Novartis Pharmaceuticals Corporation. A peer reviewer on this manuscript has disclosed that they are employed by Merck & Co., Inc. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Previous presentations
Portions of this research was presented at the AMCP Nexus 2020 Virtual meeting, October 19–23, 2020.
Supplemental Material
Download MS Word (68.3 KB)Acknowledgements
The authors wish to thank Dr. Rebecca Heist of Massachusetts General Hospital for kindly sharing her clinical practice experience and opinions on the utilization of therapies, which were highly valuable to the development of this work. Dr. Rebecca Heist received an honorarium from Novartis Pharmaceuticals Corporation. Medical writing assistance was provided by Shelley Batts, PhD, an employee of Analysis Group, Inc. Funding for this assistance was provided by Novartis Pharmaceuticals Corporation.
Data availability statement
The data and model included in this study contains confidential information and will therefore not be available for sharing.
References
- Surveillance Epidemiology and End Results (SEER) Program. Cancer stat facts: lung and bronchus cancer; 2020; [cited 2020 Jun 15]. Available from: https://seer.cancer.gov/statfacts/html/lungb.html
- American Cancer Society. Key statistics for lung cancer; 2020; [cited 2020 Jun 15]. Available from: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32(4):605–644.
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5(8):850–859.
- Paik PK, Drilon A, Fan P-D, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015;5(8):842–849.
- Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol. 2016;11(9):1493–1502.
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–550.
- Vuong HG, Ho ATN, Altibi AMA, et al. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer – a systematic review and meta-analysis. Lung Cancer. 2018;123:76–82.
- Novartis Pharmaceuticals. Novartis announces FDA approval of MET inhibitor Tabrecta™ for metastatic non-small cell lung cancer with METex14; 2020; [cited 2020 Jul 16]. Available from: https://www.novartis.com/news/media-releases/novartis-announces-fda-approval-met-inhibitor-tabrecta-metastatic-non-small-cell-lung-cancer-metex14
- Hsu F, De Caluwe A, Anderson D, et al. Patterns of spread and prognostic implications of lung cancer metastasis in an era of driver mutations. Curr Oncol. 2017;24(4):228–233.
- Awad MM, Leonardi GC, Kravets S, et al. Impact of MET inhibitors on survival among patients with non-small cell lung cancer harboring MET exon 14 mutations: a retrospective analysis. Lung Cancer. 2019;133:96–102.
- United States Food and Drug Administration. Highlights of prescribing information: TABRECTA (capmatinib); 2020; [cited 2020 Jul 5]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213591s000lbl.pdf
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944–957.
- National Comprehensive Cancer Network. Non-small cell lung cancer guidelines (version 5); 2019; [cited 2020 Mar 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/28404761/
- National Comprehensive Cancer Network. Non-small cell lung cancer guidelines (version 6); 2020; [cited 2020 Aug 4]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- United States Bureau of Labor Statistics. Consumer price index – all urban consumers, not seasonally adjusted US city average for medical care, 2006–2019; 2019; [cited 2019 Sep 16]. Available from: http://download.bls.gov/pub/time.series/cu/cu.data.15.USMedical
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices-budget impact analysis. Value Health. 2007;10(5):336–347.
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14.
- United States Census Bureau. Health insurance coverage in the United States: 2018; 2019; [cited 2019 Nov 11]. Available from: https://www.census.gov/content/dam/Census/library/publications/2019/demo/p60-267.pdf
- The Henry J. Kaiser Family Foundation. An overview of Medicare; 2019; [cited 2019 Nov 11]. Available from: https://www.kff.org/medicare/issue-brief/an-overview-of-medicare/
- Surveillance Epidemiology and End Results (SEER) Program. Prevalence database: US estimated complete prevalence (including counts) by age on 1/1/2016; 2016; [cited 2019 Sep 5]. Available from: https://surveillance.cancer.gov/prevalence/canques.html
- Surveillance Epidemiology and End Results (SEER) Program. Cancer stat facts: lung and bronchus cancer; 2016; [cited 2019 Nov 11]. Available from: https://seer.cancer.gov/statfacts/html/lungb.html#survival
- IBM Micromedex. RED BOOK; 2019; [cited 2019 Apr 9]. Available from: http://www.micromedexsolutions.com/micromedex2/librarian/
- Centers for Medicare & Medical Services. April 2020 ASP pricing file; 2020; [cited 2020 Mar 16]. Available from: https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2020-asp-drug-pricing-files
- Centers for Medicare & Medical Services. CMS physician fee schedule; 2019; [cited 2019 Sep 10]. Available from: https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx
- Huang M, Lopes GL, Insinga RP, et al. Cost-effectiveness of pembrolizumab versus chemotherapy as first-line treatment in PD-L1-positive advanced non-small-cell lung cancer in the USA. Immunotherapy. 2019;11(17):1463–1478.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047.
- Centers for Medicare & Medical Services. CMS clinical laboratory fee schedule 2020 Q1 2020; 2020; [cited 2020 Mar 6]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html
- Agency for Healthcare Research and Quality. Healthcare cost and utilization project (HCUP); 2016; [cited 2019 Oct]. Available from: https://hcupnet.ahrq.gov/#setup
- Skinner KE, Fernandes AW, Walker MS, et al. Healthcare costs in patients with advanced non-small cell lung cancer and disease progression during targeted therapy: a real-world observational study. J Med Econ. 2018;21(2):192–200.
- Novartis Pharmaceuticals. A phase II, multicenter study of oral cMET inhibitor INC280 in adult patients with EGFR wild-type (wt) advanced non-small cell lung cancer (NSCLC). Clinical study report; 2019.
- Bittoni MA, Arunachalam A, Li H, et al. Real-world treatment patterns, overall survival, and occurrence and costs of adverse events associated with first-line therapies for Medicare patients 65 years and older with advanced non-small-cell lung cancer: a retrospective study. Clin Lung Cancer. 2018;19(5):e629–e645.
- Dalal AA, Guerin A, Mutebi A, et al. Economic analysis of BRAF gene mutation testing in real world practice using claims data: costs of single gene versus panel tests in patients with lung cancer. J Med Econ. 2018;21(7):649–655.
- Gong J, Pan K, Fakih M, et al. Value-based genomics. Oncotarget. 2018;9(21):15792–15815.
- Velcheti V, Patwardhan PD, Liu FX, et al. Real-world PD-L1 testing and distribution of PD-L1 tumor expression by immunohistochemistry assay type among patients with metastatic non-small cell lung cancer in the United States. PLoS One. 2018;13(11):e0206370.
- Kim JH, Kim HS, Kim BJ. Prognostic value of MET copy number gain in non-small-cell lung cancer: an updated meta-analysis. J Cancer. 2018;9(10):1836–1845.
- Guo B, Cen H, Tan X, et al. Prognostic value of MET gene copy number and protein expression in patients with surgically resected non-small cell lung cancer: a meta-analysis of published literatures. PLoS One. 2014;9(6):e99399.