?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Hemophilia A (HA) is a genetic bleeding disorder characterized by a deficiency of clotting factor VIII (FVIII) requiring lifelong prophylactic treatment typically with conventional standard half-life recombinant FVIII (rFVIII). Lifelong prophylaxis impacts budget, patient adherence, and long-term outcomes. The consequent economic and treatment burden may be reduced by using novel extended half-life rFVIII. The objective of this analysis was to estimate the budget impact of introducing Jivi (damoctocog alfa pegol, BAY 94-9027), hereafter referred to as BAY 94-9027, as an on-demand and prophylactic treatment for severe HA from a Japanese payer’s perspective.
Methods
A global budget impact model was adapted to the Japanese setting using data obtained via a targeted literature review of Japanese sources. The model considered a five-year time horizon for a market without and with BAY 94-9027. Using annual per-patient costs, the total cost of on-demand and prophylactic treatment of adolescent and adult patients with severe HA (without inhibitors) were analyzed. The model used summary of product characteristics (SmPC) and clinical trial dosing, and unit costs from the National Health Insurance (NHI) drug price database. Comparators considered in the model comprised of currently available products in Japan. Projected BAY 94-9027 uptake ranged from 4% to 9% over the five years (2020–2024).
Results
Introduction of BAY 94-9027 for the treatment of severe HA is estimated to decrease the overall budget by 1.5%, with a cost saving of approximately $67 million USD (¥7.4 billion JPY) over five years. Estimated cost savings associated with BAY 94-9027 ranged from $1.4 million USD (¥156 million JPY) in 2020 to $23 million USD (¥2.6 billion JPY) in 2024 for the Japanese healthcare system.
Limitations
There were limitations associated with the study. The Japanese guidelines consulted during the targeted literature review of national data sources in Japan were based on global data as reference sources. Also, studies reporting the bleeding rate, dosing guidelines, and economic burden in the Japanese population identified by the targeted literature review were limited hence global studies were used and may not have been representative of the Japanese population.
Conclusions
BAY 94-9027 can reduce total severe HA treatment costs, driven by lower annual rFVIII utilization, and a narrow weekly dosing range compared to competitor products in the Japanese market.
Introduction
Hemophilia A (HA) is an X-chromosome-linked genetic bleeding disorder characterized by a deficiency of factor VIII (FVIII) due to genetic mutationsCitation1 accounting for 80% of all hemophiliaCitation2 cases. Globally, HA ranks second amongst the hereditary bleeding disorders, after Von Willebrand diseaseCitation3, with a greater prevalence amongst males than females and a global incidence of one case per 5,000 live male birthsCitation4. In 2019, a national survey of coagulation disorders in Japan (the most recent Japanese epidemiological data on HA identified by a targeted literature review at the time of study development) reported 5,410 patients with HACitation5.
Based on the level of FVIII deficiency, HA is clinically classified as mild, moderate, or severe. The activity of FVIII in patients with mild, moderate, and severe HA are 5–<40%, 1–4%, and <1%, respectivelyCitation6. Those with mild HA may experience prolonged bleeding after surgery or trauma. Whilst those with severe HA may experience prolonged bleeding even after a minor injury, more frequently in joints and muscles. In rare cases, patients with severe HA may experience simultaneous bleeding in multiple jointsCitation7, recurrent episodes of bleeding in the joints may result in chronic arthropathyCitation8.
Current treatment options for HA include intravenous infusions of recombinant FVIII (rFVIII) that are administered either episodically (on-demand) or as prophylaxisCitation9,Citation10. Prophylactic treatment is a hemostatic management that periodically replenishes clotting factors that are deficient in non-bleeding situations over a long period of time, and it is used to reduce the frequency of bleeding and preventing the development of hemophilic arthropathy in severe HA patientsCitation11. Severe HA patients are typically prescribed prophylactic treatment, which reduces bleeding episodes, to improve health outcomes and overall quality of life (QoL)Citation12,Citation13. In Japan, about 90% of patients with severe HA select prophylactic treatment, and it is considered as the primary standard of care for severe HA patientsCitation14. Conventional standard half-life (SHL) rFVIII products prescribed for prophylactic treatment need to be administered every two daysCitation15–19; in some cases, infusions may be necessary three-to-four times a week. Also, the exact dosage and optimal regimen of these products are difficult to determine because of patient-specific adjustmentsCitation12. Since HA patients require prophylactic treatment throughout their life, the total treatment cost and healthcare resource use can impose an important financial burden on healthcare systems, in addition to a clinical burden for patientsCitation20,Citation21.
Due to the economic and treatment burden of SHL rFVIII products, extended half-life (EHL) rFVIII products, which require fewer infusions and can potentially alleviate the clinical and economic burden of HA patients, whilst improving QoLCitation22, have grown in use in recent years. BAY 94-9027 is a site-specific PEGylated rFVIII (polyethylene glycol moiety attached to rFVIII) molecule with EHL and prolonged duration of action. It has demonstrated significant clinical efficacy and safety for the treatment of severe HA patients in clinicalCitation23,Citation24 as well as real-world settingCitation25,Citation26. However, the economic impact of using BAY 94-9027 for the treatment of severe HA in Japan has not been studied following its approval in September 2018. Also, the efficacy demonstrated by BAY 94-9027 in terms of annual bleed rate compared to other rFVIII products has not been studied.
The objective of this study is to estimate the impact of the introduction of BAY 94-9027 for the treatment of severe HA on the Japanese healthcare payer’s budget.
Methods
Model structure
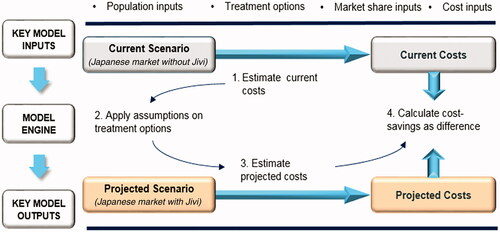
A Microsoft Excel budget impact model was adapted for Japan based on a global budget impact model originally developed using France as the base case and latterly modified for other countries prior to this study. This model assesses the economic impact of introducing BAY 94-9027 as a prophylactic and on-demand treatment for patients with severe HA without inhibitors in Japan. The model considered two scenarios – a world without BAY 94-9027 and a world with BAY 94-9027 for the Japanese market, and analyzed the difference in the per-patient costs of prophylactic and on-demand treatment. A schematic model structure is presented in . The workflow of the model adaptation is presented in Supplementary Figure S1.
Model settings
The model adopted the payer’s perspective in Japan. The base case considered a time horizon of five years (2020–2024). The prophylactic dose of BAY 94-9027 used in the model was the midpoint of the range reported in the summary of product characteristics (SmPC)Citation27. Detailed prophylactic dose calculations using SmPC label midpoints are presented in Appendix A. A dose reduction of 10% was applied for the adult (≥18 years) population to reflect the lower dose used after adolescence (based on a conservative assumptionCitation28,Citation29). All cost inputs and results were calculated in Japanese yen (¥) then converted into US dollars ($) using the average 5 year exchange rate of 0.00909 between Dec 2015 and 14 Dec 2020, when the study was conductedCitation30. Equivalent efficacy (i.e. bleeding rates) across all comparators was assumed, based on a matching-adjusted indirect comparison (MAIC)Citation31.
Model inputs
Model inputs were identified through a targeted literature review performed using national data sources in Japan.
Population inputs
The target population included male adolescent (≥12 years to <18 years) and adult (≥18 years) patients with severe HA without inhibitors who were eligible for prophylactic and on-demand treatment with rFVIII products. The average weights used for adolescent and adult patients were 54.02 kg and 66.34 kg, respectivelyCitation32. The size of the population was estimated using either the prevalence or incidence data for severe HA patients in Japan using data obtained from a targeted literature review of national sourcesCitation33–36. The proportion of patients treated with prophylactic and on-demand treatment were 87% and 13%, respectivelyCitation37. Details of the population inputs considered for the model are presented in .
Table 1. Population inputs considered in the model.
Cost inputs
The total annual patient cost was considered for calculating the budget impact of BAY 94-9027. The total annual prophylactic and on-demand treatment cost per patient were calculated as follows:
Detailed calculations of total treatment cost used in the model are described in Appendix B. The total annual patient cost considered for the adult and adolescent population for prophylactic and on-demand treatment are presented in .
Table 2. Cost inputs for adults and adolescents for each type of treatment.
Comparators
The other rFVIII products considered as the comparators for BAY 94-9027 in the model included rurioctocog alfa (Adynovate), octocog alfa (Advate), efmoroctocog alfa (Eloctate), lonoctocog beta (Afstyla), turoctocog alfa (NovoEight), emicizumab-kxwh (Hemlibra), and octocog beta (Kovaltry). Amongst these, Advate, NovoEight and Kovaltry are SHL rFVIII products, Adynovate and Eloctate are EHL rFVIII products while Hemlibra is a non-factor replacement therapy. Afstyla has been positioned as both SHL and EHL in different markets.
Market share
BAY 94-9027 was launched in Japan in September 2018. To conduct the budget impact analysis, the market share of the rFVIII products (BAY 94-9027 and other EHL rFVIII products) were compared using two scenarios – the world without BAY 94-9027 and the world with BAY 94-9027 for the Japanese market. These two scenarios were compared for both types of treatments: prophylactic and on-demand. Market share data for BAY 94-9027 and its comparators were provided by Bayer from sales volume data using the following assumptions.
Total market size was calculated based on the forecasted sales data (2020–2024) shared by Bayer for Jivi (BAY 94-9027), Kovaltry and Hemlibra, and expected share of the market for Jivi (BAY 94-9027) as a percentage of total sales. Proportions of the rest of the market occupied by Advate and Adynovate (Takeda) were estimated for 2019 and assumed to remain constant through 2020-2024. The relative proportions for each individual product were calculated and projected forward using the trend observed (i.e. Adynovate’s share is growing at the expense of Advate). The proportion of the non-Bayer and Hemlibra market that is occupied by each of the other comparators (Eloctate, NovoEight, Afstyla) was estimated for 2019 and was assumed to remain constant for 2020-2024. The relative proportion for each individual product was calculated and projected forward using the trend observed. For the scenario analysis where BAY 94-9027 has a higher market share uptake, the additional market share was gained at the expense of Advate and Adynovate. The shares of the comparators were assumed to be the same in the on-demand and prophylaxis market.
Market share data used in the model for all the products for prophylactic and on-demand treatment are presented in and , respectively.
Table 3. Market share – without and with BAY 94-9027 as prophylactic treatment.
Table 4. Market share – without and with BAY 94-9027 as on-demand treatment.
Results
Base case results for prophylactic treatment of HA
The total cost of prophylactic treatment of HA in the world without BAY 94-9027 scenario was estimated to be $4.4 billion USD (¥484 billion JPY), compared to $4.3 billion USD (¥476 billion JPY) in the world with BAY 94-9027 scenario over five years for the Japanese market. This decrease in cost upon the introduction of BAY 94-9027 corresponds to 1.5% ($67 million USD (¥7.4 billion JPY)) of the total Japanese budget for prophylactic treatment of HA across five years (). It is important to note that the cost saving of 1.5% with BAY 94-9027 is achieved while maintaining the same clinical efficacy as the comparators.
Table 5. Budget impact with the introduction of BAY 94-9027 as prophylactic treatment.
Base case results for on-demand treatment of HA
The budget impact analysis estimated that over five years, the total cost of on-demand treatment of HA in the world without BAY 94-9027 scenario is $61.56 million USD (¥6.772 billion JPY) compared to $61.52 million USD (¥6.768 billion JPY) in the world with BAY 94-9027 scenario for the Japanese market. Hence, the introduction of BAY 94-9027 corresponds to a 0.1% ($34 thousand USD (¥3.7 million JPY)) decrease in the total budget for the on-demand treatment for severe HA across five years in Japan ().
Table 6. Budget impact with the introduction of BAY 94-9027 as on-demand treatment.
Base case results for net budget impact
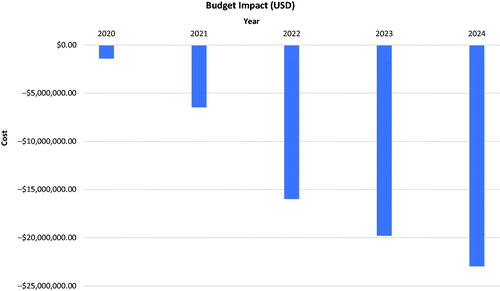
The introduction of BAY 94-9027 was estimated to precipitate a negative budget impact of 1.5% across five years for the Japanese budget for the treatment of severe HA. In 2020 and 2021, the model estimated a slight decrease of $1.4 million USD (¥156 million JPY) and 6.5 million USD (¥717 million JPY), respectively; however, in the total budget, greater decreases of $16.15 million, $19.98 million, and $23.20 million USD (¥1.776 billion, ¥2.198 billion, and ¥2.552 billion JPY) were observed in the total budget of year 2022, 2023, and 2024, respectively (). Across the five years, an overall decrease of $67.26 million USD (¥7.4 billion JPY) is expected in the total Japanese budget for the treatment of severe HA ().
Table 7. Total budget impact with the introduction of BAY 94-9027 over five years.
The model also anticipated that BAY 94-9027 could be used for treating additional HA patients without leading to any increase in the Japanese budget. Using the cost-saving achieved after introducing BAY 94-9027 in Japan, it estimated that over five years, approximately 305 additional HA patients could be treated without an increase in the current budget ().
Table 8. Additional patients anticipated to be treated within same budget.
Scenario analysis results
BAY 94-9027 is associated with the longest EHL rFVIII products, facilitating fewer administrations for patients. Due to this patient benefit, it is plausible that more patients may switch from the other EHL products than has been forecast in the world with BAY 94-9027 market share projections for the Japanese market. A scenario analysis was conducted where an additional 5% market share is transferred from Adynovate (the most expensive EHL product) to BAY 94-9027 to estimate the additional cost savings that the Japanese healthcare payer could realize. This scenario shows an increased total cost saving of 1.6% ($71.91 million USD (¥7.911 billion JPY)) over five years, ranging from $2.29 million USD (¥252 million JPY) in 2020 to $24.18 million USD (¥2.66 billion JPY) in 2024.
Discussion
The treatment of HA is associated with a marked economic burden due to the prolonged nature of the disease which requires lifelong prophylaxisCitation20,Citation21. The conventional SHL rFVIII products require a high number of infusions during prophylactic treatment, thereby increasing the overall healthcare resource use and total costCitation12,Citation20,Citation21. In contrast, EHL rFVIII products require less frequent infusions, which leads to better patient adherence, improved health outcomes and better QoL, resulting in lower healthcare resource use and overall costCitation22. Treatment costs for HA are fully covered by public funds in Japan with no requirement for co-payment by HA patients, thus cost sensitivity of patients is not a consideration. Regardless, cost containment efforts are increasing and needed as the overall burden of healthcare is rising in Japan. Given that HA treatment is lifelong associated with high costs it is important to discuss and consider the budget impact. The economic impact of the introduction of EHL products for the treatment of HA has been analyzed in different countriesCitation38,Citation39. The present study explored the economic impact of introducing BAY 94-9027 (an EHL rFVIII product) for the treatment of severe HA in Japan. The study showed a decrease of 1.5% and 0.1% in the budget upon introduction of BAY 94-9027 as a prophylactic and on-demand treatment, respectively. A previous study conducted by McMullen et al. in the United States of America reported a decrease in the budget only with on-demand treatment but not in the prophylactic treatment using rFVIII because of the lower number of patients receiving on-demand treatmentCitation38. Prophylactic treatment is considered as the primary standard of care for the treatment of HA in JapanCitation14 and is recommended over on-demand treatment to maintain the QoL of patients and reduce the number of bleeding eventsCitation14,Citation40,Citation41. Hence, even with only a slight decrease of the budget in the on-demand treatment, introduction of BAY 94-9027 as prophylactic treatment is expected to significantly decrease the overall budget by 1.5%, owing to its direct and indirect cost savings. A scenario analysis where an additional 5% of the Adynovate market share is occupied by BAY 94-9027 showed an increased cost saving of 1.6%. The overall budget initially showed a gradual decrease in the first two years of the introduction of BAY 94-9027, followed by a substantial decrease from the third year to the fifth year. This may be attributed to the expected initial gradual increase in the market share of BAY 94-9027 in Japan followed by more widespread uptake from the third year, which would correspondingly result in a decrease in the market share of other rFVIII products.
This study is the first of its kind to present the budget impact analysis of various EHL rFVIII products in Japan. Also, it considered both prophylactic and on-demand treatments of HA, and the inputs were validated with a Japanese expert. However, there were limitations associated with the study. The Japanese guidelines consulted during the targeted literature review of national data sources in Japan were based on global data as reference sources. Also, studies reporting the bleeding rate, dosing guidelines and economic burden in the Japanese population identified in the targeted literature review were limited, and the global studies used may not be representative of the Japanese population. Furthermore, given the rapid changes in HA treatment such as gene therapy, treatments and healthcare costs are expected to change dramatically. As gene therapies are more likely to be applied to a limited patient group, a fundamentally different model would be required. The model in the study presented seeks only to evaluate existing treatment options.
Despite these limitations, the present study showed that the introduction of BAY 94-9027 as a prophylactic or on-demand treatment for HA should result in the reduction of total economic burden for Japanese payers. This study can be leveraged to inform future health policy decisions about rFVIII products and their budget impact in the Japanese population.
Conclusions
This study analyzed the economic impact of the introduction of BAY 94-9027 for the prophylactic and on-demand treatment of HA on Japanese payers based on a budget impact model used in other countries prior to being adapted for Japan. The budget impact model estimates a decrease in the prophylactic, on-demand and total budget for the treatment of HA which could reduce the economic burden for Japanese payers over five years. The study is unique as it predicts a decrease in the long-term treatment costs of HA with the introduction of BAY 94-9027 in Japan. Compared to the currently used EHL products in Japan, BAY 94-9027 is the most cost saving EHL product for payers.
Transparency
Declaration of funding
This study was funded by Bayer Yakuhin, and Yuko Kidoguchi, Noriko Takahashi are employees of Bayer Yakuhin.
Declaration of financial/other interests
Teruhisa Fujii received personal fees as a medical advisor from Bayer Yakuhin, Shire Japan, Sanofi Japan and Chugai Pharmaceutical.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous presentations
This study result was accepted for the 82nd Annual Meeting of the Japanese Society of Hematology (JSH2020) as a poster presentation.
Supplemental Material
Download MS Word (102.1 KB)Acknowledgements
Medical writing support was provided by Ishneet Kaur and Rosario Vivek on behalf of HEOR, IQVIA, India. They have given their permission to be acknowledged by name.
References
- Franchini M, Mannucci P. Past, present and future of hemophilia: a narrative review. Orphanet J Rare Dis. 2012;7(1):24.
- Mansouritorghabeh H. Clinical and laboratory approaches to hemophilia A. Iran J Med Sci. 2015;40(3):194–205.
- Greer J, Arber DA, Glader B, List A, et al. Wintrobe’s Clinical Hematology. 13th ed. Philadelphia, USA: Lippincott Williams & Wilkins; 2014.
- National Hemophilia Foundation Fast Facts. 2017 [cited 2020 Dec]. Available from: https://www.hemophilia.org/bleeding-disorders-a-z/overview/fast-facts
- Project entrusted by Ministry of Health LaW. Nationwide Survey on Coagulation Disorders 2017. Published by Japan Foundation for AIDS Prevention. [cited 2020 March]. Available from: https://api-net.jfap.or.jp/library/alliedEnt/02/images/h29_research/h29_research.pdf.
- Mistry T, Dogra N, Chauhan K, et al. Perioperative considerations in a patient with hemophilia A: a case report and review of literature. Anesth Essays Res. 2017;11(1):243–245.
- Majid Z, Tahir F, Qadar L, et al. Hemophilia A with a rare presentation of hemarthrosis and arthropathy involving multiple joints in a young male child. Cureus. 2019;11(4):e4524.
- Knobe K, Berntorp E. Haemophilia and joint disease: pathophysiology, evaluation, and management. J Comorb. 2011;1(1):51–59.
- Cafuir L, Kempton C. Current and emerging factor VIII replacement products for hemophilia A. Ther Adv Hematol. 2017;8(10):303–313.
- Bachelet D, Albert T, Mbogning C, ABIRISK consortium, et al. Risk stratification integrating genetic data for factor VIII inhibitor development in patients with severe hemophilia A. PLoS One. 2019;14(6):e0218258.
- Society JTH. Hemostasis treatment guidelines for hemophilia patients without inhibitors (2013 revised ed.). Jpn J Thromb Hemost. 2013;24(6):619–639.
- Powell J. Recombinant factor VIII in the management of hemophilia A: current use and future promise. Ther Clin Risk Manag. 2009;5(2):391–402.
- Manco-Johnson M, Abshire T, Shapiro A, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544.
- Dunkley S, Lam J, John M, Asia-Pacific Haemophilia Working Group (APHWG), et al. Principles of haemophilia care: the Asia-Pacific perspective. Haemophilia. 2018;24(3):366–375.
- Aledort L, Haschmeyer R, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor‐VIII‐deficient haemophiliacs. J Intern Med. 1994;236(4):391–399.
- Löfqvist T, Nilsson I, Berntorp E, et al. Haemophilia prophylaxis in young patients – a long-term follow-up. J Intern Med. 1997;241(5):395–400.
- Prelog T, Dolničar M, Kitanovski L. Low-dose continuous infusion of factor VIII in patients with haemophilia A. Blood Transfus. 2016;14(5):474.
- Valentino L, Mamonov V, Hellmann A, et al.; for the Prophylaxis Study Group. A randomized comparison of two prophylaxis regimens and a paired comparison of on‐demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2012;10(3):359–367.
- Gringeri A, Lundin B, Von Mackensen S, THE ESPRIT STUDY GROUP, et al. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J Thromb Haemost. 2011;9(4):700–710.
- Paisley S, Wight J, Currie E, et al. The management of inhibitors in haemophilia A: introduction and systematic review of current practice. Haemophilia. 2003;9(4):405–417.
- Elander J. A review of evidence about behavioural and psychological aspects of chronic joint pain among people with haemophilia. Haemophilia. 2014;20(2):168–175.
- Ar MC, Balkan C, Kavaklı K. Extended half-life coagulation factors: a new era in the management of hemophilia patients. Turk J Haematol. 2019;36(3):141–154.
- Reding M, Ng H, Poulsen LH, et al. Safety and efficacy of BAY 94‐9027, a prolonged‐half‐life factor VIII. J Thromb Haemost. 2017;15(3):411–419.
- Santagostino E, Saxena K, Kenet G, et al. PROTECT VIII Kids trial results: BAY 94-9027 safety and efficacy in previously treated children with severe hemophilia A. Haemophilia. 2016;22(Suppl 4):41.
- Miesbach W, Di Minno G, Santagostino E, et al. Efficacy and safety of BAY 94-9027 (damoctocog alfa pegol) prophylaxis in patients with severe hemophilia A and comorbidities: a post hoc analysis of PROTECT VIII data. Washington, DC: American Society of Hematology; 2019.
- Oldenburg J, Alvarez-Román M, Castaman G, et al. Real-world effectiveness and safety of BAY 94-9027 (damoctocog alfa pegol) in previously treated patients with hemophilia A (HEM-POWR): online patient portal and LIFE-ACTIVE sub-study. Washington, DC: American Society of Hematology; 2019.
- Summary of Product Characteristics. Jivi. Summary of product characteristics (SmPC). [cited 2020 January]. Available from: https://www.ema.europa.eu/en/documents/product-information/jivi-epar-product-information_en.pdf.
- Ljung R, Gretenkort Andersson N. The current status of prophylactic replacement therapy in children and adults with haemophilia. Br J Haematol. 2015;169(6):777–786.
- Fischer K. Prophylaxis for adults with haemophilia: one size does not fit all. Blood Transfus. 2012;10(2):169–173.
- Japanese Yen to US Dollar Chart 2020. [cited 2020 14 Dec, 10:14 AM]. Available from: https://www.xe.com/currencycharts/?from=JPY&to=USD&view=5Y.
- Pocoski J, Li N, Ayyagari R, et al. Matching-adjusted indirect comparisons of efficacy of BAY 81-8973 vs two recombinant factor VIII for the prophylactic treatment of severe hemophilia A. JBM. 2016;7:129–137.
- Agency JS. Results of physique measurements by age. [cited 2020 February]. Available from: http://www.e-stat.go.jp/SG1/estat/Xlsdl.do?sinfid=000031462537.
- World Population review. Worldpopulationreview.com. 2019 [cited 2020 February]. Available from: http://worldpopulationreview.com/countries/japan-population/.
- Stonebraker J, Bolton‐Maggs P, Michael Soucie J, et al. A study of variations in the reported haemophilia A prevalence around the world. Haemophilia. 2010;16(1):20–32.
- O'Hara J, Hughes D, Camp C, et al. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis. 2017;12(1):106.
- Ministry of Health LaW. Welcome to Ministry of Health, Labour and Welfare. [cited 2020 February]. Available from: https://www.mhlw.go.jp/english/.
- Report of National Survey in Blood Clotting Disorders. 2018. [cited 2020 February]. Available from: https://api-net.jfap.or.jp/library/alliedEnt/02/images/h30_research/h30_research.pdf.
- McMullen S, Buckley B, Hall IIE, et al. Budget impact analysis of prolonged half-life recombinant FVIII therapy for hemophilia in the United States. Value Health. 2017;20(1):93–99.
- Lorenzoni V, Triulzi I, Turchetti G. Budget impact analysis of the use of extended half-life recombinant factor VIII (efmoroctocog alfa) for the treatment of congenital haemophilia a: the Italian National Health System perspective. BMC Health Serv Res. 2018;18(1):596.
- Horiuchi T, Hide M, Yamashita K, et al. The use of tranexamic acid for on‐demand and prophylactic treatment of hereditary angioedema – a systematic review. J Cutan Immunol Allergy. 2018;1(4):126–138.
- Tagliaferri A, Feola G, Molinari A, for the POTTER Study Group, et al. Benefits of prophylaxis versus on-demand treatment in adolescents and adults with severe haemophilia A: the POTTER study. Thromb Haemost. 2015;114(07):35–45.