?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims
Endovascular coiling is a common modality for treating intracranial aneurysms; however, recanalization occurs in approximately 1 in 5 cases, with downstream consequences of regrowth and rupture. Aneurysm packing density >24% reduces recanalization risk; packing density can be increased by inserting additional coils or by using coils with larger volumetric filling. Coil volume depends on length and primary wind diameter (PWD). This study evaluated the influence of PWD on packing density and total case costs.
Materials and methods
Two hypothetical scenarios and one case study were analyzed. In scenario one, the number of coils required to achieve packing density >24% in a hypothetical aneurysm was determined for 0.012″ vs. 0.010″ PWD coils. In scenario two, the total length of 0.010″ vs. 0.012″ PWD coils required to achieve a packing density >24% was analyzed relative to aneurysm volume. In the case study, packing densities with one 0.012″ PWD coil (actual scenario) and one 0.010″ PWD coil (theoretical scenario) were compared.
Results
In scenario one, cost savings would be realized by using four 0.012″ PWD coils vs. seven 0.010″ PWD coils to achieve packing density >24%. In scenario two, greater volumetric filling of 0.012″ vs. 0.010″ PWD coils was correlated with lower total length of coil required. In the case study, a 0.012″ PWD coil achieved packing density >24%, whereas an equivalent length 0.010″ PWD coil would not.
Limitations
Theoretical modeling was used to explore the impact of coil PWD on aneurysm packing density. In clinical practice, packing density depends not only on PWD but on its length, shape, distribution within an aneurysm, and other recanalization risk factors.
Conclusions
Coil PWD influences packing density, the number of coils required to achieve a specific packing density, and total case costs. Using 0.012″ PWD coils may provide cost and procedural efficiencies.
Introduction
Intracranial aneurysms occur in up to 2% of the general populationCitation1, with an estimated 9 million Americans having unruptured intracranial aneurysmsCitation2. Approximately 65,000 unruptured intracranial aneurysms are treated annually in the U.S.Citation3,Citation4, with the primary goals of preventing aneurysm rupture and consequent morbidity and mortality to patientsCitation5. Aneurysm rupture results in roughly 27,000 new cases of subarachnoid hemorrhage each yearCitation6. The devastating effects of subarachnoid hemorrhage include a mortality rate of 25–50% and permanent disability in nearly 50% of survivors, underscoring the importance of interventions to prevent hemorrhage recurrenceCitation6 or to prevent aneurysm rupture in the first place.
Two interventional modalities exist for the treatment of ruptured and unruptured intracranial aneurysms: (1) surgical management with craniotomy and clip ligation (i.e. clipping) and (2) endovascular management, which may include packing the aneurysm with detachable platinum coils (i.e. coiling), the use of flow-diverting stents, or the use of novel intrasaccular technologyCitation1,Citation6,Citation7.
Coil embolization is the most common form of aneurysm treatment in the U.S.Citation8,Citation9, with patients experiencing significantly better survival and improved functional outcomes in cases of aneurysmal rupture and shorter hospitalizations compared with patients treated with surgical clippingCitation10–13. Despite these advantages, about 1 in 5 patients (21–24% of cases) treated with endovascular coiling experience aneurysm recanalizationCitation14,Citation15, thereby increasing the risk of ruptureCitation9. An inverse correlation exists between aneurysm recanalization and how densely the aneurysm is packed with endovascular coils (i.e. packing density)Citation16–18. Several studies have demonstrated that aneurysm packing densities exceeding 24% have lower rates of coil compaction and recanalizationCitation16,Citation17,Citation19,Citation20.
As medical costs in the U.S. continue to rise, cost-efficient purchasing is one approach to optimize healthcare expenditures. In the context of endovascular coiling, the price of each embolic coil and the number of coils inserted drive the total materials cost in such procedures [9]. Aneurysm packing density can be increased by either inserting more coils or using coils that occupy a larger volume, the latter of which may be a more cost-efficient approachCitation9,Citation21–23.
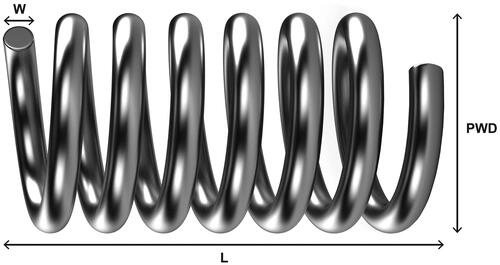
A coil’s volume is directly related to its length from end-to-end and its primary wind diameter (PWD) ()Citation18. The effect of coil length on cost efficiency has been demonstratedCitation9; however, studies that assess the relationship between coil PWD and cost efficiency are lacking. This study uses a hypothetical aneurysm model and a case study to evaluate the influence of PWD on packing density, the number of coils required to achieve a desired packing density, and the economic impact of PWD.
Methods
To analyze the effect of PWD on coil volume and subsequent packing density, a hypothetical aneurysm model was employed. The hypothetical aneurysms were filled with a set of embolic coils, and the resulting packing density was determined by first calculating the aneurysm volume and total sum of coil volumes. The volume of the hypothetical spherical aneurysm was calculated using the formula for the volume of a sphere (4/3πr3).
To assess the impact of PWD on packing density, an analysis was conducted using a set of coils that differed only by their PWD (i.e. cost per coil, coil lengths, and coil shape were the same). Coils in this analysis had a PWD of either 0.010″ or 0.012″, and all coils were complex in shape. Coils with 0.010″ PWD were selected as a comparator in these analyses because they represent the majority (∼78%) of the unit market share for one embolic coil distributor (Cerenovus, data on file). The cost per coil used was $1,429, based on the mean cost of coils (in 2015–2016 U.S. dollars) described in a previous studyCitation8, and a scenario analysis was conducted using a range of coil costs from other published literature sources ($1,295–1,695)Citation24. The formula used to calculate coil volume was [(π)(PWD/2)2(length)], and packing density was calculated by [(Σ coil volumes/aneurysm volume)(100%)]Citation18.
Two hypothetical scenarios were used to assess the influence of PWD on coil volume and packing density. In scenario one, the number of coils required to achieve a packing density >24% was compared for 0.010″ and 0.012″ PWD coils. The diameter of the spherical aneurysm was set at 6.7 mm in alignment with the mean aneurysm size reported in a recent analysisCitation8. In the second scenario, the total length of 0.010″ and 0.012″ PWD coils required to achieve a packing density >24% was analyzed as a function of aneurysm volume.
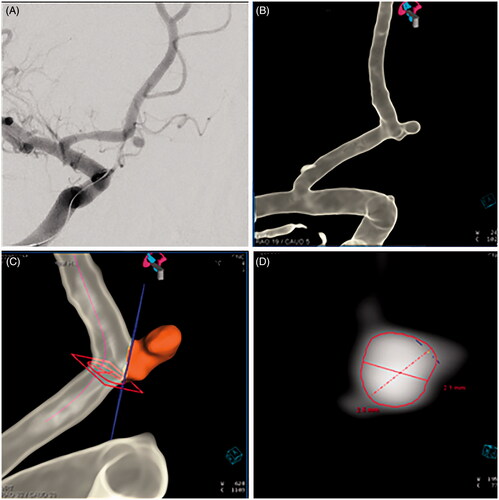
We also report a real-world application of this model by providing a case study. In this case, the dimensions of an ovoid-shaped, saccular aneurysm were determined using measurements from the 2D digital subtraction angiogram, with confirmation on the 3D computer reformatted images using Aneurysm Analysis software on a Siemens Syngo 4D workstation (Siemens Healthineers, Erlangen, Germany) (). Aneurysm volume was calculated using the formula for an ovoid-shaped aneurysm [(π)(width)(depth)(height)/6]. The patient underwent an endovascular coiling procedure using one 0.012″ PWD embolic coil, and the subsequent packing density was calculated.
Figure 2. (A) A 35-year-old woman presented with a subarachnoid hemorrhage from rupture of a 2.1 × 2.5 × 2.8 mm aneurysm located at the junction of the A1 and A2 divisions of the right anterior cerebral artery. Digital subtraction angiography (oblique view) was used to visualize the small, ovoid-shaped aneurysm. Three-dimensional computer reformatted images (B), along with Aneurysm Analysis Software, Siemens Syngo 4D workstation (Siemens Healthineers, Erlangen, Germany) (C, D), were used to confirm the dimensions of the ruptured aneurysm.
Results
Scenario 1
In the first scenario, the number of coils required to achieve packing density >24% was analyzed. Whereas seven 0.010″ PWD coils were required to achieve a packing density of 24.1%, only four 0.012″ PWD coils were required to achieve a packing density of 24.6% (). The total cost of four 0.012″ PWD coils was $5,716, compared with seven 0.010″ PWD coils costing $10,003. Therefore, by using 0.012″ PWD coils, the cost per patient was $4,287 less (a relative cost reduction of 43%). In this scenario, cost equivalence would be achieved if the 0.010″ PWD coil were priced at $802 per coil compared with the price of $1,429 per 0.012″ PWD coil.
Table 1. Scenario one. Number of 0.012″ vs. 0.010″ PWD coils required to achieve packing density >24% in a 157.5-mm3 aneurysm.
Based on the results of scenario one, sensitivity analyses were performed to assess uncertainty related to coil costs, using the upper and lower bounds of a range of coil costs ($1,295–1,695)Citation24. If a per-coil cost of $1,295 had been used, the total coil cost using 0.012″ and 0.010″ PWD coils would have been $5,180 and $9,065, respectively. In this case, the cost per patient was $3,885 less when using 0.012″ PWD coils (a relative cost reduction of 43%). Had a cost of $1,695 per coil been used, the total coil cost using 0.012″ and 0.010″ PWD coils would have been $6,780 and $11,865, respectively. In this analysis, using 0.012″ PWD coils resulted in a total case cost that was $5,085 less than if 0.010″ PWD coils had been used.
Scenario 2
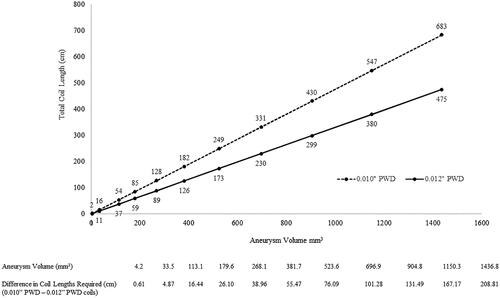
In scenario two, the total length of 0.010″ and 0.012″ PWD coils required to achieve a packing density >24% (i.e. 24.1%) was analyzed as a function of spherical aneurysm volume. The relationship between volumetric filling of coils and aneurysm size is illustrated in . Regardless of aneurysm size, 0.012″ PWD coils required less total length to achieve the target density >24%. Conversely, substantially more 0.010″ PWD coil is needed to achieve this threshold.
Case study
A 35-year-old female patient was transferred to our institution with a Hunt & Hess Grade 4 subarachnoid hemorrhage. After appropriate resuscitation and stabilization in the neurocritical care unit, she was brought to the neurointerventional suite where angiography demonstrated a 2.1 × 2.5 × 2.8 mm ruptured saccular aneurysm with an ovoid shape located at the junction of the A1 and A2 divisions of the right anterior cerebral artery (). She underwent treatment using a single 2.5 mm × 3.5 cm 0.012″ PWD embolic coil (ORBIT GALAXY, Cerenovus, SARL, Switzerland) (). Aneurysm volume was estimated to be 7.69 mm3, and the total volume of the inserted coil was 2.55 mm3. The packing density achieved was calculated to be 33.16% (). Had a 0.010″ PWD coil of the same length been used instead of the 0.012″ PWD coil, the resulting coil volume and packing density would have been 1.77 mm3 and 23.02%, respectively. Absolute and relative packing density differences of 10.14% and 44.04%, respectively, were obtained in favor of the 0.012″ PWD coil. Because the number of coils inserted was assumed to be the same in the actual case and theoretical analysis, the total cost of coils was equivalent. Therefore, for the same cost, using a single 0.012″ PWD coil was estimated to achieve greater packing density compared with using a single 0.010″ PWD coil.
Figure 4. The ruptured aneurysm was treated using a single 2.5 mm × 3.5-cm 0.012″ PWD embolic coil, resulting in a packing density of 33.16% with complete aneurysm occlusion on digital subtraction angiography (A, unsubtracted image, oblique view; B, subtracted image, oblique view).
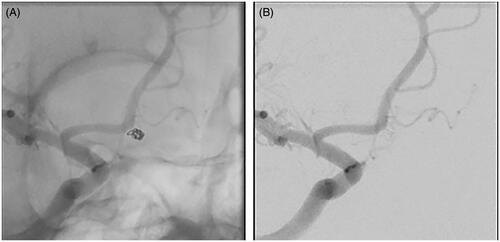
Table 2. Case study comparative analysis of a single 0.012″ PWD and a single 0.010″ PWD embolic coil in a patient with a ruptured, ovoid-shaped aneurysm.
Discussion
In an era of increasing awareness of treatment costs, being mindful of cost efficiency when purchasing products may assist healthcare providers in offsetting expenses while optimizing patient care. In the U.S., coil embolization is the most common form of aneurysm treatment. With the number of endovascular coiling procedures performed annually on the riseCitation8, the cost efficiency of embolic coils is an important consideration. In this analysis, we evaluated the influence of a coil’s PWD on aneurysm packing density using a hypothetical aneurysm model and a case study.
The results of scenario one demonstrated that, compared with 0.010″ PWD coils, fewer 0.012″ PWD coils were needed to achieve a packing density associated with lower recanalization risk (i.e. >24%Citation16,Citation20) (). Because three fewer 0.012″ PWD coils were needed to achieve the packing density threshold, a cost savings of $4,287 per patient (assuming one aneurysm per patient) was estimated. Although annual case volumes differ between centers, savings of $364,395 were estimated for an annual 85-patient cohort (assuming one aneurysm per patient)Citation8. The total cost per total coil volume was lower for 0.012″ PWD coils at $148/mm3 compared with $263/mm3 for 0.010″ PWD coils.
In scenario one, the unit price of 0.010″ PWD coils would need to be reduced by $627 (i.e. 44%) to achieve cost equivalence with the 0.012″ PWD coils (). In addition to the estimated cost savings, important clinical efficiencies may also be realized by inserting fewer coils, including shorter anesthesia time, reduced overall procedure time (and therefore, fewer potential complications), and lower radiation doses for the patient and healthcare teamCitation8,Citation9,Citation24.
We hypothesized that because an embolic coil’s volume depends on its PWD and length, 0.012″ PWD coils would afford greater volumetric filling than 0.010″ PWD coils irrespective of aneurysm size. To test this, we performed analyses with aneurysms of increasing size while holding packing density steady at 24.1% (). The results indicated that 0.012″ PWD coils have greater volumetric efficiency than 0.010″ PWD coils, irrespective of aneurysm size, and that the disparity between the required coil length increases along with increasing aneurysm size ().
The superior packing density achieved by 0.012″ PWD coils was further illustrated in the case study where a small, ruptured, ovoid-shaped aneurysm was packed with a single 0.012″ PWD coil ( and ). Had this patient’s aneurysm been packed with a 0.010″ PWD coil, the packing density would have been 23.02%, an absolute value of 10.14% less than what was achieved in the actual case (). The results of the case study also support the clinical and economic efficiencies of using 0.012″ vs. 0.010″ PWD coils. If a minimum packing density of 24% was required, an additional 0.010″ PWD coil would need to be inserted (). With a typical cohort of 85 patients per yearCitation8, the use of 0.012″ PWD coils may result in one less coil inserted per case and, therefore, an annual cost savings of $121,465 compared with if 0.010″ PWD coils were used. However, in the clinical setting, inserting additional coils is not always feasible or without consequent risks to the patient, particularly in the case of patients presenting with small, ruptured aneurysms. Thus, in this case study, the use of a larger volume coil may have minimized patient risk in addition to total procedural costs.
Clinical studies have also demonstrated that coils with larger PWD achieved greater packing density compared with coils with smaller PWDCitation21–23. In these studies, significantly greater packing density was achieved by complex-shaped 0.012″ PWD coils compared with 0.010″ PWD coils (helical or 3D)Citation21–23. Because complex-shaped coils may allow for denser packing compared with helical coilsCitation25, the superior packing density achieved in these clinical studies was likely due to a combination of coil shape and PWD. For the purpose of our analyses, we assumed all coils to be complex in shape to avoid introducing confounding factors and to highlight the impact of PWD on packing density.
In scenario one, we assumed an equivalent coil cost of $1,429 based on the mean cost per coil (in 2015–2016 U.S. dollars) described in a previous studyCitation8. Although we acknowledge that coil prices may vary by brand and size, the assumed cost of $1,429 is supported by other studies that noted a relatively flat pricing structure across the same brand, irrespective of coil sizeCitation9, and a price range of $1,295–$1,695 (assumed average retail price of $1,595) for 0.012″ PWD coilsCitation24. The results of the coil cost scenario analysis indicated that with a lower or higher cost per coil, cost savings are realized with 0.012″ PWD coils compared with 0.010″ PWD coils.
Taken together, the results of these analyses indicate that the PWD of an embolic coil is an important metric from both a clinical and an economic perspective. During coil selection, it is important for a neurointerventionalist to consider the relationship between a coil’s PWD and aneurysm packing density. However, other factors should be evaluated in coil selection such as the coil’s stiffness, which may influence its ability to be placed inside an aneurysmCitation26. Because coil stiffness is inversely proportional to PWD, coils with a larger PWD are softer and, therefore, more amenable to placement within an aneurysmCitation26. This underscores the importance of PWD during coil selection in clinical practice.
In scenario one, 0.012″ PWD coils were more cost efficient than 0.010″ PWD coils. Because this scenario focused strictly on the impact of PWD on coil volume and packing density, we assumed all coil lengths to be equal. In a recent analysis where coil efficiency was evaluated based on volume per cost, the length of an embolic coil was described as the most important contributor to volumetric efficiencyCitation9. Although we agree that coil length influences volume and cost efficiency, we demonstrated that PWD is also an important consideration in determining a coil’s volumetric and cost efficiency, perhaps especially when inserting a longer-length coil is not clinically feasible.
The two scenarios described used theoretical modeling to explore the contribution of an embolic coil’s PWD to packing density. In clinical practice, packing density is dependent not only on PWD, but also coil lengthCitation26, coil shapeCitation22, and the uniform distribution of coils within an aneurysmCitation5. It is not uncommon that a mixture of coils with varying PWD would be used to fill an aneurysm. For example, 0.015″ or 0.018″ PWD coils may provide structure and stability in endovascular coiling procedures whereas coils with smaller PWD (e.g. 0.009″) are useful in filling remaining spaces within the coil mass. Packing density is also not independent of other recanalization risk factors including aneurysm diameter and neck sizeCitation27,Citation28, coil permeabilityCitation5, local hemodynamicsCitation22, vessel geometryCitation22, the presence of intraluminal thrombusCitation22, and the use of helical vs. complex coilsCitation20,Citation21,Citation23. Regardless, packing density is an important metric to consider in terms of clinical outcomes and healthcare resource useCitation16–20,Citation25,Citation29. The results of this study underscore the importance of considering PWD in embolic coil selection to optimize packing density and total procedural costs.
Conclusions
The PWD of an embolic coil influences the total number of coils needed to fill an aneurysm, the overall packing density, and the total coil-related cost. Assuming equal coil length and unit cost, fewer 0.012″ PWD coils were required to achieve a packing density >24% compared with 0.010″ PWD coils. Regardless of aneurysm size, 0.012″ PWD coils provide greater volumetric filling compared with 0.010″ PWD coils. Using coils with a larger PWD per given length may provide cost and procedural efficiencies by limiting the number of coils required to achieve an aneurysm packing density with reduced recanalization risk. The results of this study emphasize the clinical and economic value in considering the PWD of an embolic coil in the treatment of intracranial aneurysms.
Transparency
Declaration of funding
This study was funded by Cerenovus, a subsidiary of Johnson & Johnson.
Declaration of financial/other relationships
RG is a consultant for Cerenovus, Medtronic Neurovascular, and Balt Neurovascular.
EK is an employee of Cerenovus, a subsidiary of Johnson & Johnson.
HLC and STK are paid consultants for Cerenovus.
PT is a consultant for Cerenovus, Medtronic Neurovascular, and Stryker.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
RG and PT contributed details pertaining to the case study. RG, EK, SK, and HLC conducted the analysis and wrote and edited the paper. All authors discussed the results and contributed to the final manuscript.
Previous presentation
Parts of this study were presented at the 12th Annual Meeting of the Society of Vascular and Interventional Neurology, November 20–23, 2019, Atlanta GA, USA.
Acknowledgements
The authors would like to thank Alia Khaled, Aleeshah Ahmad, and Kristin Kraus for their assistance in preparing the paper.
References
- Ajiboye N, Chalouhi N, Starke RM, et al. Unruptured cerebral aneurysms: evaluation and management. ScientificWorldJournal. 2015;2015:954954.
- Vlak MH, Algra A, Brandenburg R, et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–636.
- Brinjikji W, Rabinstein AA, Nasr DM, et al. Better outcomes with treatment by coiling relative to clipping of unruptured intracranial aneurysms in the United States, 2001-2008. AJNR Am J Neuroradiol. 2011;32:1071–1075.
- United States Census Bureau. U.S. Population 2019. [2018 Dec 6]. Available from: https://www.census.gov/topics/population.html
- Chueh JY, Vedantham S, Wakhloo AK, et al. Aneurysm permeability following coil embolization: packing density and coil distribution. J NeuroIntervent Surg. 2015;7:676–681.
- Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355:928–939.
- Arthur AS, Molyneux A, Coon AL, WEB-IT Study investigators, et al. The safety and effectiveness of the Woven EndoBridge (WEB) system for the treatment of wide-necked bifurcation aneurysms: final 12-month results of the pivotal WEB Intrasaccular Therapy (WEB-IT) Study. J Neurointerv Surg. 2019;11:924–930.
- Gandhoke GS, Pandya YK, Jadhav AP, et al. Cost of coils for intracranial aneurysms: clinical decision analysis for implementation of a capitation model. J Neurosurg. 2018;128:1792–1798.
- Wang C, Ching EC, Hui FK. Aneurysm coil embolization: cost per volumetric filling analysis and strategy for cost reduction. J Neurointerv Surg. 2016;8:541–543.
- Molyneux AJ, Kerr RS, Yu LM, et al., International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–817.
- McDougall CG, Spetzler RF, Zabramski JM, et al. The barrow ruptured aneurysm trial. J Neurosurg. 2012;116:135–144.
- Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427–433.
- Zhang X, Tang H, Huang Q, et al. Total hospital costs and length of stay of endovascular coiling versus neurosurgical clipping for unruptured intracranial aneurysms: systematic review and meta-analysis. World Neurosurg. 2018;115:393–399.
- Naggara ON, White PM, Guilbert F, et al. Endovascular treatment of intracranial unruptured aneurysms: systematic review and meta-analysis of the literature on safety and efficacy. Radiology. 2010;256:887–897.
- Ferns SP, Sprengers ME, van Rooij WJ, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke. 2009;40:e523–e529.
- Sluzewski M, van Rooij WJ, Slob MJ, et al. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology. 2004;231:653–658.
- Kawanabe Y, Sadato A, Taki W, et al. Endovascular occlusion of intracranial aneurysms with Guglielmi detachable coils: correlation between coil packing density and coil compaction. Acta Neurochir (Wien). 2001;143:451–455.
- Tamatani S, Ito Y, Abe H, et al. Evaluation of the stability of aneurysms after embolization using detachable coils: correlation between stability of aneurysms and embolized volume of aneurysms. AJNR Am J Neuroradiol. 2002;23:762–767.
- Uchiyama N, Kida S, Nomura M, et al. Significance of volume embolization ratio as a predictor of recanalization on endovascular treatment of cerebral aneurysms with guglielmi detachable coils. Interv Neuroradiol. 2000;6 (Suppl 1):59–63.
- Slob MJ, Sluzewski M, van Rooij WJ. The relation between packing and reopening in coiled intracranial aneurysms: a prospective study. Neuroradiology. 2005;47:942–945.
- Slob MJ, van Rooij WJ, Sluzewski M. Coil thickness and packing of cerebral aneurysms: a comparative study of two types of coils. AJNR Am J Neuroradiol. 2005;26:901–903.
- Slob MJ, van Rooij WJ, Sluzewski M. Influence of coil thickness on packing, re-opening and retreatment of intracranial aneurysms: a comparative study between two types of coils. Neurol Res. 2005;27:116–119.
- van Rooij WJ, Sluzewski M. Packing performance of GDC 360 degrees coils in intracranial aneurysms: a comparison with complex orbit coils and helical GDC 10 coils. AJNR Am J Neuroradiol. 2007;28:368–370.
- Milburn J, Pansara AL, Vidal G, et al. Initial experience using the Penumbra coil 400: comparison of aneurysm packing, cost effectiveness, and coil efficiency. J Neurointerv Surg. 2014;6:121–124.
- Bendok BR, Rahme RJ, Complex Registry Group. Complex shaped detachable platinum coil system for the treatment of cerebral aneurysms: the Codman Trufill DCS and Trufill DCS Orbit Detachable Coil System COMPLEX Registry final results. J Neurointerv Surg. 2013;5:54–61.
- White JB, Ken CG, Cloft HJ, et al. Coils in a nutshell: a review of coil physical properties. AJNR Am J Neuroradiol. 2008;29:1242–1246.
- Mascitelli JR, Oermann EK, De Leacy RA, et al. Predictors of treatment failure following coil embolization of intracranial aneurysms. J Clin Neurosci. 2015;22:1275–1281.
- Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403.
- Maud A, Lakshminarayan K, Suri MF, et al. Cost-effectiveness analysis of endovascular versus neurosurgical treatment for ruptured intracranial aneurysms in the United States. J Neurosurg. 2009;110:880–886.