Abstract
Aims and Objectives
Patients diagnosed with post-transplant lymphoproliferative disease (PTLD) experience high mortality within the first 2 years of diagnosis; however, few data exist on the economic burden of PTLD in these patients. We determined the healthcare resource utilization (HRU) and cost burden of post-kidney transplant PTLD and evaluated how these differ by survival status.
Materials and Methods
Utilizing data from the United States Renal Data System and the Scientific Registry of Transplant Recipients, we identified 83,818 Medicare-covered kidney transplant recipients between 2007 and 2016, of which 347 had at least one Medicare claim during the first year after diagnosis of PTLD. We tabulated Medicare Part A and Part B and calculated per patient-year (PPY) costs.
Results
Patients diagnosed with PTLD in the first year post-transplant had Part A + B costs of $222,336 PPY, in contrast with $83,546 PPY in all kidney transplants. Post-transplant costs in the first year of PTLD diagnosis were similar regardless of the year of diagnosis. Cost burden for PTLD patients who died within 2 years of diagnosis was >3.3 times higher than PTLD patients still alive after 2 years. Of those who died within 2 years, the majority died within 6 months and costs were highest for these patients, with almost 7 times higher costs than PTLD patients who were still alive after 2 years.
Limitations
Medicare costs were the only costs examined in this study and may not be representative of other costs incurred, nor be generalizable to other insured populations. Patients were only Medicare eligible for 3 years after transplant unless aged ≥62 years, therefore any costs after this cut-off were not included.
Conclusions
PTLD represents a considerable HRU and cost burden following kidney transplant, and the burden is most pronounced in patients who die within 6 months.
Introduction
Post-transplant lymphoproliferative disease (PTLD) is an aggressive type of lymphoma that can develop following allogeneic hematopoietic cell transplant (HCT) or solid organ transplant (SOT)Citation1,Citation2. Only patients post-transplant are at risk of PTLD and it is regarded as rare in the post-transplant populationCitation1,Citation3. Patients who have had a transplant are severely immunocompromised due to use of immunosuppressive agents and have a substantially higher risk (between 1.5 and 2.2 times) of developing a lymphoma after transplant (PTLD), compared with the general populationCitation2,Citation4,Citation5. While HCT patients can receive immunosuppressants for up to 5 years after a transplant, SOT patients with a functioning transplant need to remain immunosuppressed for the rest of their lives to prevent allograft rejection and are therefore at risk of PTLD throughout their livesCitation1,Citation6,Citation7. There were a total of 39,718 SOTs performed in the US in 2019, of which kidney transplants were the most frequent, with 23,401 (59%) performedCitation8.
Kidney transplant recipients who develop PTLD within the first 2 years have high mortality rates compared with recipients without PTLDCitation9, with 1-year survival rates after diagnosis ranging from 51 to 73%, and 5-year survival between 39 and 61% in international registries and between 59 and 64% in US studiesCitation10–15. The high mortality in PTLD is in part due to the complexity of the disease and its wide range of histologic features, which makes PTLD diagnosis challenging and management difficult, as well as the limited treatment options available for these patients, and lack of consistent treatment approach indicated for PTLDCitation4,Citation13. Survival has improved in recent decades with better management and understanding of transplants and PTLD; however, many patients with PTLD do not respond to available therapies and die quickly after developing the diseaseCitation4,Citation9,Citation16,Citation17.
The quick onset of death and high mortality in PTLD can lead to increased costs and healthcare resource utilization (HRU); however, with the difficulties in diagnosing PTLD and the small number of patients, data on the economic burden of this disease in SOTs are limited. SOTs represent a significant financial investment: kidney transplants, the least expensive SOT, have a chargeable amount of ∼$442,500 per transplant procedure in the USCitation18. Despite the rarity and low incidence of PTLD, limited data suggest that the disease is associated with increased hospitalization and HRU. A German database analysis showed that in SOT patients with PTLD in whom initial rituximab treatment failed, ∼20% of the time after PTLD diagnosis was spent in hospital, with approximately half of that time spent in the intensive care unitCitation19. No such data have been published from US databases. Therefore, the full extent of costs and HRU, especially with paid amounts, in transplant recipients with and without PTLD in the US is still unclear.
PTLD can compromise the substantial clinical and economic investments made towards a successful kidney transplant and add to the overall costs. In this study, we sought to determine the HRU and cost burden of PTLD following diagnosis and evaluate whether this differs by survival status within the first 2 years post-transplant.
Methods
Kidney transplants were examined in this study as they are the most frequent transplants in the US. This study used data from the Scientific Registry of Transplant Recipients (SRTR)Citation20. The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This study also utilized data from The United States Renal Data System (USRDS)Citation21. End-stage kidney disease (ESKD) patients are eligible for Medicare following transplantation or dialysis, and their Medicare data are collected by the USRDS with paid amounts. Patients with successful kidney transplants are only eligible for Medicare for up to 3 years following the date of transplant. Patients aged ≥62 years at transplant may age into Medicare by crossing the age threshold of 65 years as their ESKD eligibility expires. To gain information about PTLD diagnoses following transplants, the SRTR was examined. The SRTR is a national database that records information from transplant centers on all SOTs in the US, including data on malignancies after transplantation (e.g. PTLD). Utilizing USRDS and SRTR data from 2007 to 2016, we identified Medicare-covered kidney transplant recipients. SRTR malignancy data were used to identify patients with a PTLD diagnosis, which were linked to the USRDS. Patients were required to have a USRDS claim on the date of transplantation.
There are four components of Medicare insurance: Part A (hospital insurance), Part B (medical insurance – office visit and durable medical equipment), Part C (private Medicare health plan), and Part D (prescription drug coverage)Citation22. Due to the focus of this study on HRU and medical costs, only patients enrolled in Medicare Part A and Part B fee-for-service were included. Patients who underwent single-organ transplants and re-transplants were included in our analysis, whereas multi-organ transplant recipients were excluded due to concerns of likely differences in HRU and costs. We tabulated Medicare Part A (inpatient [including intensive care unit admission], outpatient, home health, hospice, skilled nursing facility costs, dialysis and emergency room [ER] visits) and Part B (physician/carrier and durable medical equipment claims) HRU and paid amounts leading up to and after the PTLD diagnosis date, as provided by the OPTN report date.
We examined pre-PTLD costs 3–6 months (91–180 days) and 3 months (≤90 days) before diagnosis, as well as post-PTLD costs in the first 2 years after diagnosis. In addition, costs were analyzed annually for the first 3 years following transplantation and reported by PTLD status. Each year post-transplant was calculated as 365.25 days per year following the kidney transplant. Kidney recipients with a PTLD diagnosis any time within 365.25 days following their kidney transplant procedure were considered as PTLD patients within 1-year post-transplant.
In the pre-PTLD diagnosis cost analysis, costs were censored to exclude those occurring before and on the transplant date and subsequent transplant hospitalization. For instance, in a recipient with a PTLD diagnosis 60 days post-transplantation, we included costs occurring after the transplant date up to the day before the PTLD diagnosis date. Such patients would contribute no data from the 3–6 months pre-PTLD period. Furthermore, cost information was censored at the loss of Medicare eligibility, death, or at the end of the 3-year post-transplant period.
Medicare payment data for those diagnosed with PTLD were stratified by death within <6 months, 6 months to 1 year, and 1–2 years of diagnosis, to allow for meaningful comparisons of cost and HRU data. Per patient-year (PPY), per patient-6 months (PP6M), per patient-3 months (PP3M), and per patient-month (PPM) were computed to standardize costs and were stratified by patient survival status. PPY costs were calculated as the sum of costs per claim type divided by the number of observed patient-years or patient-months; 95% confidence intervals were computed using percentile bootstrap methods using 2,000 samples. Since this study was conducted using an existing database of de-identified healthcare claims, it did not require institutional review board/independent ethics committee approval. For aggregate data fewer than 10 individuals, results have not been disclosed to prevent patient identification; in such cases, counts and costs are suppressed and replaced with an asterisk instead.
Results
A total of 165,098 kidney transplant recipients were identified between 2007 and 2016, 83,818 (50.77%) of which were Medicare-covered kidney transplant recipients and 81,280 (49.23%) were non-Medicare covered recipients. Among the Medicare-covered kidney transplant recipients, 347 had PTLD and were Medicare eligible on the date of PTLD diagnosis and had at least one claim during the first year after diagnosis. Of the PTLD patients, 67% were male, with an average age of 56 years at transplant (standard deviation [SD] ± 18 years) and 58 years at PTLD diagnosis (SD ± 19 years). Pediatric patients (those aged 0–17 years) comprised 5.5% of the total PTLD population examined. In this total PTLD population, median time from transplant to PTLD diagnosis was 1.30 years (interquartile range 0.68–2.70 years). In total, 214 (62%) patients were alive 2 years post-PTLD diagnosis.
Medicare payments after kidney transplant
Part A + B Medicare costs in all kidney transplant patients are reported in . Part A + B costs were $83,546 PPY in the first year post-transplant (n = 83,818), with approximately two-thirds of the cost coming from inpatient hospitalization (Table S1 in Supplementary Information). In the second and third years post-transplant, Part A + B expenditure was $26,148 PPY (n = 61,563) and $25,326 PPY (n = 48,120), respectively, with costs primarily coming from inpatient (39%) and Part B costs (37–38%; , Table S1). First-year post-transplant costs were considerably higher than the second and third years as they included the costs of transplant hospitalization.
Figure 1. Part A + B Medicare costs by year after transplant. Part B costs consisted of office visit and durable medical equipment claims. Abbreviations. n, number of patients; PPY, per patient-year; PTLD, post-transplant lymphoproliferative disease.
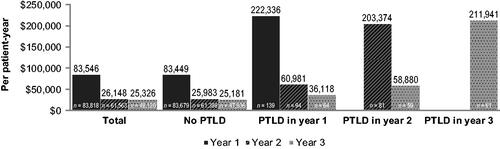
Table 1. Medicare payments by time from PTLD diagnosisa.
Medicare payments in kidney transplant patients were further examined by PTLD status. Patients who developed PTLD in the first year post-transplant had Part A + B costs of $222,336 PPY (n = 139), while patients without PTLD diagnosis in the same year had Part A + B costs of $83,449 PPY (n = 83,679; ). In both cases, two-thirds of the costs were inpatient costs (Table S1, S2). Patients who received a PTLD diagnosis in the first year post-transplant and survived had PPY costs of $60,981 (n = 94) in year 2 and $36,118 (n = 64) in year 3 ().
Table 2. HRU by time from PTLD diagnosisa.
Patients who were diagnosed with PTLD in the second year post-transplant incurred costs of $203,374 PPY (n = 81), 60% of which were inpatient costs (, Table S2). Those who survived into year 3 incurred costs of $58,880 PPY (n = 59). Furthermore, patients who were diagnosed with PTLD in the third year following kidney transplantation faced average costs of $211,941 PPY (n = 61), 68% of these coming from inpatient costs (, Table S2).
Medicare payments and HRU among patients diagnosed with PTLD
Medicare costs were examined in PTLD patients by time from diagnosis, including 3–6 months prior to diagnosis, 3 months prior to diagnosis, and in the first and second years after PTLD diagnosis. For the first 3 months prior to PTLD diagnosis, Part A + B costs were $12,351 PP3M (n = 356), while for the 3–6 months prior to diagnosis Part A + B costs were $7,493 PP3M (n = 350; ). For the 3 months prior to PTLD diagnosis, costs were driven by inpatient (44%) and then Part B costs (35%), while for the 3–6 months prior to PTLD diagnosis costs were driven by both Part B costs (37%) and inpatient costs (30%; ). Part A + B costs were $145,871 PPY 1 year after PTLD diagnosis (n = 347; ). Inpatient hospitalization represented up to 60% of PPY costs in this year. Part A + B costs were $40,289 PPY (n = 175) in year 2 after PTLD diagnosis, with inpatient costs representing 42% of PPY in this year (). This was a 3.6-fold decrease in Part A + B costs from year 1 to year 2 after PTLD diagnosis.
HRU was examined in PTLD patients over the same timeframes. The average number of inpatient claims per PTLD patient was 0.12 in the 3–6 months before diagnosis and 0.18 in the 3 months before diagnosis, increasing to 1.93 in year 1 and 0.51 in year 2 post-diagnosis ().
A higher percentage of patients had ER visit claims in the 3 months prior to diagnosis (35%, n = 165) than in the 3–6 months prior to diagnosis (15%, n = 72; ). Following PTLD diagnosis, the percentage of patients with ER claims increased substantially, to 53% (n = 185) and 51% (n = 91) in the first and second year, respectively. The percentage of patients with outpatient claims was consistently high in years 1 and 2 (∼97%); however, the percentage of patients with inpatient claims decreased in the second year post-PTLD diagnosis, from 69% (n = 240) to 30% (n = 53; ).
Cost burden of PTLD by survival status
Costs at 2 years from PTLD diagnosis were examined based on patient survival status: those who died within 2 years versus those who were alive at 2 years. All cost components by PPY were markedly higher for patients who died within 2 years compared with those who were alive (). Inpatient hospitalization accounted for the majority of costs; that is, 68% of costs for patients who died within 2 years of PTLD diagnosis and 51% for those who were alive at 2 years (). The cost burden for PTLD patients who died within 2 years of PTLD diagnosis was >3.3 times higher than PTLD patients who were still alive 2 years after diagnosis: $249,850 PPY (n = 133) versus $75,001 PPY (n = 214), respectively (). Part B costs in particular were substantially higher in those who died within 2 years at $39,447 PPY (n = 131), which compares with $16,014 PPY (n = 213) for those alive at 2 years ().
Table 3. Medicare payments by survival status at 2 years after PTLD diagnosisa.
Of those PTLD patients who died within 2 years, the majority died within 6 months of diagnosis (65%, n = 86; ). These patients had 6.7 times higher costs compared with those patients who were alive at 2 years (n = 214): $250,045 PP6M versus $37,501 PP6M, respectively (, ). Costs were lower in patients who died between 6 months and 1 year after diagnosis ($29,736 PP6M, n = 21), and lower still in those who died between 1 and 2 years after diagnosis ($24,738 PP6M, n = 26; ).
Table 4. Part A + B Medicare payments in PTLD patients who died within 2 years of diagnosis by time of deatha.
Discussion
In this study, we demonstrated that Medicare expenditure among kidney transplant recipients with PTLD patients are high, at $222,336 PPY for those diagnosed in the first year post-transplant. If these patients survived into their second and third year post-transplant, their costs reduced considerably, to $60,981 and $36,118 PPY, respectively. This cost contrasts with Medicare expenditures in all kidney transplants, at $83,546 PPY in the first year, $26,148 PPY in the second year and $25,326 PPY in the third year post-transplant. Patients diagnosed with PTLD in year 2 or 3 post-transplant had similar high annual costs (∼$200,000 PPY) for the first year of diagnosis as patients diagnosed with PTLD in year 1, demonstrating that PTLD costs are high in the first year of diagnosis regardless of the timing after transplant. These high costs were coupled with the rapid onset of PTLD, as most PTLD diagnoses occurred in the first year post-transplant (n = 139). This suggests that the main cost burden of PTLD occurs within 1 year of diagnosis and represents a substantial Medicare expenditure, in addition to the already high year 1 expenses of a kidney transplant.
This study suggests the cost burden of PTLD becomes apparent as early as 6 months prior to PTLD diagnosis. While these costs are dwarfed by those incurred in the first year post-PTLD diagnosis, they show that the burden of PTLD may start early, as costs and HRU claims rise 3–6 months prior to diagnosis. Earlier or improved treatments may therefore be important to decrease costs in these patients. The average number of HRU inpatient claims per PTLD patient increased as time approached the date of PTLD diagnosis. This was consistent with the higher proportion of patients with hospitalization claims and Medicare payments seen in these patients during the 3–6 and 3 months prior to diagnosis and further exhibited the increase in economic burden the closer the patients came to diagnosis. The percentage of patients with inpatient claims increased in the 3 months pre-PTLD diagnosis and then again in the year after diagnosis. Percentage of inpatient claims dropped off after this time period, suggesting that patients with PTLD were more likely to be unwell and admitted to hospital immediately before and after diagnosis. This increase in HRU and Medicare expenditures, coupled with a decrease in patient health, has both clinical and resource use implications: the correlation of costs, HRU and health could be used to more accurately predict the overall costs of kidney transplants and PTLD to insurance providers and hospitals.
By examining PTLD based on survival status, we could ascertain the end-of-life costs and if this was different over time. PTLD patients who died within 2 years of diagnosis had costs >3.3 times higher than PTLD patients who were alive at 2 years, showing that the majority of PTLD costs are incurred by patients who do not survive. More importantly, most PTLD patients who died within 2 years died within the first 6 months, and those who died within the first 6 months had almost 7 times higher costs than patients who survived. The majority of these costs came from inpatient hospitalizations, which is consistent with high end-of-life care in these patients, particularly in those who die within the first 6 months. The considerable risk of death within 6 months of diagnosis combined with its quick onset serve as a major driving factor for the high cost and HRU burden observed in the first 2 years after a PTLD diagnosis, and further illustrate the disproportionate burden of PTLD on the US healthcare system.
To the authors’ knowledge, this study is the first to examine the cost burden of PTLD in kidney transplant patients. There is a lack of literature on costs and HRU for PTLD following kidney transplants, particularly based on survival status. There is currently no literature examining Medicare expenditures of PTLD in the US; clearly a substantial data gap exists. Despite the rarity of PTLD, this study has shown its significant burden on the healthcare system; therefore, additional studies examining its economic burden are needed. A recent registry analysis by Francis et al. showed that transplant recipients with PTLD had increased excess mortality in the first 2 years post-transplant compared with controls, but not afterCitation9. Our study demonstrated that most of these patients die within the first 6 months, providing a clearer picture of where the cost and HRU burden of PTLD lies. Further research is needed in this area to confirm our findings and to establish if there is a cost difference between rapid- and late-onset PTLD and determine what risk factors of PTLD impact survival. In addition, there is an urgent need for new PTLD treatments with better outcomes and survival, as many patients do not respond to available therapies and succumb to this extremely aggressive and often fatal diseaseCitation4,Citation9,Citation16,Citation17. Better treatments for PTLD will help ensure that transplants are successful and reduce the cost of care in a successful transplant.
This study has certain limitations. Medicare costs were the only costs examined as part of this analysis and may not be representative of other costs incurred, or generalizable to other insured populations such as commercially insured patients, those with dual Medicaid-Medicare coverage, or government-insured patients. PTLD is more common in pediatric kidney recipients, who have a higher percentage of private health insurance than adult kidney recipients, therefore, our analysis of Medicare claims data may be incomplete for the entire PTLD populationCitation23–25. It should be noted that patients were only Medicare eligible for 3 years after transplant unless aged ≥62 years, therefore any costs after this cut-off were not included in the analysis, and the costs presented in this study are associated only with PTLD within the first 3 years after transplantation. The data examined in this study do not provide information on PTLD severity or type of PTLD, therefore it was not possible to assess the relationship between disease severity and cost or HRU burden. Kidney transplants were the only SOTs examined; kidney transplants are among the least expensive SOTs and therefore the costs reported here are not representative of other SOTs. PTLD costs may be substantially higher in transplants such as heart and lung, in which the cost of care is proportionally more expensiveCitation18,Citation26. Furthermore, treatment costs within the inpatient setting are not separate from the diagnosis-related group costs, therefore treatment costs for PTLD and non-PTLD patients could not be determined.
This analysis included 347 PTLD patients who were Medicare-eligible and had at least one Medicare claim during the first year after PTLD diagnosis; however, PTLD may be under-reported, as this study only included and followed patients who had transplant hospitalization claims and those whose PTLD diagnosis was confirmed via malignancy forms. The USRDS and OPTN databases have not been validated for PTLD, therefore it is possible that the true number of PTLD patients in these databases is higher than that reported in this studyCitation27. Consequently, the HRU and costs of PTLD post-kidney transplant could be different in the full PTLD population to what is reported here.
Despite its limitations, the current analysis is strengthened by the robust data sources used for the patient-level information examined. The USRDS and SRTR registries are among the most comprehensive kidney registries in the US, and our analysis comprises a large and representative sample of 83,818 patients. To ensure costs were standardized as much as possible, we computed PPY and PPM costs based on Medicare paid amounts. We used one set of costs as a basis for our analysis (paid amounts) to ensure consistent reporting and analysis of these data.
Conclusions
PTLD represents a considerable HRU and cost burden following a kidney transplant, regardless of the year of diagnosis. This cost burden for PTLD patients who died within 2 years of diagnosis was >3.3 times higher than PTLD patients still alive after 2 years. Among those who died within 2 years, the majority died within the first 6 months and costs were highest for these patients, with almost 7 times higher costs than PTLD patients who were still alive after 2 years. Additional studies are needed to determine whether this high cost burden and healthcare utilization is modifiable, through earlier or improved treatments.
Transparency
Declaration of funding
This study was funded by Atara Biotherapeutics.
Declaration of financial/other interests
Crystal Watson and Arie Barlev are employees and stockholders of Atara. There are no other financial/other interests to declare by the authors.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed equally to the concept/design, drafting, critical revision and approval of this original article. Statistics and data collection were contributed by Yoon Son Ahn, MS; Bryn Thompson, MPH and Melissa Skeans, MS. Funding for this study was secured by Crystal Watson, MS. All authors agree to be accountable for all aspects of this article.
Acknowledgements
Medical writing assistance was provided by Manca Povsic, PhD, and Max Harris, of AMICULUM, funded by Atara Biotherapeutics. The authors would like to thank all participating patients and their families.
Geolocation information
Registry data used in this study were collected across the US, while the analyses were conducted at Hennepin Healthcare Research Institute, Chronic Disease Research Group, Minneapolis, Minnesota, US and Atara Biotherapeutics, South San Francisco, California, US 94080 (as available per author affiliations).
Previous presentations
The analyses reported in this study have previously been presented as abstracts at the International Society of Pharmacoeconomic and Outcomes Research US Congress 2020 and the 62nd American Society of Hematology Annual Meeting.
Data disclaimers
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government.
Supplemental Material
Download MS Word (33.6 KB)References
- National Organisation for Rare Disorders. Post-transplant lymphoproliferative disease 2018. [cited 2020 October]. Available from: https://rarediseases.org/rare-diseases/posttransplant-lymphoproliferative-disorders/
- Liu M, Husain S, Famure O, et al. Incidence, risk factors, clinical management, and outcomes of posttransplant lymphoproliferative disorder in kidney transplant recipients. Prog Transplant. 2019;29(2):185–193.
- Sardella M, Belcher G. Pharmacovigilance of medicines for rare and ultrarare diseases. Ther Adv Drug Saf. 2018;9(11):631–638.
- Al-Mansour Z, Nelson B, Evens A. Post-transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep. 2013;8(3):173–183.
- Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2018 annual data report: kidney. Sci Registry Transpl Recipients. 2018;20:20–130.
- Orlando G. Finding the right time for weaning off immunosuppression in solid organ transplant recipients. Expert Rev Clin Immunol. 2010;6(6):879–892.
- Pidala J, Lee S, Quinn G, et al. Variation in management of immune suppression after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(10):1528–1536.
- United Network for Organ Sharing. Transplant Trends 2020. [cited 2020 September]. Available from: https://unos.org/data/transplant-trends/.
- Francis A, Johnson D, Craig J, et al. Post-transplant lymphoproliferative disease may be an adverse risk factor for patient survival but not graft loss in kidney transplant recipients. Kidney Int. 2018;94(4):809–817.
- Faull R, Hollett P, McDonald S. Lymphoproliferative disease after renal transplantation in Australia and New Zealand. Transplantation. 2005;80(2):193–197.
- Caillard S, Cellot E, Dantal J, et al.; French PTLD Registry. A French cohort study of kidney retransplantation after post-transplant lymphoproliferative disorders. Clin J Am Soc Nephrol. 2017;12(10):1663–1670.
- Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(2):222–230.
- Caillard S, Lelong C, Pessione F, et al.; French PTLD Working Group. Post-transplant lymphoproliferative disorders occurring after renal transplantation in adults: report of 230 cases from the French Registry. Am J Transplant. 2006;6(11):2735–2742.
- Caillard S, Dharnidharka V, Lawrence A, et al. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80(9):1233–1243.
- Hauke R, Smir B, Greiner T, et al. Clinical and pathological features of posttransplant lymphoproliferative disorders: Influence on survival and response to treatment. Ann Oncol. 2001;12(6):831–834.
- Fox C, Burns D, Parker A, et al. EBV-associated post-transplant lymphoproliferative disorder following in vivo T-cell-depleted allogeneic transplantation: clinical features, viral load correlates and prognostic factors in the rituximab era. Bone Marrow Transplant. 2014;49(2):280–286.
- Styczynski J, van der Velden W, Fox C, et al.; on behalf of the Sixth European Conference on Infections in Leukemia, a joint venture of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation (EBMT-IDWP), the Infectious Diseases Group of the European Organization. Management of Epstein-Barr virus infections and post-transplant lymphoproliferative disorders in patients after allergenic hematologist stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101(7):803–811.
- Statista. Average amount charged for select organ transplantations in the U.S. as of 2020 2020. [cited 2020 October]. Available from: https://www.statista.com/statistics/808471/organ-transplantation-costs-us/.
- Zimmermann H, Xu H, Barlev A, et al. Burden of hospitalizations due to Epstein-Barr virus associated post-transplant lymphoproliferative disorder (EBV + PTLD) in patients who failed first line rituximab or rituximab plus chemotherapy following solid organ transplant (post-SOT): a retrospective chart review study of German PTLD registry. Blood. 2019;134(Suppl 1):abst 65.
- Scientific Registry of Transplant Recipients. Data that drives development [cited 2020. October]. Available from: https://www.srtr.org/about-the-data/the-srtr-database/.
- US Renal Data System. 2019 Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2019.
- Medicare.gov. Your Medicare coverage choices [cited 2021 February]. Available from: https://www.medicare.gov/what-medicare-covers/your-medicare-coverage-choices
- Hyun H, Park E, Cho M, et al. Post-transplant lymphoproliferative diseases in pediatric kidney allograft recipients with Epstein-Barr virus viremia. J Korean Med Sci. 2019;34(30):e203.
- Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2018 Annual Data Report: Kidney. Rockville, MD: US Department of Health and Human Services; 2018. p. 1–70.
- Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. National Data - Transplants in the U.S. by Recipient Age and Primary Source of Payment. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration; 2016. p. 1–4.
- Milliman Research Report. 2020. U.S. organ and tissue transplants: cost estimates, discussion, and emerging issues 2020 [cited 2020 October]. Available from: https://milliman-cdn.azureedge.net/-/media/milliman/pdfs/articles/2020-us-organ-tissue-transplants.ashx.
- Kasiske BL, Kukla A, Thomas D, et al. Lymphoproliferative disorders after adult kidney transplant: epidemiology and comparison of registry report with claims-based diagnoses. Am J Kidney Dis. 2011;58(6):971–980.
