Abstract
Aims
Nivolumab has been approved for advanced squamous and non-squamous non-small cell lung cancer (NSCLC) following platinum-based chemotherapy in both Canada and Sweden. We aimed to determine the value-for-money of nivolumab versus docetaxel in a Canadian and Swedish setting based on 5-year data.
Methods
These cost effectiveness analyses used partitioned survival models with three mutually exclusive health states: progression-free, progressed disease, and death. All clinical parameters were derived from two registration phase 3 randomized trials, CheckMate 017 and CheckMate 057, with a minimum follow-up of 5 years. Treatment duration was based on time-on-treatment data from the clinical trials. Costs were derived from published sources. The primary outcomes of the analyses were quality-adjusted life-years (QALYs), life-years gained, and incremental cost-effectiveness ratios (ICERs). The model input parameters for each analysis were chosen in line with guidance from the respective HTA authorities.
Results
From a Canadian payer perspective, the ICERs were CAN$140,753 per QALY in the squamous population, and CAN$173,804 per QALY in the non-squamous population, assuming a 10-year time horizon and a 5% discount rate for both costs and outcomes. Sensitivity analyses demonstrated that changes to the discount rates for outcomes had the highest impact on the ICERs. In the Swedish analysis, the ICERs were SEK568,895 per QALY in the squamous population and SEK662,991 per QALY in the non-squamous population, assuming a 15-year time horizon, a 3% discount rate, and a 2-year maximum treatment duration for nivolumab. Sensitivity analyses demonstrated that the ICERs were most sensitive to changes in the discount rate for outcomes.
Conclusion
These updated analyses, based on more mature trial data with a minimum follow-up of 5 years, generate more favorable ICERs versus the previously submitted HTA assessments that resulted in approval of nivolumab for patients with previously treated NSCLC in Canada and Sweden.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide, with an estimated 1.8 million deaths in 2018Citation1. The four most commonly diagnosed cancers (lung, followed by breast, colorectal, and prostate) were expected to account for about half (48%) of all cancers diagnosed in Canada in 2019Citation2. An estimated 1 in 15 Canadians are expected to be diagnosed with lung cancer in their lifetimeCitation2. Lung cancer was also the leading cause of cancer death in Sweden, with 4004 new cases and 3849 deaths in 2018Citation3.
Lung cancer is associated with a poor prognosis. The 5-year survival rate for metastatic non-small cell lung cancer (NSCLC) is ∼2 to ∼13% in CanadaCitation4 and 1% in SwedenCitation5 with survival rates strongly associated with stage at diagnosisCitation4,Citation6. The introduction of immune checkpoint blockade to restore antitumor immunity has substantially changed the landscape of NSCLC and other cancer types.
Nivolumab is a fully human immunoglobulin G4 programmed death-1 (PD-1) immune checkpoint inhibitor antibody that disrupts PD-1–mediated signaling and restores host antitumor immunity. The efficacy of nivolumab in locally advanced or metastatic NSCLC after prior chemotherapy was demonstrated in two pivotal, randomized, phase 3 studies in squamous and non-squamous NSCLC: CheckMate 017 and CheckMate 057. CheckMate 017 recruited 272 patients with stage IIIB/IV squamous NSCLC who progressed after platinum-based chemotherapyCitation7. CheckMate 057 recruited 582 patients with stage IIIB/IV or recurrent non-squamous NSCLC after failure of platinum doublet therapyCitation8.
CheckMate 017 and CheckMate 057 are the first randomized phase 3 trials to report 5-year outcomes for a PD-1 inhibitor in patients with previously treated advanced NSCLCCitation9. In both studies, nivolumab was associated with a clinically and statistically significant improvement in the primary endpoint of overall survival (OS) compared with docetaxel. In CheckMate 017, median OS was 9.2 months (95% confidence interval [CI], 7.3–12.6) for nivolumab versus 6.0 months (95% CI, 5.1–7.3) for docetaxel (hazard ratio [HR], 0.62; 95% CI, 0.48–0.79; p < .001) with 5-year survival rates of 12.3 and 3.6%, respectively. In CheckMate 057, median OS was 12.2 months (95% CI, 9.7–15.1) for nivolumab versus 9.5 months (95% CI, 8.1–10.7) for docetaxel (HR, 0.70; 95% CI, 0.58–0.83; p < .001) with 5-year survival rates of 14.0 and 2.1%, respectivelyCitation9.
Nivolumab was associated with a significantly improved adverse event (AE) profile in both studies. In CheckMate 017, grade 3–4 treatment-related AEs were reported in 7% of nivolumab-treated versus 55% of docetaxel-treated patientsCitation7. In CheckMate 057, grade 3–4 treatment-related AEs were reported in 10% of nivolumab-treated versus 54% of docetaxel-treated patientsCitation8.
In 2014, nivolumab was approved by the US Food and Drug Administration (FDA) for the treatment of patients with advanced (metastatic) squamous and non-squamous NSCLC with progression on or after platinum-based chemotherapyCitation10,Citation11. Nivolumab was later granted approval by the European Medicines Agency for the treatment of patients with squamous NSCLC in 2015, and this approval was expanded to non-squamous disease in 2016Citation12. After regulatory approval in the United States (US) and Europe, cost-effectiveness analyses were undertaken in order to support health technology assessment (HTA) submissions in markets across the globe.
Canadian and Swedish settings
There is a strong alignment between the Canadian and Swedish healthcare systems and HTA procedures. In both countries, the national health services rely on a publicly funded healthcare payer model and cover all residents. Both countries also have national HTA agencies—the Canadian Agency for Drugs and Technologies in Health (CADTH) and the Swedish Dental and Pharmaceutical Benefits Agency (Tandvårds- och läkemedelsförmånsverket [TLV]), with legislated guidelines to inform evidence-based decisions on the pricing and reimbursement of new and existing healthcare technologies.
Nivolumab is approved in both Sweden and Canada for the treatment of adults with locally advanced or metastatic squamous and non-squamous NSCLC following prior chemotherapyCitation13,Citation14. At the time of HTA submission in Canada and Sweden, only 12 months of minimum follow-up for CheckMate 017 and 18 months of minimum follow-up for CheckMate 057 were available. The cost-effectiveness analyses developed using these data sets have been described in previous publicationsCitation13,Citation14.
With the availability of more mature data for CheckMate 017 and CheckMate 057, there is an interest to revisit the previously published cost-effectiveness analyses for squamous and non-squamous NSCLC. The 5-year data are expected to be more robust with less inherent uncertainty and can provide a valid reference point for the extrapolations in previous HTA submissions. A recently published paper revisited previous cost-effectiveness analyses for nivolumab in this population in the United Kingdom (UK) setting, and updated the clinical outcomes parameters using the 5 year data from CheckMate 017 and CheckMate 057 trialsCitation15. Further, an assessment of the cost effectiveness of nivolumab from the US perspective has been accepted for publicationCitation16. Here, we present the updated cost-effectiveness analysis of nivolumab in pre-treated NSCLC patients using a minimum of 5-years of follow up in both Canada and Sweden. The underlying model structure and the choice of fitted parametric survival functions remain the same as in the UK and US assessments.
Methods
Model framework
Cohort-based, partitioned survival models were developed to evaluate the incremental cost-effectiveness of nivolumab versus docetaxel in patients with advanced (metastatic; stage IIIb/IV) squamous and non-squamous NSCLC that had progressed during or after platinum-doublet chemotherapy. Models were developed with three mutually exclusive health states: progression-free (PF), progressed disease (PD), and death. In each cycle, patients were partitioned to each state based on cumulative PF and OS probabilities using individual patient data from the registrational trials, which had a minimum of 5 years of follow-up at the time of this analysis. The dosage of nivolumab applied was 240 mg every 2 weeks for Sweden and 480 mg every 4 weeks for Canada. This flat-dose regimen was clinically equivalent to the weight-based dose of 3 mg/kg used in the clinical trialsCitation7,Citation8. The flat-dose regimen for nivolumab was approved by the FDA in 2018Citation17,Citation18 and by Health Canada in 2018Citation19. The dosage of docetaxel applied was 75 mg/m2 every 3 weeks. Models were run over a patient’s lifetime in weekly cycles, applying a half-cycle correction.
The analysis perspective was that of third-party payers in both countries. The Canadian analysis assumed a 10-year time horizon and 5% discount rate on costs and outcomes in line with previous cost-effectiveness analysis used to support HTA submissionCitation13. A scenario was explored using a discount rate of 1.5% on costs and outcomes in line with updated local HTA guidanceCitation20. The Swedish analysis assumed a 15-year time horizon and 3% discount rate on costs and outcomes in line with local HTA guidanceCitation13,Citation14,Citation21.
The primary outcomes of the models were incremental cost per quality-adjusted life-years (QALYs) gained and incremental cost per life-year gained (LYG). Costs were expressed in CAN$ for Canada and SEK for Sweden.
Model inputs
Survival analyses
Parametric curves were fitted to the OS and progression-free survival (PFS) data from CheckMate 017 and CheckMate 057 and were extrapolated over a patient’s lifetime. The analysis for patients with squamous and non-squamous NSCLC was conducted separately comparing nivolumab with docetaxel using the intent-to-treat (ITT) population from CheckMate 017 and CheckMate 057 trials respectively, each with a minimum of 5 years of follow-up. Cumulative survival probabilities for PFS and OS were used to estimate the number of patients in the PF, PD, and death states at weekly intervals. Consistent with guidelines from the Decision Support Unit at the National Institute for Health and Care Excellence, the choice of parametric model for OS was informed by goodness-of-fit statistics assessed by Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC), visual fit to the CheckMate 017 and CheckMate 057 Kaplan–Meier (KM) data as well as external validation of model-predicted landmark survival against observed landmark survival from the 6-year follow-up data from CheckMate 003Citation22 (data on file, Bristol Myers Squibb), and clinical expert opinion.
For the squamous NSCLC population (CheckMate 017 trial), the assessment of log-cumulative hazards, log-cumulative odds, and Schoenfeld residual plots demonstrated that the proportional hazards assumption was not violated for OS for nivolumab versus docetaxel. Therefore, a single parametric model was fitted to the nivolumab and docetaxel data. A dependent two-knot spline on the hazards scale was determined to be the best fitting model for both nivolumab and docetaxel following the criteria described above.
Statistical testing demonstrated that the proportional hazards assumption was violated for PFS in the squamous population. A number of parametric and cubic spline-based models were fit to the PFS data for each arm independently. The one-knot spline model on the hazards scale was chosen to model PFS for both the nivolumab and docetaxel arms based on AIC/BIC, visual inspection, and clinical plausibility.
For the non-squamous population (CheckMate 057), the proportional hazards assumption was violated for OS for nivolumab versus docetaxel as the KM curves crossed at approximately 7 months. Therefore, independent parametric survival models were fitted separately to the nivolumab and docetaxel arms. The lognormal model was selected to model OS for both the nivolumab and docetaxel arms per the criteria described above.
The proportional hazards assumption was also not demonstrated for PFS for nivolumab versus docetaxel as the KM curves crossed at approximately 7 months in the non-squamous population. The two-knot spline model on the odds scale was selected as the best fitting curve for PFS for both nivolumab and docetaxel arms.
The final OS and PFS distributions used in the base-case analyses for nivolumab and docetaxel for both squamous and non-squamous NSCLC patients are shown in the Supplementary material (Figure S1). Distribution characteristics for the selected OS and PFS curves are shown in Supplementary material Table S5.
Figure 1. Tornado diagram for squamous (a) and non-squamous (b) NSCLC in Canada. Lower and upper limits of the OWSA shown in parentheses; all costs shown in CAN$. Abbreviations. ICER, incremental cost-effectiveness ratio; PF, progression-free; PD, progressed disease.
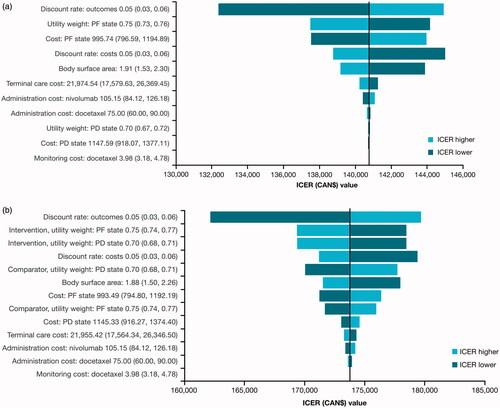
It is worth noting that these analyses remain unadjusted for treatment switching. In both CheckMate 017 and CheckMate 057, at the time when the initial analysis showed that nivolumab met its primary endpoint of superior OS, protocol amendments were developed as part of an extension study to offer crossover to nivolumab treatment for surviving patients who had been initially randomized to docetaxel. Although the impact in CheckMate 017 was fairly limited, analysis of the 2-year data for CheckMate 057 demonstrated that more than half (25 of 45) of docetaxel-randomized patients who were alive at 2 years had received treatment with an immunotherapy due to treatment crossover or subsequent treatmentCitation23.
Treatment duration
The proportion of patients on treatment in each cycle was modeled based on KM time-on-treatment data from CheckMate 017 and CheckMate 057. The base-case analysis for Sweden assumed a maximum treatment duration of 2 years for nivolumab, which is consistent with the HTA report and recommendations by TLVCitation24.
Similar guidelines are observed in the UK, where nivolumab is an option for patients with locally advanced or metastatic squamous NSCLC after chemotherapy for a treatment duration of up to 2 yearsCitation25. In addition, analyses of CheckMate 003, a phase 1/2 trial in previously treated patients with various solid tumors including NSCLC, which applied a 96-week treatment-stopping rule, demonstrated a similar long-term survival profile for nivolumab patients to CheckMate 017 and CheckMate 057Citation26. A 2-year treatment-stopping rule was also applied in KEYNOTE-010, a randomized phase 2/3 trial comparing pembrolizumab and docetaxel in patients with previously treated PD-L1–positive NSCLCCitation27.
In the Canadian setting, treatment duration was based on KM time-on-treatment data and a 2-year stopping rule was explored as a scenario.
The analysis accounted for patients receiving subsequent treatment following disease progression on nivolumab and docetaxel. For each comparator, the proportions of patients who received subsequent treatment were obtained from the clinical trials (Supplementary material Table S1)Citation7,Citation8. The mean duration of subsequent treatment was estimated as 3.5 months based on data from the LENS observational studyCitation28.
Costs
For both the Swedish and Canadian analyses, healthcare resource use was consistent with the analyses submitted to the TLV and CADTH, with 2019 unit costs applied. The analyses accounted for costs associated with disease management, drug acquisition, drug administration, monitoring, AEs, and subsequent treatment ().
Table 1. Cost and utility inputs in the models.
Acquisition costs for nivolumab, docetaxel, and subsequent treatments were based on retail prices. Drug costs for Sweden were obtained from a regional price list and for Canada were obtained from CADTH Economic Guidance for each drug respectively ()Citation29–33. Drug administration costs were estimated based on 2019 reimbursement rates for a 30-minute intravenous infusion in an outpatient setting from regional price lists in Canada and Sweden: Ontario Schedule of BenefitsCitation34 and TLV and Södra Regionvårdsnämnden prislista 2019Citation35, respectively (). Monitoring costs were based on health resources required for recommended clinical monitoring strategy ()Citation35. The aggregate subsequent treatment cost was computed based on the mean duration of treatment and weighted by the proportion of patients receiving each subsequent treatment (). Disease management costs were estimated based on monthly (4-week) health resources used for supportive care derived from published literature. These included routine physician office visits, radiotherapy, computerized tomography scans, and X-rays in the PF state; and additional blood transfusion, oxygen, and oncologist visits in the PD state (Supplementary material Table S2). The health resource use estimates were multiplied by unit costs derived from regional price lists and tariffs (2019) for the CanadianCitation34 and SwedishCitation35 diagnosis-related groups and adjusted to account for the weekly cycles used in the model. One-off end-of-life/terminal-care costs of CAN$21,975 and SEK76,927, based on published literature, were applied to each patient who died in Canada and Sweden, respectively (Supplementary material Table S3)Citation36,Citation37.
The frequency of grade 3–4 AEs that occurred in ≥5% of patients in CheckMate 017 and CheckMate 057 were included in the analysis. AE costs were based on a previously published trial-based analysis of nivolumab versus docetaxel. Unit costs for each AE, obtained from Wehler et al. for Canada and Smare et al. for SwedenCitation14,Citation38, were multiplied by the proportion of patients experiencing AEs from the 5-year update of the clinical trials to obtain the per-patient cost of managing AEs (). AE costs were applied as one-off costs in the first cycle.
Health-state utilities
Utility values for the PF and PD health states were derived from EQ-5D questionnaire data collected in the CheckMate 017 and CheckMate 057 trials. Canadian and Swedish specific weights were applied to derive country-specific utility valuesCitation39,Citation40, as shown in .
Utility decrements (disutilities) associated with AEs were identified in a review of previous HTA submissions and publications (Supplementary material Table S4)Citation41–43. Where disutility was not available for a specific AE, it was assumed to be 0. Disutilities associated with AEs were only applied in the first cycle of the model.
Sensitivity analyses
One-way sensitivity analysis (OWSA), scenario analysis and probabilistic sensitivity analysis (PSA) were conducted to assess the impact of uncertainty in model inputs on the outcomes. In the OWSA, individual parameters were varied over plausible ranges based either on 95% CIs for utilities, and ±20% for costs and body surface area. Discount rates were varied from 3 to 6% for Canada and 1 to 5% for Sweden. In the scenario analysis, time horizon, discount rate, utilities and nivolumab acquisition costs were varied. In the PSA, probability distributions were assigned to model parameters and 1000 Monte Carlo simulations were performed to explore the impact of joint uncertainty on outcomes. OS and PFS parameters were drawn from their corresponding parametric survival distributions accounting for correlation between shape and scale parameters. Costs and AE disutilities were drawn from gamma distributions and utilities were drawn from beta distributions.
Results
In Canada, in the squamous NSCLC population, the incremental cost per QALY gained was CAN$140,753 and the incremental cost per LYG was CAN$112,921 (), whereas in the non-squamous population, the incremental cost per QALY was CAN$173,804 and the incremental cost per LYG was CAN$139,530 for nivolumab versus docetaxel (). The incremental LYs and QALYs were 0.85 and 0.69 for the squamous population and 0.67 and 0.54 for the non-squamous population, respectively.
Table 2. Base-case results for the squamous (a) and non-squamous (b) NSCLC populations in Canada (CAN$).
In the Swedish setting, for the squamous population, the incremental cost per QALY gained was SEK568,895 and the incremental cost per LYG was SEK417,693 () and in the non-squamous NSCLC, the incremental cost per QALY gained was SEK662,991 and the incremental cost per LYG was SEK483,531 for nivolumab versus docetaxel (). The exchange rate in February 2021 was 1CAN$∼ 6.55SEK. The incremental LYs and QALYs were 1.28 and 0.94 for the squamous population and 1.03 and 0.75 for the non-squamous population, respectively.
Table 3. Base-case results for the squamous (a) and non-squamous (b) NSCLC populations in Sweden (SEK).
Sensitivity analyses were conducted with tornado diagrams and cost-effectiveness acceptability curves drawn for both Canada ( and ) and Sweden ( and ). Sensitivity analyses showed that the ICERs did not change significantly (remained within ±10%). The most sensitive parameter was the discount rate for outcomes for both countries. Additional scenario analyses varying time horizon, discounts rates, utilities, nivolumab dose and acquisition costs (±20%) and a medical cap at 2 years were conducted ( and ). Capping the cost of nivolumab at 2 years, varying the time horizon of analysis and the nivolumab acquisition cost had a significant impact on the ICERs in both Canada and Sweden. For Canada, a scenario was run with 15 years of time horizon to help compare with the Swedish base-case results. PSA results are consistent with the base-case analyses for each indication ( and ).
Figure 2. CEAC for squamous (a) and non-squamous (b) NSCLC in Canada. Abbreviations. CEAC, cost-effectiveness acceptability curve; QALY, quality-adjusted life-year; WTP, willingness to pay.
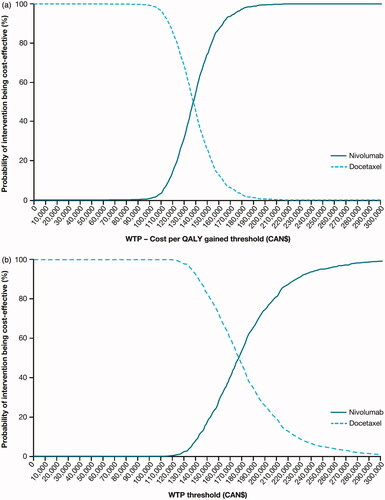
Figure 3. Tornado diagram for squamous (a) and non-squamous (b) NSCLC in Sweden. Lower and upper limits of the OWSA shown in parentheses; all costs shown in SEK. Abbreviations. ICER, incremental cost-effectiveness ratio; PF, progression-free; PD, progressed disease.
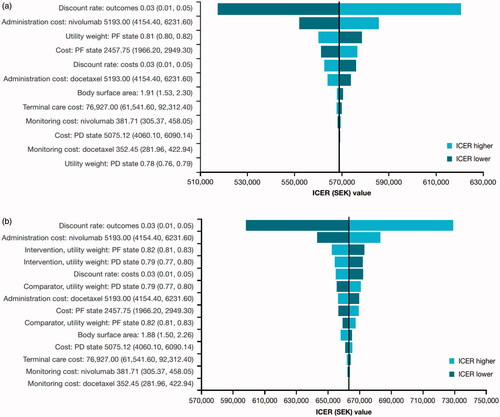
Figure 4. CEAC for squamous (a) and non-squamous (b) NSCLC in Sweden. Abbreviations. CEAC, cost-effectiveness acceptability curve; QALY, quality-adjusted life-year; WTP, willingness to pay.
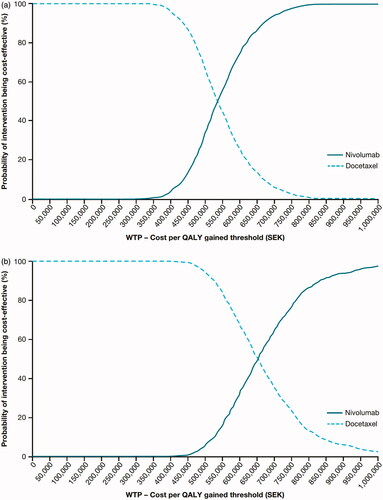
Table 4. Scenario analyses results for the squamous (a) and non-squamous (b) NSCLC populations in Canada (CAN$).
Table 5. Scenario analyses results for the squamous (a) and non-squamous (b) NSCLC populations in Sweden (SEK).
Discussion
The analyses used to support HTA submission for nivolumab in Canada and Sweden were based on 12-month data from CheckMate 017 and 18-month data from CheckMate 057. In the Canadian HTA submission, the estimated incremental cost per QALY was CAN$151,560 (vs. CAD$140,753 in updated 5-year analysis) for squamous and CAN$133,520 (vs. CAD$173,804 in 5-year analysis) for non-squamous NSCLCCitation13,Citation31. The Economics Guidance Panel conducted a review of the submitted economic evaluations and estimated an ICER between CAN$193,918 and CAN$219,660 per QALY for squamous and between CAN$183,386 and CAN$236,851 per QALY for non-squamous NSCLC. These ICERs were higher than those presented in current or previous resultsCitation13, mainly because of a shorter time horizon (5 instead of 10 years) and applying utilities from the literature (instead of trial-specific)Citation44. In the current analysis, a 5-year time horizon was deemed too short as 12% of squamous and 14% of non-squamous patients were alive at 5 years, based on KM OS data from CheckMate 017 and CheckMate 057, respectively. The review also assumed 10% drug wastage and increased the treatment duration of nivolumab for a mean duration of 2.9 (squamous) and 2.8 months (non-squamous) after disease progression.
The increase in ICER for non-squamous in the 5-year analysis versus our 18-month analysis (CAN$173,804 vs. CAN$133,520) was driven by higher incremental nivolumab costs (CAD$93,146 vs. CAD$84,918) because of improved PFS extrapolations (the proxy for duration of treatment) and lower incremental QALYs (0.54 vs. 0.64) and LYs gained (0.67 vs. 0.78). Although in the 5-year data the survival extrapolations for nivolumab were slightly better than in the 18-month data, the survival extrapolation for docetaxel also improved. It is possible that the impact of treatment crossover resulted in the increase of the QALY benefit for patients who received docetaxel, leading to a decrease in incremental QALYs in the 5-year versus the 18-month analysis for CheckMate 057 (where the OS curve was uninfluenced by crossover)Citation31. Effects of crossover of patients from docetaxel to nivolumab would introduce an upward bias in an ICER estimate influencing the decision-problem of whether to reimburse nivolumab. The OS curve fit from our original 18-month model predicted a 5-year landmark survival of 11.6% in the nivolumab arm versus the 14.0% OS observed in the 5-year data for CheckMate 057.
For the Canadian squamous analysis, the reduction in the ICER was driven by higher incremental QALY gains (0.69 vs. 0.66) when comparing 12-month and 5-year analyses. The updated OS curves resulted in a 4% increase in incremental LYs (0.85 vs. 0.82) over the 10-year time horizonCitation13,Citation31. OS estimates for nivolumab were more favorable using the 5-year data due to the OS plateau seen in the tail of the curve. It is worth noting that, although conservative, the OS curve fit from our original 12-month model predicted a 5-year landmark survival of 10.7% in the nivolumab arm versus the 12.3% OS observed in the 5-year data cut for CheckMate 017Citation13.
When considering the sensitivity analysis conducted with a 2-year treatment stopping rule, the ICER was reduced by 31% (CAN$120,507 vs. CAN$173,804) for the non-squamous and 28% (CAN$101,495 vs. CAN$140,753) for the squamous arm, respectively.
In Sweden, the New Therapies Council recommended nivolumab as a cost-effective treatment option for squamous and non-squamous NSCLC, supported by an analysis that included treatment stopping at 2 yearsCitation14. The original analyses were later updated with a minimum of 3 years of follow-upCitation14. Assuming a 2-year stopping rule, the 3-year analysis reported an incremental cost per QALY of SEK719,268 for squamous and SEK593,702 for non-squamous NSCLCCitation14. The 5-year analyses estimated an ICER reduction of 21% (SEK568,895 per QALY) and an increase of 12% (SEK662,991 per QALY) versus the 3-year analyses for squamous and non-squamous NSCLC, respectively.
Similar to the Canadian analysis, the increase in ICER in the non-squamous 5-year analysis in Sweden was due to higher nivolumab cost (SEK499,737 vs. SEK481,556)Citation14 resulting from improved PFS (and hence longer treatment duration) and lower incremental QALYs gain (0.75 vs. 0.81) perhaps resulting from substantial treatment switching to immunotherapies by non-squamous docetaxel patients, improving OS in these patients. The impact of longer extrapolations of PFS (and treatment duration) was attenuated in this context due to the assumed 2-year stopping rule.
For the squamous population, the reduction in the ICER was driven mainly by lower incremental costs (SEK535,333 vs. SEK719,268) and higher incremental QALYs (0.94 vs. 0.72) when comparing the 3- and 5-year analyses.
Without a treatment stopping rule, the total incremental costs increased slightly when comparing the 5- and 3-year analysis (SEK756,081 vs. SEK734,573) in the squamous populationCitation14. The ICER was reduced by 21% (SEK803,482 vs. SEK1,013,697 per QALY) primarily due to the increased QALYs (0.94 vs. 0.72) between the 5- and 3-year analysesCitation14. For the non-squamous population, the incremental costs remained lower (SEK732,823 vs. SEK999,032); however, incremental QALYs decreased slightly (0.75 vs. 0.81) compared with the 3-year analysis, resulting in an overall reduction in the ICER of 21% (SEK972,222 vs. SEK1,231,664 per QALY)Citation14.
The analyses using treatment stopping rules demonstrate reductions in the ICERs. However, a limited number of patients remained on treatment beyond 2 years, based on KM time-on-treatment data from CheckMate 017 and CheckMate 057. In the squamous population, 6.1 and 4.6% remained on treatment after 3 and 5 years, respectively, and in the non-squamous 7.7 and 4.2% remained on treatment at 3 and 5 years. Although there are no randomized data evaluating the utility of treatment after 2 years, other immunotherapy trials have included a maximum treatment duration for patients receiving second-line treatment for NSCLC. The CheckMate 003 trial included a stopping rule at 96 weeks and demonstrated a similar long-term survival profile for nivolumab patients to CheckMate 017 and CheckMate 057Citation9,Citation26,Citation45. A 2-year treatment stopping rule was also included in the KEYNOTE-010 study of pembrolizumabCitation27 in a population with previously treated PD-L1–positive NSCLC. HTA assessments in the United Kingdom and Sweden have also incorporated a 2-year stopping rule as part of the HTA recommendations for nivolumab in patients with previously treated NSCLCCitation24,Citation25.
Strengths and limitations
The models were based on versions previously submitted to CADTH and TLV HTA bodies. The trial data are considered mature relative to the data previously submitted, and therefore the OS and PFS extrapolations are associated with less uncertainty. Although the original economic evaluations were based on a 12- and 18-month data for squamous and non-squamous NSCLC, respectively, it is evident that the extrapolations remain valid when compared with estimates from CheckMate 003, CheckMate 017, and CheckMate 057, all of which have at least 5 years of follow-up.
The main limitation of the analyses pertains to the lack of accounting for treatment crossover from the docetaxel arm to the nivolumab arm when estimating the OS extrapolations for squamous and non-squamous NSCLC. This might have resulted in conservative ICER estimates as it is likely that the OS extrapolations for docetaxel would be overestimated. In addition, acquisition costs were based on retail prices because wholesale acquisition costs were not publicly available. Only grade 3–4 AEs were included in the models and disutilities were derived from published literature as they were not available from the trials.
It should also be noted that HTA agencies (CADTH and TLV in this case) prefer the use of utilities estimated from the trial data using respective national tariffs in the base case analysis, since they believe this reflects most accurately the health-related quality of life of patients in the specific trial context. However, the utilities taken from the trial may be overestimated because only patients in good health and able to stay in the trial are measured, while those who do not do well may leave the trial and be lost from a measurement perspective. In principle, although there was generally a good completion rate of the patient-reported outcomes in the trials and the EQ-5D data collection plan included assessment throughout survival follow-up (after the conclusion of randomized treatment), patients who continued completion of the questionnaire may not be representative of all eligible patients, some of whom may have discontinued responding to the EQ-5D. To address this potential limitation, a scenario analysis was conducted using the utilities from Chouaid et al. (2013)Citation44. The sensitivity analysis results should, however, be regarded as a lower bound or conservative estimate of the QALYs gained from nivolumab treatment, as the Chouaid et al. analysis was conducted during a period where standard of care was chemotherapy.
Conclusions
Trial data demonstrated that nivolumab is associated with increased OS and response rates compared with docetaxel in patients with advanced pre-treated squamous and non-squamous NSCLC. These analyses, based on trial data with a minimum follow-up of 5 years, suggest that nivolumab generates more favorable ICERs versus the previously submitted HTA assessments in both Canadian and Swedish settings that resulted in approval of nivolumab in previously treated patients with NSCLC. Future research should evaluate whether these trial- and model-based outputs could be replicated with long-term follow-up in a real-world setting.
Transparency
Declaration of funding
This study was funded by Bristol Myers Squibb.
Declaration of financial/other relationships
MAC, CH, KL, and JRP are employees of Bristol Myers Squibb. PD is an employee of Parexel International, funded by Bristol Myers Squibb to conduct the analysis. CS and CT are former Parexel employees.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to study conception and design, drafted the manuscript, and approved of the final version for submission. CS and CT performed the analysis.
Acknowledgements
Editorial support was provided by Parexel and was funded by Bristol Myers Squibb.
Supplemental Material
Download MS Word (137.9 KB)Data availability statement
The analysis reported in this study uses patient-level data from the CheckMate 017 and CheckMate 057 trial. The patient-level data are not publicly available, but the results of the trial have been reported in a number of publications. The trial results supporting the findings of this analysis are presented graphically within the article. The survival analysis was implemented in R using the flexsurv package.
References
- GLOBOCAN. Global Cancer Observatory 2018 – all cancers fact sheet; 2018. https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf.
- Canadian Cancer Society. Canadian cancer statistics; 2019. http://www.cancer.ca/∼/media/cancer.ca/CW/publications/Canadian%20Cancer%20Statistics/Canadian-Cancer-Statistics-2019-EN.pdf.
- International Agency for Research on Cancer. Sweden fact sheets. https://gco.iarc.fr/today/data/factsheets/populations/752-sweden-fact-sheets.pdf.
- Canadian Cancer Society. Suvival statistics for non-small cell lung cancer. https://www.cancer.ca/en/cancer-information/cancer-type/lung/prognosis-and-survival/non-small-cell-lung-cancer-survival-statistics/?region=on.
- Regionala Cancer Centrum I Samverkan. Lung cancer. https://www.cancercentrum.se/globalassets/cancerdiagnoser/lunga-och-lungsack/kvalitetsregister/rapport/20191015_nlcr_nationell_rapport_2018.pdf.
- Nilsson J, Berglund A, Bergstrom S, et al. The role of comorbidity in the management and prognosis in nonsmall cell lung cancer: a population-based study. Acta Oncol. 2017;56(7):949–956.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639.
- Borghaei H, Gettinger S, Vokes EE, et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723–733.
- European Society for Medical Oncology. FDA expands approved use of nivolumab to squamous NSCLC. https://www.esmo.org/oncology-news/FDA-Expands-Approved-Use-of-Nivolumab-to-Squamous-NSCLC.
- Oncology Times. FDA approves Opdivo for non-squamous NSCLC. Oncol Times. 2015;37(22):47.
- European Medicines Agency. Opdivo. https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo.
- Goeree R, Villeneuve J, Goeree J, et al. Economic evaluation of nivolumab for the treatment of second-line advanced squamous NSCLC in Canada: a comparison of modeling approaches to estimate and extrapolate survival outcomes. J Med Econ. 2016;19(6):630–644.
- Smare C, Venkatachalam M, Medin E, et al. An economic evaluation of nivolumab for the treatment of squamous and non-squamous NSCLC in the Swedish setting. Nordic J Health Econ. 2019;7(1):47–64.
- Rothwell B, Kiff C, Ling C, et al. Cost effectiveness of nivolumab in patients with advanced, previously treated squamous and non-squamous non-small-cell lung cancer in England. Pharmacoecon Open. 2020. DOI:https://doi.org/10.1007/s41669-020-00245-4
- Chaudhary A, Lubinga SJ, Smare C, et al. Cost-effectiveness of nivolumab in NSCLC patients in the United States. Am J Manag Care. 2021 (in press).
- OPDIVO (nivolumab) [prescribing information]. Princeton, NJ: Bristol Myers Squibb; 2014.
- Nivolumab dosing schedule approved for every 4 weeks. Oncol Times. 2018;40(7):28.
- OPDIVO [product monograph]. Montreal, Canada: Bristol Myers Squibb Canada Co.; 2020.
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada – 4th edition. https://www.cadth.ca/dv/guidelines-economic-evaluation-health-technologies-canada-4th-edition.
- Tandvårds- och läkemedelsförmånsverket. Prissökningar i databsen. https://www.tlv.se/beslut/sok-i-databasen.html.
- Topalian SL, Hodi FS, Brahmer JR, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5(10):1411–1420.
- Barlesi F, Steins M, Horn L, et al., editors. Long-term outcomes with nivolumab vs. docetaxel in patients with advanced NSCLC: CheckMate 017 and CheckMate 057 2-y update. Copenhagen, Denmark: European Society for Medical Oncology 41st Congress; 2016, October 7–11.
- Tandvårds- och läkemedelsförmånsverket. Underlag för beslut i landstingen. Opdivo (nivolumab). Koncentrat till infusionsvätska, lösning; 2016. https://www.tlv.se/download/18.467926b615d084471ac3396d/1510316400229/Kunskapsunderlag_opdivo_lungcancer_icke_skivepitel.pdf.
- National Institute for Health and Care Excellence. Nivolumab for previously treated squamous non-small-cell lung cancer; 2020. https://www.nice.org.uk/guidance/ta655/chapter/1-Recommendations.
- Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550.
- Solem CT, Penrod JR, Lees M, et al. Resource utilization among advanced squamous and non-squamous non-small cell lung cancer patients receiving second-line treatment in France, Germany, Italy, and Spain: results of a retrospective medical chart review. Value Health. 2015;18(7):A450.
- Apoteket. Apoteket price database 2019; 2019. https://www.apoteket.se/.
- Pan-Canadian Oncology Drug Review. Final economic guidance report: atezolizumab (Tecentriq) for non-small cell lung cancer; 2018. https://www.cadth.ca/sites/default/files/pcodr/pcodr_atezolizumab_tecentriq_nsclc_fn_cgr.pdf.
- Pan-Canadian Oncology Drug Review. Final economic guidance report: nivolumab (Opdivo) for non-small cell lung cancer; 2016. https://www.cadth.ca/sites/default/files/pcodr/nivolumab_opdivo_nsclc_fn_egr.pdf.
- Pan-Canadian Oncology Drug Review. Final economic guidance report: pembrolizumab (Keytruda) for nonsquamous non-small cell lung cancer; 2019. https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10153PembroNSQ-NSCLC_fnEGR_NOREDACT-ABBREV_Post_31May2019_final.pdf.
- Pan-Canadian Oncology Drug Review. Pan-Canadian oncology drug review initial economic guidance report: osimertinib (Tagrisso) for advanced or metastatic non-small cell lung cancer; 2018. https://cadth.ca/sites/default/files/pcodr/pcodr_osimertinib_tagrisso_nsclc_1stln_in_egr.pdf.
- Ontario Ministry of Health. Ontario schedule of benefits. http://www.health.gov.on.ca/en/pro/programs/ohip/sob/.
- Södra sjukvårdsregionen. Regionala priser och ersättningar; 2020. https://sodrasjukvardsregionen.se/verksamhet/avtal-priser/.
- Walker H, Anderson M, Farahati F, et al. Resource use and costs of end-of-life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J Palliat Care. 2011;27(2):79–88.
- Norwegian Medicines Agency (NoMA). Sweden's dental and pharmaceutical benefits agency (TLV). FINOSE joint assessment report: Tecentriq (atezolizumab); 2018. https://www.tlv.se/download/18.799b0a9f16b9029968838c8/1561553375250/bes_190617_tecentriq_eng.pdf.
- Wehler E, Zhao Z, Pinar Bilir S, et al. Economic burden of toxicities associated with treating metastatic melanoma in eight countries. Eur J Health Econ. 2017;18(1):49–58.
- Bansback N, Tsuchiya A, Brazier J, et al. Canadian valuation of EQ-5D health states: preliminary value set and considerations for future valuation studies. PLoS One. 2012;7(2):e31115.
- Burstrom K, Sun S, Gerdtham UG, et al. Swedish experience-based value sets for EQ-5D health states. Qual Life Res. 2014;23(2):431–442.
- Lloyd A, van Hanswijck de Jonge P, Doyle S, et al. Health state utility scores for cancer-related anemia through societal and patient valuations. Value Health. 2008;11(7):1178–1185.
- Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6(1):84.
- Tolley K, Goad C, Yi Y, et al. Utility elicitation study in the UK general public for late-stage chronic lymphocytic leukaemia. Eur J Health Econ. 2013;14(5):749–759.
- Chouaid C, Agulnik J, Goker E, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8(8):997–1003.
- Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20(10):1395–1408.
- Pan-Canadian Oncology Drug Review. pCODR expert review committee (pERC) final recommendation: nab-paclitaxel (Abraxane); 2014. https://www.cadth.ca/sites/default/files/pcodr/pcodr-abraxane-mpc-fn-rec.pdf.
- Ontario Ministry of Health. Schedule of benefits for laboratory services; 2020. http://www.health.gov.on.ca/en/pro/programs/ohip/sob/lab/lab_mn2020.pdf.
