Abstract
Aims
There is limited published evidence for the cost-effectiveness of treatments for unresectable or metastatic endometrial cancer (mEC). The objective of this analysis was to assess the cost-effectiveness of pembrolizumab versus chemotherapy for previously treated unresectable or mEC, in women whose tumors have deficient mismatch repair (dMMR) or high microsatellite instability (MSI-H). The analysis was carried out from a US healthcare payer perspective.
Materials and methods
A lifetime partitioned survival model comprising three health states (progression-free, progressed disease and death) was constructed. Chemotherapy was represented by single-agent paclitaxel or doxorubicin. Overall survival, progression-free survival and time on treatment data for pembrolizumab were obtained from a Phase II clinical study that included women with previously treated dMMR/MSI-H unresectable or mEC (KEYNOTE-158, NCT02628067). Survival data for chemotherapy were obtained from a published Phase III study for previously treated advanced endometrial cancer. Costs included were drug acquisition and administration, health-state, end-of-life, and adverse event management. Costs were presented in 2019 US$. Outcomes were calculated as quality-adjusted life-years (QALYs), using EQ-5D data from KEYNOTE-158. Model results were tested extensively in deterministic and probabilistic sensitivity analyses.
Results
Results demonstrated that pembrolizumab is a highly cost-effective treatment option when compared with chemotherapy, with estimated deterministic and probabilistic incremental cost-effectiveness ratios (ICERs) of $58,165 and $57,668 per QALY gained, respectively. Pembrolizumab was associated with a large QALY and life-year gain per person versus chemotherapy over the model time horizon (deterministic 4.68 life year gain, 3.80 QALYs), with the majority of QALYs accrued in the progression-free health state.
Limitations
The key limitation of the analysis was the lack of comparative effectiveness data for pembrolizumab versus chemotherapy.
Conclusions
Pembrolizumab is a highly cost-effective treatment option when compared with chemotherapy for women with previously treated dMMR/MSI-H unresectable or mEC. Results were robust to the changes in parameters and assumptions explored.
Introduction
Endometrial cancer forms in the tissue lining of the uterus, are generally adenocarcinomas and occur most frequently after menopauseCitation1,Citation2. In the US, endometrial cancer is the most common cancer of the female reproductive organs and the sixth most common cause of cancer death among women. In 2020, an estimated 65,620 women will be diagnosed with endometrial cancer in the US and an estimated 12,590 women will die from the diseaseCitation3. Metastatic endometrial cancer frequently involves the ovaries and other reproductive organs, and can often involve the lymphatic system, blood, lungs and other vital organsCitation4. The probability of survival is particularly poor for women with metastatic endometrial cancer (mEC, endometrial cancer that has spread to other organs or tissues); in the US, the 5-year survival probability for early stage endometrial cancer is 95% compared with just 17% for mECCitation3.
There is currently no agreement on the standard treatment for women with mEC; treatment can comprise a combination of surgery, radiotherapy and multi- or single-agent chemotherapy regimens, the choice of which depends on multiple factorsCitation5. The NCCN Clinical Practice Guidelines In Oncology (NCCN GuidelinesFootnotei) for recurrent, metastatic or high-risk endometrial cancer outline a range of combination and single-agent chemotherapy options for consideration, with platinum compounds, anthracyclines, and taxanes most commonly used; however, rates of response are typically lowCitation6.
The NCCN Guidelines include combination lenvatinib/pembrolizumab, pembrolizumab monotherapy, nivolumab monotherapy, and larotrectinib or entrectinib as biomarker-directed systemic therapy options for second-line treatment of recurrent, metastatic, or high-risk endometrial cancer, in certain circumstancesCitation6. In European guidelines, single-agent chemotherapies are preferredCitation7,Citation8. The European Society for Medical Oncology (ESMO) Clinical Practice Guidelines advise that, while several agents have been tested, only paclitaxel has consistently shown a response rate of more than 20%. Additionally, the British Gynaecological Cancer Society (BGCS) Uterine Cancer Guidelines note that paclitaxel, pegylated liposomal doxorubicin and topotecan are options at second line for women who have relapsed within 6 months of their prior chemotherapyCitation7,Citation10.
In the NCCN Guidelines for mEC, pembrolizumab is presented as a treatment option for tumors with high tumor burden (TMB-H), high microsatellite instability (MSI-H) or deficient mismatch repair (dMMR) and nivolumab is presented as a treatment option for dMMR. dMMR/MSI-H is a form of genomic instability, with estimates in the literature for prevalence in endometrial cancer ranging from 15% to 31% across all stagesCitation6,Citation12–17. Nivolumab is not approved for use in the US for mEC; however, pembrolizumab was granted accelerated approval by the Food and Drug Administration (FDA) for the treatment of unresectable or metastatic solid tumors with MSI-H or dMMR that have progressed following prior treatment in patients who have no satisfactory alternative treatment options, and for the treatment of unresectable or metastatic, MSI-H or dMMR colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. For solid tumors, approval was supported from the demonstration of anti-tumor activity for patients in KEYNOTE-158 (NCT02628067)Citation18,Citation19. KEYNOTE-158 is a Phase II, open-label, single-arm trial in which 1,151 people with previously treated unresectable or metastatic solid tumors were enrolled and treated with 200 mg pembrolizumab every 3 weeks. Of those enrolled, 49 participants had MSI-H/dMMR unresectable or mECCitation18. For these patients, the objective response rate was 57%, with 16% patients achieving complete response and 41% achieving partial response. Median progression-free survival (PFS) was 26 months, and 73% patients remained alive at 12 monthsCitation20.
Evidence suggests that dMMR/MSI-H could be a negative prognostic factor for survival in advanced disease. In a study carried out by Arabi et al. patients with high grade endometrial cancer with three negative biomarkers for MSI-H reported markedly poorer survival when compared to microsatellite stable tumors (median survival 3 months vs 71 months, p < .04)Citation11. Pembrolizumab has shown robust clinical benefit; however, the long-term cost-effectiveness of pembrolizumab in dMMR/MSI-H mEC has not been investigated. A search of the economic literature carried out in 2019 identified a single study that evaluated the cost-effectiveness of pembrolizumab in women with mEC; however, this study was limited to the short term, and the outcomes of the analysis were reported in terms of an incremental cost per 2-year survivorshipCitation21.
The objective of this analysis was to assess the cost-effectiveness of pembrolizumab versus chemotherapy in the treatment of women with unresectable or mEC whose tumors have dMMR/MSI-H and who have been previously treated with chemotherapy. The chemotherapy comparator was represented by the single-agent chemotherapies paclitaxel and doxorubicin, since these treatments are included as second-line treatment options in published guidelinesCitation9,Citation10. The analysis was carried out from the perspective of the US healthcare payer. Cost-effectiveness results are expressed as incremental cost per additional quality-adjusted life year (QALY) and life year (LY) gained, in line with the reference case for economic evaluations published by the Institute for Clinical and Economic Review, and uses a willingness-to-pay threshold of $100,000 per QALY, in line with updated evidence for the US-based thresholdCitation22,Citation23.
Methods
Model overview
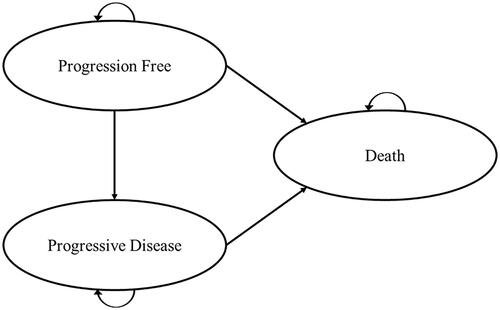
A partitioned survival model comprising three health states (progression free, progressed disease and death) was constructed in Microsoft ExcelFootnoteii to assess the cost-effectiveness of pembrolizumab versus chemotherapy for a cohort of women with previously treated dMMR/MSI-H unresectable or mEC (). Women with dMMR/MSI-H unresectable or mEC enter the model in the progression-free health state and, each cycle, can remain progression-free, transition to progressed disease, or die. Patients who transition to progressed disease can remain in the progressed disease health state or die. Death is an absorbing health state where once patients enter, they remain in this state for the remainder of the model time horizon. Overall survival (OS) and progression-free survival (PFS) were modelled based on parametric fitting of the OS and PFS KM data, while the proportion of patients in post-progression was estimated as the difference between OS and PFS. A partitioned survival model approach was selected given that models of this type are commonly used to model therapies in advanced or metastatic cancer and it was judged the most appropriate structure to incorporate the comparative trial evidence, given the limitations of the available dataCitation24.
The model has a lifetime duration (30 years), in line with the reference case for economic evaluations published by the Institute for Clinical and Economic Review, and has a cycle length of 1 week to ensure that chemotherapy regimens are accurately costedCitation22. Since the cycle length is small when compared with the model time horizon (1 week versus 30 years), a half-cycle correction was judged unnecessary.
The model considers a US healthcare payer perspective and includes all direct healthcare-related costs and effects. Healthcare costs included in the analysis were drug acquisition and administration (including for subsequent therapies), health state-related costs, end-of-life costs, and adverse event costs. Costs are presented in US$ for a cost year of 2019. Outcomes included were life years and QALYs. Costs and QALYs were accrued within each health state and were discounted at an annual rate of 3.0% from Year 1Citation22. Deterministic and probabilistic results were estimated. In addition, one-way sensitivity analysis and a range of scenario analyses were investigated to test the robustness of the model to changes in inputs and structural assumptions, in line with current health economic evaluation best practiceCitation25.
The analysis compared pembrolizumab 200 mg as an intravenous (IV) infusion administered every 3 weeks for up to 2 years (or until progression or death, if earlier), with chemotherapy. Chemotherapy was represented by the single agents paclitaxel or doxorubicin because these agents are included as second-line treatment options in published guidelinesCitation9,Citation10. Paclitaxel was assumed to be administered via IV infusion at a dose of 80 mg/m2 for 3 out of 4 weeks, until progression or withdrawal from treatment due to unacceptable toxicity. Doxorubicin was assumed to be administered via IV infusion at a dose of 60 mg/m2 every 3 weeks for a maximum of eight 3-weekly cyclesCitation26.
Model inputs
A summary of all inputs included in the analysis, and the upper and lower values tested in one-way sensitivity analysis, as well as the distributions used for probabilistic sensitivity analysis, is provided in .
Table 1. Summary of model inputs.
Efficacy data – overall survival and progression-free survival
PFS and overall survival (OS) data were included in the model for pembrolizumab and chemotherapy. For pembrolizumab, these data were obtained from KEYNOTE-158 and were extrapolated to obtain long-term estimates of survival.
For chemotherapy, PFS and OS data were obtained via a focused literature review. The focused search was designed to find studies of paclitaxel and/or doxorubicin that reported survival data for women with previously treated mEC. All the identified studies were reviewed and are presented in . None of the identified studies considered patients with dMMR/MSI-H unresectable or mEC. As MSI-H is a negative prognostic factor for disease, it is possible that these identified survival data could overestimate PFS and OS compared with women with MSI-H mECCitation11.
Table 2. Identified survival data for women with previously treated mEC treated with paclitaxel and/or doxorubicin
Of the data identified, McMeekin et al. and Miller et al. provided data from the largest cohorts of patients at n = 248 for McMeekin et al. and n = 255 for Miller et al.Citation27,Citation28 The remaining three studies included data from between 17 and 44 patientsCitation29–31. In addition, only McMeekin et al. included data for women with mEC who received either paclitaxel or doxorubicin; each other study reported data for either paclitaxel or doxorubicinCitation27–31. Therefore, in the base case analysis, the study published by McMeekin et al. in 2015 was used to estimate PFS and OS over the time horizon of the modelCitation27. McMeekin et al. presented results from a Phase III randomized trial of ixabepilone versus paclitaxel or doxorubicin for the second-line treatment of women with advanced endometrial cancerCitation27. In the comparator arm of this study, women received either paclitaxel (28%) or doxorubicin (72%), though survival data were combined, and not presented separately for paclitaxel or doxorubicin.
Women enrolled in this study were of a similar age to women with dMMR/MSI-H unresectable or mEC in KEYNOTE-158 (median 64 versus 65 years of age, respectively). At baseline, patient performance status in the McMeekin et al. study was not directly comparable with performance status from KEYNOTE-158; McMeekin et al. reported Karnofsky status and KEYNOTE-158 reported Eastern Cooperative Oncology Group (ECOG) scoresCitation18,Citation27. However, we note that women were included in the study reported by McMeekin et al. if they had locally advanced, recurrent, or metastatic diseaseCitation27. This contrasts with KEYNOTE-158, where women were enrolled with unresectable or metastatic disease only.
Data from all studies identified from the focused search were used in scenario analyses to test the robustness of the model; however, we note that McMeekin et al. reported the largest median OS of all the studies identified, and evaluated a similarly large number of patients as Miller et al. (248 patients in total)Citation27,Citation28.
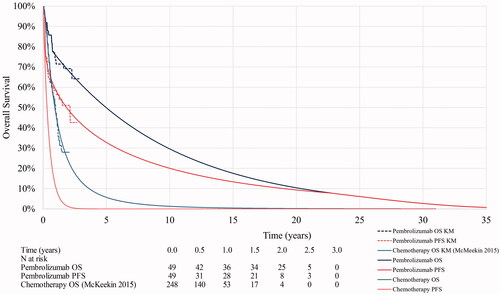
The modelled and observed OS and PFS for pembrolizumab and chemotherapy are presented in ; and are described in further detail in the sections below.
Overall survival
OS data for pembrolizumab were obtained from KEYNOTE-158 for women with dMMR/MSI-H unresectable or mEC. The cumulative hazard plot showed a change in hazard at Week 40, i.e. there was a different slope before and after around 40 weeks for pembrolizumab. Consequently, we considered that a piecewise model may be a more appropriate fit to the data and, alongside a range of one-piece parametric extrapolations (exponential, Weibull, log-normal, log-logistic, Gompertz, generalized gamma), we explored the fit of two-piece parametric extrapolations using Kaplan–Meier data to Week 40 and parametric extrapolation thereafter.
The fit of each distribution was assessed visually against Kaplan–Meier data, statistical fit was assessed using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC), and final selections were validated by two clinicians. The two-piece parametric extrapolations were considered to fit the data well, in particular, given the strong durability of response evident in the data, the two-piece Weibull and exponential produced the most clinically plausible extrapolations of the observed OS from KEYNOTE-158, and remained below the general population survival curve. The two-piece exponential curve was noted to be the most conservative option and was therefore selected for use in the base case. The selected OS curve is presented in . This selection was tested in sensitivity analysis. Visual and statistical fits are presented in the Supplementary Appendix.
OS data for chemotherapy were estimated by fitting one-piece parametric distributions to digitized Kaplan–Meier curves from the study by McMeekin et al. As no noticeable change in the rate of cumulative hazards over time was observed for this study, a two-piece approach was not explored. Instead, the log-normal parametric extrapolation was selected as the best fitting curve based on visual and statistical fit and was used in the base case; this choice was verified by two clinicians.
OS data for the general population was also included within the model and estimates of OS for pembrolizumab and chemotherapy were assumed not to exceed theseCitation32.
Progression-free survival
Similar to the approach taken for OS, PFS Kaplan–Meier data for pembrolizumab were obtained from KEYNOTE-158 for women with dMMR/MSI-H unresectable or mEC and extrapolated using one- and two-piece approaches. The cumulative hazard plot showed a change in hazard at Week 10 which coincided with the time of the first radiographic assessment in KEYNOTE-158. Thus, the two-piece approaches used Kaplan–Meier data to Week 10 and parametric extrapolation thereafter. As with OS, the fit of each distribution for PFS was assessed visually and statistically, with final selections validated by two clinicians. The two-piece parametric extrapolations were considered to fit the data well and the Weibull distribution was selected for use in the base case and was assumed not to exceed OS. The selected PFS curve is presented in . This selection was tested in sensitivity analysis. Visual and statistical fits are presented in the Supplementary Appendix.
PFS data for chemotherapy were obtained from McMeekin et al. using the reported median PFS (4 months) and applying an exponential distribution to estimate long-term survivalCitation27. No Kaplan–Meier data for PFS were reported in the study, so it was not possible to digitize these data and test a range of parametric distributions. However, PFS estimates for chemotherapy were tested extensively in scenario analysis using PFS reported and extrapolated from alternative data sourcesCitation28–31.
Adverse event management
Adverse events were included in the model as reported in the clinical trials for pembrolizumab and chemotherapy if they were Grade 3 or above and expected to require significant medical intervention or hospitalization. Data for pembrolizumab were obtained from KEYNOTE-158 for women with dMMR/MSI-H unresectable or mEC and, in the base case, adverse event data for chemotherapy were obtained from McMeekin et al. to be consistent with the source of data for PFS and OSCitation18,Citation27. Adverse event costs were sourced from the Agency for Healthcare Quality and Research or from literature and inflated to 2019 valuesCitation33. These costs were multiplied by the estimated adverse event incidence and adjusted using the within trial duration for long-term costs for fulminant type 1 diabetes and acute respiratory distress syndrome, with the resulting total cost of managing adverse events, per patient, was applied in the model for pembrolizumab ($4,041) and chemotherapy ($6,271) as a one-off cost in the first cycle (). The larger cost associated with chemotherapy is the result of the greater toxicity of chemotherapy compared to pembrolizumab.
Table 3. Adverse event incidence and cost.
Utilities
Utilities were included in the model to estimate QALYs for pembrolizumab and chemotherapy. For PFS and progressed disease, the utilities were derived from EQ-5DFootnoteiii scores collected from the population of patients with unresectable or mEC and dMMR/MSI-H in KEYNOTE-158, using a published, validated US-specific algorithmCitation18. The estimated utility values for PFS and progressed disease were 0.817 (95% confidence interval [CI] 0.797, 0.836) and 0.779 (95% CI 0.699, 0.859), respectively. Disutilities related to the loss of utility associated with experiencing an adverse event were considered in the analysisCitation34. A disutility associated with adverse events was not incorporated in the model in the base case based to a small sample of patients reporting EQ-5D values while experiencing adverse events (n = 4) with associated uncertainty. This exclusion was conservative since patients in the chemotherapy arm were more likely to experience toxicities; however, the impact of including adverse event disutility was explored in a scenario analysis.
Resource use and costs
Testing for dMMR/MSI-H status
In the model, to identify women eligible for treatment with pembrolizumab, a cost of testing for dMMR/MSI-H was applied to patients receiving pembrolizumab in the first cycle since women are eligible for pembrolizumab if they are identified as having solid tumors with dMMR/MSI-H which have progressed following prior treatment. This cost was not applied to chemotherapy since eligibility for treatment is not related to being identified as dMMR/MSI-H and would not be carried out in routine clinical practice.
The cost of dMMR testing (immunohistochemistry, $389.76) and MSI-H testing (polymerase chain reaction, $348.56) were included in the base case and obtained from the CMS Physician Fee Schedule and the CMS Laboratory Fee ScheduleCitation35,Citation36. Of those tested, the majority (67%) were tested for both MSI-H and dMMR, with 22% patients were assumed to receive testing for MSI-H only, 11% for dMMR only, which equated to a testing cost per person tested of $614.23. The assumptions reflect testing practice in the US for 2018/2019, based on market research [data on file]Citation37. Assuming a prevalence of MSI-H of 14.52% for unresectable or mEC based on data reported for late stage endometrial cancer from Le et al.Citation16, seven women would need to be tested to identify one patient eligible for treatment with pembrolizumab. Based on this, the one-off cost of testing for each patient identified as eligible to be treated with pembrolizumab was $4,230. This cost was applied in the first cycle of the model as a one-off cost.
Time on treatment
In the base case, Kaplan–Meier data for pembrolizumab time on treatment (ToT) were obtained from KEYNOTE-158 for women with dMMR/MSI-H unresectable or mEC and applied directly in the model, since these data were matureCitation18. ToT was censored at 104 weeks to align with the maximum treatment duration for pembrolizumab and, as patients were assumed to discontinue treatment upon progression, ToT was not permitted to exceed PFS. Parametric curves were fitted to the ToT data in sensitivity analysis.
ToT data for paclitaxel and doxorubicin were not reported in McMeekin et al. In the absence of data for ToT, PFS was used as a proxy. Paclitaxel and doxorubicin were administered up to a cumulative dose of 500 mg/m2, in line with FDA labelling and consistent with the dosing in the McMeekin studyCitation27,Citation38,Citation39.
This may have resulted in an overestimate of ToT for chemotherapy since a proportion of patients will discontinue treatment before progression due to adverse events or other reasons. This, in turn, may have resulted in an overestimate of cost for chemotherapy in the model. However, in the absence of data, this was considered to be a reasonable simplification and is not expected to impact results since the cost of paclitaxel and doxorubicin are low.
Drug costs
In the base case, the unit costs of pembrolizumab, paclitaxel and doxorubicin were obtained from the IBM Micromedex RED BOOKFootnoteiv, a US database providing drug prices for over 300,000 prescription and over-the-counter pharmaceuticals, chemicals used for compounding, and medical devices and supplies based on the wholesale acquisition cost (WAC)Citation40.
Drug costs were estimated for each of the regimens modelled (pembrolizumab, paclitaxel, and doxorubicin) and perfect vial sharing was assumed in the base case, i.e. no wastage was costed (). To estimate drug costs for paclitaxel and doxorubicin, assumptions around patient body surface area (BSA) were required, since both paclitaxel and doxorubicin are administered on a per m2 basis. These data were obtained from KEYNOTE-158 for women with dMMR/MSI-H unresectable or mEC; KEYNOTE-158 did not provide BSA data, but mean BSA was calculated from weight and height using the Du Bois formulaCitation18,Citation41. The average BSA was estimated to be 1.76 m2. The cost of paclitaxel and doxorubicin applied in the model was weighted based on the proportions observed in McMeekin et al., which were 72% for doxorubicin and 28% for paclitaxelCitation27.
Table 4. Dosing regimens and estimated costs for pembrolizumab, paclitaxel and doxorubicin.
In addition to the cost of pembrolizumab and chemotherapy, a cost associated with subsequent therapies was included in the model and applied at progression. For women with dMMR/MSI-H unresectable or mEC in KEYNOTE-158, only six patients received subsequent treatments (10.2%). The subsequent treatments most commonly received were pembrolizumab (n = 2) and tamoxifen (n = 2). Given that patients receiving pembrolizumab who progress are unlikely to continue treatment with pembrolizumab, and given the paucity of data to reliably inform subsequent treatment, it was assumed that all patients in the model who receive subsequent therapy (10.2%) received tamoxifen as subsequent treatment for 140 days (20 weeks); 140 days was the average treatment duration of subsequent therapies reported for the five dMMR/MSI-H unresectable or mEC patients receiving these therapies in KEYNOTE-158. In addition, pembrolizumab was not available to patients at the time of conduct of the chemotherapy trials. The cost of treatment was applied as a one-off cost at the point of progression and was estimated to be $93.36 based on an oral dose of 20 mg per day, and on acquisition costs reported in the IBM Micromedex RED BOOKCitation40,Citation42.
Administration costs
The cost of administering IV treatments was estimated to be $309.60 for the first hour of infusion (CPT 96413) and $60.47 for each additional hour (CPT 96417), based on cost data obtained from the CMS Hospital Outpatient Prospective Payment SystemCitation36,Citation43. Given assumptions made around duration of infusion for pembrolizumab and chemotherapy, the total cost per administration was estimated to be $154.80 for pembrolizumab (30 min infusion), $430.54 for paclitaxel (180 min infusion) and $51.60 for doxorubicin (10 min infusion).
Disease management costs
A focused literature review was carried out to identify studies reporting costs associated with the management of progression-free and progressed disease for women with dMMR/MSI-H unresectable or mEC in the US; however, no relevant studies were identified. Consequently, a micro-costing approach was undertaken to estimate these costs. It was assumed that patients would require one monthly outpatient visit and one computed tomography (CT) scan every quarter. These frequencies were obtained from a review of the management of ovarian cancer by the Institute for Clinical and Economic Review, with costs obtained from the Medicare Physician Fee ScheduleCitation36,Citation44. The resulting estimated weekly cost of PFS and progressed disease was $83.05, and this cost was applied at each cycle for patients remaining in the PFS or progressed disease health states.
End of life costs were sourced from a study of healthcare utilization and hospital expenditures for patients in the final 30 days of life in the US and inflated to current pricesCitation45. This cost was estimated to be $10,384 and was applied in the model at the point of death.
Approach to sensitivity analysis
Deterministic and probabilistic sensitivity analyses were carried out to test the robustness of model results. Deterministic analyses present results where model parameter input values are based on their mean estimate. By contrast, probabilistic analysis involves the simultaneous varying of all model input values according to an assigned distribution. In this analysis, model results are recorded for 5,000 iterations and mean costs and QALYs are estimated using results from all iterations. The distributions assigned to each model input are described in for each variable included within the probabilistic sensitivity analysis. The choice of distribution was made based on guidance from Briggs et al.Citation46 Results of the probabilistic sensitivity analyses were presented on a cost-effectiveness plane and cost-effectiveness acceptability curve.
A series of deterministic sensitivity analyses were carried out to test the robustness of model results to changes in parameters and structural assumptions. A one-way sensitivity analysis tested the impact of varying model inputs independently between their upper and lower bounds; the inputs varied and their upper and lower values are presented in . Results of the one-way sensitivity analysis were presented on a tornado diagram. A range of scenario analyses were also carried out to test model assumptions; the scenarios considered are summarized in and include alternative options for extrapolating OS, PFS and ToT for pembrolizumab and chemotherapy relaxing the assumption of perfect vial sharing, including dis-utilities associated with adverse events.
Table 5. Scenario analyses.
Results
Base case deterministic results
Base case deterministic results are presented in . Pembrolizumab was associated with an additional 4.68 life years and an additional 3.80 QALYs versus chemotherapy over the time horizon of the analysis (30 years). The majority of these gains were accrued in the progression-free health state, with an additional 3.33 QALYs for women treated with pembrolizumab versus chemotherapy, reflecting the additional time spent in that health state for women treated with pembrolizumab.
Table 6. Base case deterministic, discounted results.
Pembrolizumab was associated with an incremental cost of $220,934 versus chemotherapy over the time horizon, with the majority of cost accrued for drug acquisition ($119,426). Combined with the incremental gain in LYs and QALYs, the estimated incremental cost per LY gained was $47,236 and per QALY gained (ICER) was $58,165 for pembrolizumab versus chemotherapy. At a willingness-to-pay of $100,000 per QALY gained, this resulted in a net monetary benefit (i.e. the value of pembrolizumab in monetary terms) of $158,907.
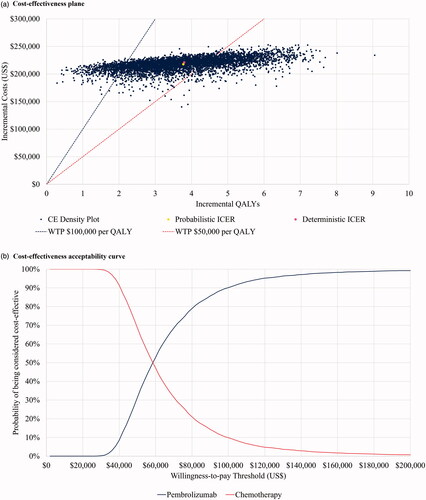
Probabilistic sensitivity analysis
The results of the probabilistic sensitivity analysis are presented in . The estimated incremental cost per LY gained was $46,334 and per QALY gained for was $57,668 for pembrolizumab versus chemotherapy for the probabilistic sensitivity analysis. We note that the results of the deterministic and probabilistic analyses are similar. The probability of pembrolizumab being considered cost-effective versus chemotherapy at a willingness-to-pay of $100,000 per QALY gained was 90.1%. The probabilistic analysis indicated the main uncertainty was related to QALY gains, with all iterations indicating a positive incremental QALY gain.
One-way sensitivity analysis and scenario analyses
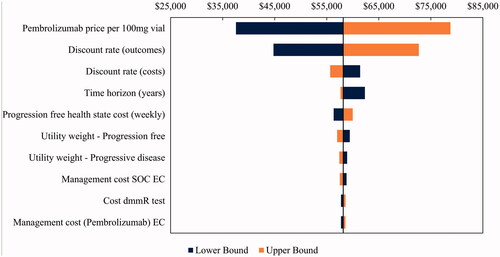
Deterministic results were tested in one-way sensitivity analysis and scenario analyses and were found to be robust to all changes explored (; ). In one-way sensitivity analysis, the model inputs which had the largest impact on the ICER were the price of pembrolizumab and the discount rates for costs and outcomes, with a maximum estimated ICER of $78,768.
Table 7. Results of the scenario analyses.
In scenario analyses, all scenarios explored reported ICERs below $100,000 per QALY threshold. The three scenarios with the highest ICERs were overall survival using the one-piece exponential approach for pembrolizumab ($89,962) and when the time horizon was reduced to 20 or 10 years ($62,341 and $87,168 respectively). At 20 years, a significant proportion of patients receiving pembrolizumab remain alive in the model (approximately 10%) and therefore these time horizons were considered implausible. Including adverse event disutilities resulted in and ICER of $57,191, which was lower than the base case. The lowest ICERs reported in scenario analysis were pembrolizumab overall survival projections with the lowest ICER $35,199 reported for one-piece Gompertz.
Discussion
For previously treated dMMR/MSI-H unresectable or mEC, results of this analysis have demonstrated that pembrolizumab is a highly cost-effective treatment option when compared with the single chemotherapy agents paclitaxel or doxorubicin, and these results are robust to changes in model parameters or assumptions.
Pembrolizumab demonstrates a considerable gain in life years (4.68) and QALYs (3.80) versus chemotherapy over the time horizon of the analysis (30 years). These gains are due to improved PFS and OS for pembrolizumab versus chemotherapy, with the majority of gains accrued in the progression-free health state (3.33 of 3.80 incremental QALYs).
The demonstrated substantial lifetime benefits of treatment with pembrolizumab versus chemotherapy are accompanied by an increase in costs, driven largely by the increased treatment cost of pembrolizumab, resulting in an ICER of $58,165 for the base case deterministic analysis. Probabilistic results are supportive, with an estimated ICER of $57,668 and the probability of pembrolizumab being considered cost-effective versus chemotherapy at a willingness-to-pay of $100,000 per QALY gained is 90.1%.
This analysis has several strengths. The model structure was chosen to be consistent with other cost-effectiveness analyses in advanced or metastatic cancer and was constructed with close reference to the Institute for Clinical and Economic Review reference case for economic evaluationCitation22. Furthermore, the analysis was underpinned by data obtained from reliable sources, in particular for the PFS and OS data incorporated in the model for pembrolizumab. These data were obtained from a Phase II clinical trial for pembrolizumab which had completed almost three years of follow-up. Extrapolations beyond the observed data were reviewed and validated by two clinical experts. In addition, EQ-5D data describing utilities for PFS and progressed disease were obtained from patients with dMMR/MSI-H unresectable or mEC within KEYNOTE-158 and valued using a US tariff, and standard US sources for costs were used within the analysisCitation33,Citation35,Citation40.
We note that the results of the analysis may be conservative. Firstly, adverse event disutility was not included in the model base case due to small patient numbers informing the disutility values; however, pembrolizumab is associated with a good tolerability profile and including these disutilities reduces the deterministic ICER to $57,191. Moreover, there is some evidence to suggest that MSI-H could be a negative prognostic factor in advanced disease; consequently, given that chemotherapy data were obtained from women with unknown MSI-H status, and noting that some patients within McMeekin et al. had locally advanced or recurrent endometrial cancer, it is possible that the survival data obtained from McMeekin et al. overstate PFS and OS in these patientsCitation27.
The limitations of this analysis relate to areas where data are unavailable or unclear:
The OS and PFS data in the model for pembrolizumab were obtained from a single-arm trial, and no matched-adjusted indirect comparison was carried out to assess a relative treatment effect versus paclitaxel or doxorubicin. Such analyses were not possible since individual patient data were not available from McMeekin et al.Citation27 Instead, chemotherapy data were included in the model from a published source without adjustment. This was necessary given the lack of published comparative data within this patient group. However, it is possible that inclusion of these data in this way could overestimate survival for chemotherapy since a major difference in patient characteristics between KEYNOTE-158 and McMeekin et al. was that patients within McMeekin et al. did not have confirmed MSI-H endometrial cancer, and that McMeekin et al. included patients with locally advanced or recurrent diseaseCitation27 rather than only unresectable or mEC patients, as for KEYNOTE-158.
Further to this, we note that there is some evidence to suggest that MSI-H could be a negative prognostic factor in advanced disease; consequently, it is possible that the survival data obtained from McMeekin et al. overestimate PFS and OS in these patientsCitation11,Citation27.
Survival data for pembrolizumab were based on small patient numbers given that the data were obtained from a small subset of patients in the larger KEYNOTE-158 clinical trial who had MSI-H expression and had endometrial cancer (n = 49). However, we note that the data obtained from these patients reflect the patient group of interest, and results are robust to changes in modelled OS and PFS.
Chemotherapy in the treatment of unresectable or mEC varies, and we have only compared with paclitaxel or doxorubicin in this analysis. Future analyses could consider comparing additional chemotherapies where data allow; however, we consider paclitaxel and doxorubicin to be important comparators for this patient group based upon published guidelinesCitation9,Citation10.
While the analysis has limitations, we have mitigated these factors where possible via clinical validation of the survival analyses. We also extensively tested the model in sensitivity analysis and note that the results were robust to the changes investigated.
Conclusions
The results of this analysis suggest that, from a US healthcare perspective, pembrolizumab is a highly cost-effective treatment option compared with the chemotherapy single agents paclitaxel and doxorubicin for women with previously treated dMMR/MSI-H unresectable or mEC. Results are robust to changes in model parameters and assumptions.
Future analyses should consider incorporating comparative survival data for pembrolizumab versus chemotherapy within a dMMR/MSI-H unresectable or mEC patient group, using this model structure. Finally, it would be beneficial to capture real-world data to inform disease management costs and testing rates.
Transparency
Declaration of funding
This study was supported by funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Declaration of financial/other relationships
Elizabeth Thurgar, Suzette Matthijsse, and Mark Gouldson are employees of BresMed Health Solutions, which received consultancy fees from Merck and Co. in connection with this study. Mayur Amonkar, Patricia Marinello, Navneet Upadhyay, Chizoba Nwankwo and Raquel Aguiar-Ibáñez are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
A peer reviewer on this manuscript has disclosed that they have received research grants from Intuitive Surgical and Hope Against Cancer, and an honorarium from GlaxoSmithKline. Another reviewer has disclosed that they have received research funding from Merck. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Raquel Aguiar-Ibáñez, Suzette Matthijsse, Mark Gouldson and Elizabeth Thurgar were involved in the development of the economic model, which was reviewed by all named authors. Elizabeth Thurgar developed the text for this manuscript, which was reviewed and edited by all named authors.
Supplemental Material
Download MS Word (438.2 KB)Acknowledgements
The authors would like to thank the following individuals for their contributions to the analyses of clinical, safety and quality-of-life data from KEYNOTE-158 to populate the model: Ruifeng Xu, Principal Scientist Outcomes Research; Qian Wang, Principal Scientist Statistical Programming; Amit Bhaniani, Sr. Scientist, Stat. Programming; and Li Ma, Scientist, Statistical Programming, and to Robert Orlowski, Sr. Prin. Scientist, Clinical Research Oncology Clinical Development, for providing clinical expertise regarding the validity of long-term projections used within the model.
Notes
i NCCN Guidelines is a registered trademark of National Cancer Comprehensive Network, Plymouth Meeting, PA, USA.
ii Microsoft Excel is a registered trademark of Microsoft Corporation, Redmond, Washington, USA.
iii EQ-5D is a registered trademark of EuroQol, Rotterdam, The Netherlands.
iv Micromedex RED BOOK is a registered trademark of IBM, Armonk, NY, USA.
References
- National Cancer Institute. NCI – Endometrial Cancer. 2021 [cited 2021 Feb 2]. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/endometrial-cancer
- Purdie DM, Green AC. Epidemiology of endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2001;15(3):341–354.
- Cancer.Net. Uterine Cancer: Statistics. 2020 [cited 2020 Feb 4]. Available from: https://www.cancer.net/cancer-types/uterine-cancer/statistics
- Kurra V, Krajewski KM, Jagannathan J, et al. Typical and atypical metastatic sites of recurrent endometrial carcinoma. Cancer Imaging. 2013;13:113–122.
- Rauh-Hain JA, Del Carmen MG. Treatment for advanced and recurrent endometrial carcinoma: combined modalities. Oncologist. 2010;15(8):852–861.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Uterine Neoplasms v1.2021 © National Comprehensive Cancer Network, Inc. 2021. All rights reserved. [cited 2021 Jan 12]. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. National Comprehensive Cancer Network.
- Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26(1):2–30.
- Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31(1):12–39.
- Colombo N, Preti E, Landoni F, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi33–8.
- Sundar S, Balega J, Crosbie E, et al. BGCS uterine cancer guidelines: recommendations for practice. 2017. https://www.bgcs.org.uk/wp-content/uploads/2019/05/BGCS-Endometrial-Guidelines-2017.pdf
- Arabi H, Guan H, Kumar S, et al. Impact of microsatellite instability (MSI) on survival in high grade endometrial carcinoma.Gynecol Oncol. 2009;113(2):153–158.
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. J Clin Oncol Precis Oncol. 2017;2017:PO.17.00073.
- Cortes-Ciriano I, Lee S, Park WY, et al. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun. 2017;8:15180.
- Cristescu RLK, Pruitt S. Prevalence of high microsatellite instability in cancer patients in the real world. Washington (DC): SITC; 2018.
- Hause RJ, Pritchard CC, Shendure J, et al. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22(11):1342–1350.
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413.
- Lorenzi MAM, Zhang J, Mehta S, et al. P585 structured literature review and meta-analyses of the prevalence of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in endometrial and ovarian cancers. Washington (DC): SITC; 2018.
- Merck S, Dohme C. A clinical trial of pembrolizumab (MK-3475) evaluating predictive biomarkers in subjects with advanced solid tumors (KEYNOTE 158). P158V02MK3475. 2018.
- US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017.
- O'Malley D, Marabelle A, De Jesus-Acosta A, et al. Pembrolizumab in patients with MSI-H advanced endometrial cancer from the keynote-158 study. ESMO 2019 Congress. Ann Oncol. 2019;30:v425–v34.
- Barrington DA, Dilley SE, Smith HJ, et al. Pembrolizumab in advanced recurrent endometrial cancer: a cost-effectiveness analysis. Gynecol Oncol. 2019;153(2):381–384.
- Institute for Clinical and Economic Review. ICER’s reference case for economic evaluations: principles and rationale. Institute for Clinical and Economic Review; 2020. http://icer-review.org/wp-content/uploads/2020/01/ICER_Reference_Case_013120.pdf.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797.
- Beth Woods ES, Stephen P, Nick L, et al. NICE DSU technical support document 19: partitioned survival analysis for decision modelling in health care: a critical review. NICE Decision Support Unit; 2017.
- Neumann PJ, Sanders GD, Russell LB, et al. Cost-effectiveness in health and medicine. Oxford: Oxford University Press, 2016.
- Merck S, Dohme C. Lenvatinib in combination with pembrolizumab versus treatment of physician's choice in participants with advanced endometrial cancer [KEYNOTE-775]. 2020.
- McMeekin S, Dizon D, Barter J, et al. Phase III randomized trial of second-line ixabepilone versus paclitaxel or doxorubicin in women with advanced endometrial cancer. Gynecol Oncol. 2015;138(1):18–23.
- Miller DS, Scambia G, Bondarenko I, et al. ZoptEC: phase III randomized controlled study comparing zoptarelin with doxorubicin as second line therapy for locally advanced, recurrent, or metastatic endometrial cancer (NCT01767155). J Clin Oncol. 2018;36(15):5503.
- Di Legge A, Trivellizzi IN, Moruzzi MC, et al. Phase 2 trial of nonpegylated doxorubicin (Myocet) as second-line treatment in advanced or recurrent endometrial cancer. Int J Gynecol Cancer. 2011;21(8):1446–1451.
- Lincoln S, Blessing JA, Lee RB, et al. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88(3):277–281.
- Makker V, Hensley ML, Zhou Q, et al. Treatment of advanced or recurrent endometrial carcinoma with doxorubicin in patients progressing after paclitaxel/carboplatin: Memorial Sloan-Kettering Cancer Center experience from 1995 to 2009.Int J Gynecol Cancer. 2013;23(5):929–934.
- National Center for Health Statistics. United States life tables. NVSR. 2017;68(7):66. (PHS) 2019-1120. Centers for Disease Control and Prevention.
- Agency for Healthcare Research and Quality. HCUPnet – healthcare costs and utilization project. 2019 [cited 2019 Dec 10]. Available from: https://hcupnet.ahrq.gov/#setup
- Disutility [Internet]. York; York Health Economics Consortium; 2016 [cited 2021 Feb 2]. Available from: https://yhec.co.uk/glossary/disutility/
- CMS. Centres for Medicare and Medicaid Services, Clinical Laboratory Fee Schedule. 2020 [cited 2020 May 5]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files
- CMS. Physician Fee Schedule – CY 2020 Physician Fee Schedule Final Rule. 2020 [cited 2020 Feb 3]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched
- IQVIA. MSI/MMR treatment. Market Research [Merck - Data on file]. 2020.
- US Food and Drug Administration. FDA. Taxol (paclitaxel) injection - Prescribing information. 2021 [cited 2021 Feb 2]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
- US Food and Drug Administration. FDA. Doxorubicin hydrochloride injection - Prescribing information. 2021 [cited 2021 Feb 2]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050467s073lbl.pdf
- IBM Micromedex. Red Book Search Results. 2020 [cited 2020 Feb 11]. Available from: https://www.micromedexsolutions.com/micromedex2/librarian/CS/F4EC4C/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/58F466/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/redbook.FindRedBook?navitem=topRedBook&isToolPage=true
- Du Bois D and Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871.
- Electronic Medicines Compendium. Tamoxifen 20mg Film-Coated Tablets, Summary of Product Characteristics. 2020.
- CMS. Centres for Medicare and Medicaid Services, Hospital Outpatient Prospective Payment System. 2020 [cited 2020 May 5]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS
- Institute for Clinical and Economic Review. Poly ADP-Ribose Polymerase (PARP) Inhibitors for Ovarian Cancer: Effectiveness & Value. 2017 [cited 2020 May 5]. Available from: http://icerorg.wpengine.com/wp-content/uploads/2020/10/MWCEPAC_OVARIAN_FINAL_EVIDENCE_REPORT_10112017-1.pdf
- Bekelman JE, Halpern SD, Blankart CR, et al. Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA. 2016;315(3):272–283.
- Briggs A, Claxton, K, Sculpher, M. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- Simeone JC, Shah S, Ganz ML, et al. Healthcare resource utilization and cost among patients with type 1 diabetes in the United States. J Manag Care Spec Pharm. 2020;26(11):1399–1410.
- Bice T, Cox CE and Carson SS. Cost and healthcare utilization in ARDS–different from other critical illness? Semin Respir Crit Care Med. 2013;34(04):529–536.
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220.