Abstract
Aim
We aimed to determine the incidence of and identify the factors associated with treatment-resistant depression (TRD), psychiatric conditions, hospitalization, and cost in patients with major depressive disorder (MDD) who were treated using second-line strategies after an inadequate response to initial antidepressants (AD).
Materials and methods
Using South Korean National Health Insurance claims data (1 January 2013 to 30 June 2018), we conducted a retrospective cohort analysis in newly treated patients with MDD who subsequently switched or added AD, or added atypical antipsychotics (AAPs) as a second-line treatment. We assessed the incidence of treatment-resistant depression (TRD), psychiatric conditions, and hospitalization for the first 2 years and costs in the third year. Odds ratios (ORs) or relative ratios were estimated using logistic and linear regression models to identify the risk factors for clinical and economic outcomes.
Results
In 15,887 patients, the TRD was 16.81% during the 24-month follow-up period (14.14% in switching AD, 19.65% in adding AD, and 19.91% in adding AAP; p < 0.0001). When adding AD or AAP, the OR of TRD was 1.43 (95% confidence interval (CI): 1.30–1.56) and 1.42 (95% CI: 1.23–1.65), respectively, compared to switching AD. However, these factors were not associated with the incidence of psychiatric conditions. Adding AAP increased hospitalization (OR = 1.25, 95% CI: 1.11–1.41), the number of inpatient days by 2.57-fold (95% CI: 1.75–3.76), and cost by 1.20-fold (95% CI: 1.02–1.40), compared to switching AD; adding AD did not show a significant association with these outcomes.
Conclusions
In patients with MDD with inadequate responses to initial AD, TRD still occurred after subsequent treatments according to clinical guidelines. Since the effectiveness of second treatment strategies can differ in reality, further analysis of the clinical and economic evidence regarding second treatment strategies, such as adding AD or AAP, is needed using real-world data.
Introduction
Major depressive disorder (MDD) is a relatively common mental illness that is highly recurrentCitation1. According to the World Health Organization (WHO), the global burden of MDD has been shown to be the third largest and is expected to become the largest by 2030Citation2. Despite pharmacological advances, the response rate to initial antidepressants (AD) is 50–70% but the remission rate is only 30%Citation3. Treatment-resistant depression (TRD) is defined as having no meaningful therapeutic response, even though patients have continuously received two or more AD treatments with different pharmacological classificationsCitation4. The most important factor in drug failure is lack of efficacy, and according to treatment guidelines and expert opinion, efficacy should be determined after at least 4–6 weeks of drug administrationCitation5. In addition, comorbid psychiatric conditions, including psychotic conditions, anxiety disorders, insomnia, and substance use disorders, are well-known predictors of treatment resistance in MDD. Patients with MDD and psychotic conditions have a very low treatment response compared to those with non-psychotic MDDCitation6. In particular, psychiatric conditions are often observed in patients with TRD compared to patients who do not develop TRD; therefore, psychiatric conditions are known risk factors for TRDCitation7.
Patients who do not have a therapeutic response at the beginning are less likely to improve even if administration of the same drug is continued; hence, it is critical to consider changes in the treatment strategy at the earliest. General treatment guidelines do not provide the most effective treatment strategy for inadequate responders in MDDCitation8. In the past, dose increases or switching to other ADs was preferred, but the preference gradually shifted to the addition of other ADsCitation9. Accumulating evidence has shown that combination therapy in the treatment of MDD may produce higher remission rates and lower relapse rates than traditional monotherapyCitation10,Citation11, either as an initial treatment plan or as a strategy following an inadequate response to initial treatmentCitation12. When the initial treatment fails, recent treatment guidelines recommend the addition of atypical antipsychotics (AAPs), and their usefulness is well elucidatedCitation13. Since the guideline was developed based on clinical evidence and expert opinion, it may differ from real-world data. It is necessary to confirm the clinical and economic effectiveness of different treatment strategies—switching AD, adding AD, and adding AAP—following the initiation of AD monotherapy based on clinical guidelines in a real-world setting.
This study observed the clinical burden, including TRD and psychiatric conditions, and economic factors such as healthcare resource use and the medical costs of patients with MDD who had an inadequate response to initial AD monotherapy and were treated with second treatment strategies based on clinical guidelines. We also identified clinical and economic risk factors through real-world data analysis.
Materials and methods
Data sources
This retrospective cohort study utilized the Korea Health Insurance Review and Assessment Service (HIRA) database, which contains claims from a universal healthcare system to capture patient-level demographic and clinical characteristics, medical and pharmacy utilization, and expenditures. Source data, including 1.4 billion in annual medical bills and 65 trillion Korean won (KRW) in medical expenses, are built into an open database of 525.8 billion in claims. These nationwide claims developed by HIRA under the National Health Insurance Service (NHIS) system can be used in various studies, such as risk factor analyses, comparative effectiveness studies, and pharmacoeconomic analyses, as real-world dataCitation14. Since this was an analysis of all citizens’ health insurance data, it is representative of the Korean population. We used claims data from 1 January 2013 to 30 June 2018 and indexed patients from 1 July 2013 to 30 June 2015 (hereafter, the ‘index period’). Therefore, we allowed 6-month pre-index periods to capture pre-existing comorbidities and exclusionary criteria and followed up patients for at least 3 years. Since Korea has a single-payer healthcare system, we did not need to check the patients’ enrollment throughout the study period.
Patient selection
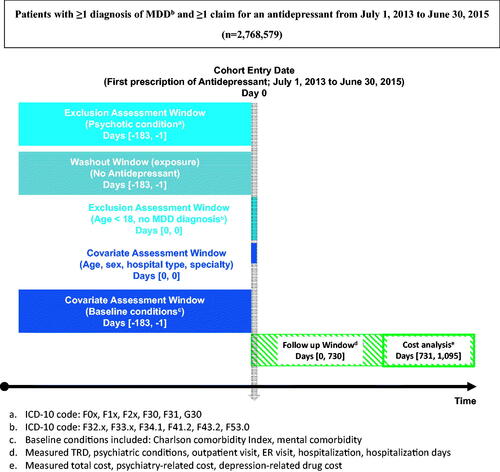
To select newly treated patients with MDD, patients were included if they had a prescription history of AD monotherapy, including at least one medical claim indicating a clinical visit coded for MDD (International Classification of Disease (ICD) 10-codes F32.x, F33.x, F34.1, F41.2, F43.2, F53.0) during the index period. Patients’ first AD monotherapy claims during the index period served as the study index date. To clarify patients as newly AD-treated, patients who had been prescribed ADs within 6 months prior to the index date were excluded. The list of drugs included in this study is presented in Supplementary Table S1. The exclusion criteria were <18 years of age on the index date or a main disease or sub-disease diagnosis of psychotic conditions [ICD 10-codes, F0x (dementia), F1x (addiction), F2x (schizophrenia), F30 (manic), F31 (bipolar affective disorder), G30 (Alzheimer's)] during the 6-month pre-index period. presents a summary of the patient cohorts.
With at least 42 d of continuous eligibility following the index date, these patients were categorized into three groups according to a subsequent regimen after the initiation of their first AD monotherapy: switching to another class of AD, adding AD, or adding AAP. As mutually exclusive treatment groups, the first group only switched to different pharmacological classes from an initial AD, the second added another AD, and the third added AAP. These treatment strategies were consistent with recently published clinical guidelinesCitation15. A flowchart depicting the patient selection process is presented in Supplementary Figure S1.
Study measures
The clinical outcome was the incidence of TRD and psychiatric conditions 2 years after the index date. TRD was regarded as having no meaningful therapeutic response, even though patients continued to receive two or more AD therapies with different pharmacological classifications. The clinical information available in the claims database is quite limited and does not include direct measures of clinical status. Thus, we assessed treatment failure based on treatment regimen change. Based on a consensus from the literature review and a panel discussion, TRD in this study was defined as the start of the third treatment regimenCitation4,Citation5. In previous studies, the evaluation period was 6 weeks, and in this study, the first regimen was 42 d or longer in order to set an appropriate period to sufficiently evaluate the AD responseCitation16. In our study, if there were three or more treatment regimens spanning 42 d while allowing a 30-d interval and the prescriptions of the three regimens were all different, the third regimen’s start date was regarded as the TRD date. Since the side effects of a drug occur soon after it is taken, changes in the regimen after administration for more than 42 d can be attributed to a lack of effectiveness or decreased complianceCitation17. It was impossible to evaluate the adequacy of medication compliance in the claims database; therefore, it was defined as TRD based on the number of failed usagesCitation18. Psychiatric conditions including dementia, schizophrenia, bipolar disorders, anxiety disorders, obsessive-compulsive disorder, personality disorders, substance use disorders, and insomnia were also identified during the 2-year follow-up period after the index date.
We assessed economic outcomes, namely healthcare resource utilization for the first two years and medical costs in the third year. Healthcare resource utilization, including the number of outpatient visits, emergency room visits, and hospitalization and inpatient days, was assessed over the 24 months period following the index date. The economic burden is high in patients with TRDCitation19,Citation20. Since the costs during the 24-month follow-up period were incurred during TRD, it was difficult to confirm whether TRD affects cost. Therefore, to confirm the costs incurred after TRD, in this study, the three groups’ expenses were measured after the end of the 24-month follow-up period in the following year. The total medical cost estimates included all medical and prescription claims regardless of diagnosis or drug type. In contrast, psychiatry-related costs included only those medical claims with a psychiatry specialty, and depression-related drug costs included only those for AD and AAP.
In this study, age, gender, the severity of illness, hospital category at the index date, treating physician’s specialty at the index date, and index year were considered as the variables that can be identified in the claims database. Depression severity based on the diagnosis code was observed: non-psychotic mild depression (F32.0, F33.0), non-psychotic moderate depression (F32.1, F33.1), non-psychotic severe depression (F32.2, F33.2), and psychotic severe depression (F32.3, F33.3). In addition, mental comorbidities were included as variables indicating depression severity: anxiety disorders, obsessive disorder, personality disorder, and insomniaCitation21. A list of mental comorbidities is presented in Supplementary Table S2. The Charlson comorbidity index (CCI) was assessed during the pre-index period. If the treating physician’s specialty at the index date was psychiatry, the specialty at the index date was considered to be a covariate in the analyses, since treatment for psychiatric disorders was performed aggressively. In addition, since there may be changes in healthcare-related policies or drug use within the index year, the index year was also considered to be an explanatory variable.
Statistical analyses
For categorical variables, the chi-squared or Fisher’s exact test was used. Continuous variables are shown as mean ± SD and were compared using ANOVA. Patient demographics and clinical characteristics were summarized. TRD, psychotic disorders, healthcare resource use (outpatient visits, emergency room visits, hospitalization, and inpatient days), and medical costs were summarized and compared according to the second regimen’s treatment pattern.
Logistic regression analyses were conducted to identify the factors associated with TRD, psychiatric conditions, and hospitalization after adjusting for nine covariates: age, sex, coded depression severity, CCI, mental comorbidity, hospital category at index date, specialty at index date, year of index date, and second regimen treatment pattern. Mental comorbidity and coded depression severity were used to adjust for depression severity. Given that TRD is a potential confounder of other outcome variables, it was taken as a covariate for the multivariable analyses. We conducted regression models to examine the effects of TRD on log-transformed medical costs and inpatient days, adjusting for confounding factors. The exponential form of the coefficients indicates the multiplied value of y, given each one-unit increase in an independent variable. We defined this as the relative ratio. Statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA).
This study was approved by the Institutional Review Board of Sungkyunkwan University (approval number: SKKU-2018-04-004).
Results
Of the 15,887 patients, 8,270 (52.06%) patients were switched to different pharmacological classes of AD, 6,025 (37.92%) added another AD, and 1,592 (10.02%) added an AAP in the second regimen. summarizes the patients’ baseline demographic and clinical characteristics. The average age at the index date was 54.35 ± 17.12 years old, where 10.50% of the patients were under 30 years old, 20.76% were in their 50s, 20.58% were in their 60s, and 21.98% were in their 70s. The proportion of males was 40.17%, whereas that of females was 59.83%. The average CCI score was 1.68 ± 1.97 points, and 36.10% of patients had a score of 0 for comorbid diseases; however, 25.96% of patients had more than 3 points. Mental comorbidities included anxiety disorders (36.55%) and insomnia (14.49%). As for the hospital category at the index date, 54.23% of patients sought consultation at tertiary hospitals, 8.28% at general hospitals, 37.50% in clinics, and 50.24% of patients visited a psychiatry specialty physician at the index date.
Table 1. Patients’ baseline demographics and clinical characteristics.
presents a summary of outcomes. Considering measures indicative of TRD, 16.81% of the total population had at least two regimens containing AD (14.14% switched AD, 19.65% added AD, and 19.91% added AAP, p < 0.0001). In patients with major psychiatric conditions during the 24-month follow-up period, the addition of AAP was associated with a significantly higher rate of dementia (11.81% vs. 9.70% and 6.95%, p < 0.001), schizophrenia (34.55% vs. 4.23% and 5.18%, p < 0.001), and bipolar disorders (43.22% vs. 8.95% and 10.76%, p < 0.001) than switching to AD and adding another AD, respectively.
Table 2. Details regarding treatment-resistant depression, psychiatric conditions, healthcare resource use, and cost.
The hospitalization and mean number of outpatient visits in switching AD were significantly higher than in adding AD or AAP (48.04% vs. 42.80% or 43.28%, respectively, p < 0.001; 87.82 ± 66.57 vs. 82.98 ± 64.55 or 67.83 ± 54.27, respectively). In contrast, the number of inpatient days was significantly higher when adding AAP compared to when switching or adding AD (34.08 ± 115.60 vs. 20.68 ± 75.77 or 18.73 ± 78.47). Additionally, we observed differences in psychiatric conditions and healthcare resource use between patients with and without TRD (Supplementary Table S3). The mean number of outpatient visits in the patients with TRD during the 24-month follow-up period was significantly higher (88.30 ± 63.12 vs. 83.10 ± 65.26, p < .0001), and the mean number of inpatient days was also higher (25.79 ± 94.76 vs. 20.37 ± 78.82, p = .0056) than in the patients who did not develop TRD.
To compare the cost at the time point after TRD, 8,942 (56.29%) patients who were followed up for 3 years after the index date were included. There was no significant difference in the total direct medical cost in the third year after the initial treatment, but psychiatry-related costs and depression-related drug costs were significantly higher in the AAP group. The mean total 12-month psychiatry-related costs in switching AD were KRW 342,811 ± 1,371,521; KRW 462,753 ± 1,511,229 in the addition of AD; and KRW 902,256 ± 2,777,215 in the addition of AAP (p < .0001). The 12-month depression-related drug costs in switching AD were KRW 93,182 ± 163,735; KRW 141,966 ± 197,711 for adding AD; and KRW 264,787 ± 377,496 in the addition of AAP (p < .0001).
and report significant differences in each outcome after the multivariable adjustment. In the logistic regression analysis, the odds ratio (OR) of TRD when adding AD or AAP was 1.43 (95% CI: 1.30 − 1.56) or 1.42 (95% CI: 1.23 − 1.65), respectively, compared to switching AD. TRD was significantly higher in patients with anxiety disorders (OR = 1.10) and insomnia (OR = 1.20) than in those without anxiety disorders and insomnia. However, it was lower in those over 70 years of age than in those under 30 years old (OR = 0.82). The second treatment strategy was not associated with the incidence of psychiatric conditions. The incidence of psychiatric conditions was significantly higher in patients with TRD (OR = 1.40) than in those without TRD. It was also significantly higher in patients in their 40s and 50s than in those under 30 years old (OR = 1.21), female patients (OR = 1.12), and those who visited clinics at the index date compared to tertiary hospitals (OR = 1.42). Higher CCI scores were associated with the incidence of psychiatric conditions.
Table 3. Risk of TRD and psychiatric conditions for the first 2 years.
Table 4. Risk regarding healthcare resource use and cost.
Adding AAP increased the incidence of hospitalization (OR = 1.25, 95% CI: 1.11–1.41), the number of inpatient days by 2.57-fold (95% CI: 1.75–3.76), and cost by 1.20-fold (95% CI: 1.02–1.40) compared to switching AD but the effect of adding AD was not significant. TRD onset is associated with increased hospitalization, inpatient days, and costs. Hospitalization was significantly higher in patients with insomnia (OR = 1.19) than in those without. Hospitalization was also significantly higher in those in their 50s (OR = 1.29) and 60s (OR = 1.42) and in those over 70years old (OR = 1.89) than in those under 30. The number of inpatient days was significantly higher in patients with insomnia (1.83-fold), those in their 50s (2.12-fold) and 60s (3.17-fold), and in those over 70 years old (8.54-fold) than in those aged under 30 years. Compared to those under 30 years of age, the medical costs in all other age groups increased significantly. Higher CCI scores and the treating physician’s specialty at the index date were associated with increased hospitalization, inpatient days, and medical costs.
Discussion
In our study, using a nationwide claims database, an average of 16.81% of newly treated patients with MDD did not respond, even after subsequent treatments. It was found that not only younger age, anxiety disorders, and insomniaCitation7,Citation22 but also second-line treatment strategies such as adding AD or AAP might be associated with the incidence of TRD. Psychiatric conditions were more common in patients who developed TRD than in those without TRD. However, similar to the previous researchCitation7, there was no significant difference in the incidence of psychiatric conditions according to the second treatment strategy.
The incidence of TRD in our study was within the range of values reported in the literature, that is, ranging from 2 to 35%Citation23,Citation24. The variance across the studies seemed to result from the application of various definitions of TRD. Therefore, this study set the response evaluation period at 6 weeks and confirmed the incidence of TRD after a sufficient response evaluation period based on clinical guidelines and expert opinions. This study confirmed that TRD still occurs in patients with MDD who demonstrate an inadequate response to initial AD treatment even after the provision of a second treatment regimen; previous studies assessed TRD incidence in patients with MDD who were previously treated with ADCitation23,Citation24.
The incidence of TRD was significantly different among the three treatment strategies: 14.14% in those who switched to AD, 19.65% in those that added AD, and 19.91% in those that added AAP, although it would be expected that patients who added AAP would show a lower TRD incidence than those who switched or added AD regardless of the usual doseCitation25,Citation26. In clinical practice, antipsychotics are often used when the following symptoms are shown: clinical symptoms that may suggest (temporary) psychotic symptoms, depressive symptoms that do not improve, difficulty controlling emotions, and increased impulsiveness, anxiety, and insomnia, such as bipolar or schizoaffective disordersCitation6. Since anxiety disorders, personality disorders, and insomnia are known risk factors for TRD, the presence of these psychiatric conditions were considered as covariates in our study. However, psychotic symptoms, especially those that were temporary, could not be confirmed in the claims database; therefore, there was a limitation in that they could not be adjusted, although patients with psychotic symptoms were likely to be treated with AAP. In another study using the claims database, 1.2% of patients with pharmaceutically treated depression (PTD) had at least one AAP regimen. The prescription of AAPs was not high, even with second-line agents. It is likely that the recommended minimum dose of AAP did not followCitation16. Considering the low prescription frequency of AAP in the second regimen in this previous study, it is known that AAP as an augmentation therapy for AD was used at less than the minimum recommended dose in Korea; thus, sufficient effectiveness may not have been achievedCitation27. In our study, 12.5 mg and 25 mg of quetiapine, which are used for sleep control purposes, were excluded; therefore, it can be said that AAPs used at low doses were excluded. Since our study did not consider the required minimum dosage of other AAPs as a regimen, we could not check the incidence of psychotic symptoms and the standardization of the prescribed AAP dose. Therefore, these results should be interpreted with caution. Further research is necessary to confirm the effectiveness of AAPs in treating TRD.
The results of our study are consistent with the idea that patients with TRD have higher healthcare resource utilization and higher medical costs than those without TRDCitation28,Citation29. Compared to healthcare costs in patients without TRD, those in patients with TRD increased to 29.3% and 57% in the United StatesCitation30 and JapanCitation31, respectively. In this study, the development of TRD, as mentioned in previous studies, as well as the presence of insomnia, being elderly, and having a higher CCI score and a higher number of visits to an internal medicine specialist at the index date were found to be factors that increased overall medical costs. Additionally, our study confirmed that the addition of AAPs has been shown to significantly increase hospitalization and inpatient days compared to switching AD. These patients spent more on psychiatry- and depression-related costs. Despite adjusting for psychiatric conditions, depression severity, and comorbidity index scores, the patients that were prescribed AAP were found to incur more medical expenses than the other groups. There may have been effects of psychotic symptoms that could not be confirmed in the claims database, and the cost analysis did not take into account the unit price of each drug.
This study has several limitations. First, only the conditions or comorbidities that can be confirmed in the claims database were considered. As is true of all retrospective studies where cohort identification was based on the use of diagnostic medical coding, we have to consider the possibility that there was an inaccuracy in the coding and that there were unexplained comorbidity differences among the study groups before adjustment. Although there may be uncertainties in diagnostic medical coding, its validity has been evaluated in the previous studiesCitation32. In our study, the primary outcome was defined based on drug use instead of the diagnostic code, and diagnostic coding was used only as a covariate. Second, although the regression model controls for the measured confounders, unmeasured covariates can remain. In this study, the pre-index period for confirming the patient’s medical history was set at 6 months, since MDD is a chronic and recurrent disease, and other accompanying details in the medical history that were not confirmed in the pre-index period may have influenced the type of second-line agents prescribed. Due to the lack of information related to patient compliance for each treatment regimen in this study, treatment compliance can be considered in further studies when evaluating the response, taking into account the appropriate dose and duration of drug use. The side effects of the treatments, quality of life, and social functioning were not considered in this study because these factors represent clinical information that is difficult to identify in claims databases. This study has found some interesting associations; second-line treatment strategies such as adding AD or AAP were identified as risk factors for TRD but the interpretation of the meaning of these associations needs to be with caution. Third, the analysis was limited to the insured Korean population and was reflective of healthcare practice patterns and patient management in Korea. The study results may not be generalizable to different patient populations.
Nevertheless, there is little evidence of the comparative effectiveness of the second treatment strategies for managing patients with MDD and inadequate responses to initial AD; hence, the findings of the current analysis may serve as the basis for predicting the potential utility of these treatment strategies.
Conclusions
In patients with MDD who have inadequate responses to initial AD, TRD still occurs even after subsequent treatments, as recommended in the clinical guidelines. With well-known risk factors including age, anxiety disorders, and insomnia, a second-line treatment strategy, such as adding AD or AAP, may have an impact on the clinical and economic burden on patients, unlike those in the clinical guidelines. To optimize the second treatment regimen for patients with MDD, such as adding AD or AAP, there is a need to accumulate evidence to further evaluate their clinical and economic burden. Therefore, additional real-world studies that take into account the prescription patterns and patient characteristics for each treatment are needed in order to evaluate the most effective treatment strategy to reduce the clinical and economic burden resulting from TRD.
Transparency
Declaration of funding
No sponsorship or funding was provided for the conduct of this study.
Declaration of financial/other relationships
The authors have no conflicts of interest or financial relationships to disclose. The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed that they have received manuscript or speaker’s fees from Astellas, Dainippon Sumitomo Pharma, Eisai, Eli Lilly, Elsevier Japan, Janssen Pharmaceuticals, Kyowa Yakuhin, Lundbeck, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Nihon Medi-Physics, Novartis, Otsuka Pharmaceutical, Shionogi, Shire, Tsumura, Wiley Japan, and Yoshitomi Yakuhin, and research grants from Eisai, Mochida Pharmaceutical, Meiji Seika Pharma and Shionogi. The reviewers have no other relevant financial relationships or otherwise to disclose.
Author contributions
All authors were involved in the conception, study design, interpretation of the data, writing of the manuscript, and final approval of the version to be published.
Previous presentations
None.
Supplemental Material
Download MS Word (46.8 KB)Supplemental Material
Download MS Word (18.9 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
Data availability statement
The data that support the findings of this study are available from the authors upon reasonable request and with permission from the Health Insurance Review and Assessment Service (HIRA) in the Republic of Korea.
References
- Bromet E, Andrade LH, Hwang I, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. Jul 26
- Mrazek DA, Hornberger JC, Altar CA, et al. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr Serv. 2014;65(8):977–987.
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or serveral treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917.
- McIntyre RS, Filteau MJ, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1–7.
- Ng CH, Kato T, Han C, et al. Definition of treatment-resistant depression – Asia Pacific perspectives. J Affect Disord. 2019;245:626–636.
- Dold M, Bartova L, Kautzky A, et al. Psychotic features in patients with major depressive disorder: a report from the European group for the study of resistant depression. J Clin Psychiatry. 2019;80(1):e1–e7.
- Cepeda MS, Reps J, Ryan P. Finding factors that predict treatment-resistant depression: results of a cohort study. Depress Anxiety. 2018;35(7):668–673.
- Han K-M, Park S-C, Won E-S, et al. Evidence-based Korean pharmacological treatment guideline for depression, revised edition (III): dose increment, switching, combination, and augmentation strategy in antidepressnt therapy. J Korean Neuropsychiatr Assoc. 2013;52(5):386–401.
- Zhou X, Ravindran AV, Qin B, et al. Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J Clin Psychiatry. 2015;76(4):e487–98.
- Carter B, Strawbridge R, Husain MI, et al. Relative effectiveness of augmentation treatments for treatment-resistant depression: a systematic review and network meta-analysis. Int Rev Psychiatry. 2020;32(5–6):477–490.
- Bschor T, Kern H, Henssler J, et al. Switching the antidepressant after nonresponse in adults with major depression: a systematic literature search and meta-analysis. J Clin Psychiatry. 2018;79(1):11–18.
- Rogoz Z. Combined treatment with atypical antipsychotics and antidepressants in treatment-resistant depression: preclinical and clinical efficacy. Pharmacol Rep. 2013;65(6):1535–1544.
- Wang HR, Bahk WM, Seo JS, et al. Korean medication algorithm for depressive disorder: comparisons with other treatment guidelines. Clin Psychopharmacol Neurosci. 2017;15(3):199–209.
- Kim JA, Yoon S, Kim LY, et al. Towards actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32(5):718–728.
- Seo JS, Bahk WM, Wang HR, et al. Korean medication algorithm for depressive disorders 2017: third revision. Clin Psychopharmacol Neurosci. 2018;16(1):67–87.
- Kim N, Cho SJ, Kim H, et al. Epidemiology of pharmaceutically treated depression and treatment resistant depression in South Korea. PLoS One. 2019;14(8):e0221552.
- Dold M, Kasper S. Evidence-based pharmacotherapy of treatment-resistant unipolar depression. Int J Psychiatry Clin Pract. 2017;21(1):13–23.
- Cepeda MS, Reps J, Fife D, et al. Finding treatment-resistant depression in real-world data: how a data-driven approach compares with expert-based heuristics. Depress Anxiety. 2018;35(3):220–228.
- Gibson TB, Jing Y, Smith Carls G, et al. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16(5):370–377.
- Lepine BA, Moreno RA, Campos RN, et al. Treatment-resistant depression increases health costs and resource utilization. Braz J Psychiatry. 2012;34(4):379–388.
- Hassan AK, Farmer KC, Brahm NC, et al. Evaluating antidepressant treatment prior to adding second-line therapies among patients with treatment-resistant depression. Int J Clin Pharm. 2016;38(2):429–437.
- Balestri M, Calati R, Souery D, et al. Socio-demographic and clinical predictors of treatment resistant depression: A prospective European multicenter study. J Affect Disord. 2016;189:224–232.
- Tsukazawa SK, Fife D, Shimamoto K, et al. Treatment resistant depression development from major depressive disorder: a claims database analysis in Japan. Value in Health. 2016;19(7):A843.
- Fife D, Feng Y, Wang MY, et al. Epidemiology of pharmaceutically treated depression and treatment resistant depression in Taiwan. Psychiatry Res. 2017;252:277–283.
- Zhou X, Keitner GI, Qin B, et al. Atypical antipsychotic augmentation for treatment-resistant depression: a systematic review and network meta-analysis. IJNPPY. 2015;18(11):pyv060.
- Wang HR, Woo YS, Ahn HS, et al. Can atypical antipsychotic augmentation reduce subsequent treatment failure more effectively among depressed patients with a higher degree of treatment resistance? a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2015;18(8):pyv023.
- Davies P, Ijaz S, Williams CJ, et al. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12(12):CD010557.
- Corey-Lisle PK, Birnbaum HG, Greenberg PE, et al. Identification of a claims data "signature" and economic consequences for treatment-resistant depression. J Clin Psychiatry. 2002;63(8):717–726.
- Crown WH, Finkelstein S, Berndt ER, et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63(11):963–971.
- Amos TB, Tandon N, Lefebvre P, et al. Direct and indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a US commercial claims database. J Clin Psychiatry. 2018;79(2):17m11725.
- Mahlich J, Tsukazawa S, Wiegand F. Estimating prevalence and healthcare utilization for treatment-resistant depression in Japan: a retrospective claims database study. Drugs Real World Outcomes. 2018;5(1):35–43.
- Kimm H, Yun JE, Lee SH, et al. Validity of the diagnosis of acute myocardial infarction in korean national medical health insurance claims data: the Korean heart study (1). Korean Circ J. 2012;42(1):10–15.