Abstract
Alzheimer’s disease (AD) is the predominant cause of dementia and a leading cause of death globally. With no cure or treatment to slow disease progression, AD-related healthcare costs are substantial and increase as the severity of the disease progresses. Given the complexity of this disease, including initial pathophysiological damage occurring decades before clinical manifestation, finding new impactful treatments for AD relies on highly innovative research and development. However, such sizable and sustained investments bring into question whether conventional value assessment models are fit for this purpose. In this article, we examine the importance and challenges of assimilating the perspectives of varied stakeholders, including patients, caregivers, health systems, payers, and society at large, into a comprehensive value assessment model that may be well suited for a breakthrough treatment for AD.
Introduction
Dementia refers to a group of symptoms, including difficulties with memory, language, and cognitive skills, that affect a person’s ability to perform daily activities. Alzheimer’s disease (AD) is estimated to represent 60–80% of all dementia casesCitation1. Symptoms in AD progress gradually from a baseline of normal cognition to dementiaCitation2. There is no treatment currently that prevents, cures, or slows the progression of AD; current medications are indicated for the symptomatic treatment of mild to moderately severe AD dementia, ameliorating symptoms such as cognitive and functional decline, for a limited timeCitation3. Current symptomatic therapies available in the United States include cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and memantine, an N-methyl-d-aspartate receptor antagonistCitation4. In addition, a memantine and donepezil combination therapy is approved in the United StatesCitation4. Despite the availability of symptomatic treatments, AD remains both progressive and irreversible, making it one of the leading causes of death globallyCitation5. Of the top 10 causes of death in the United States in 2017Citation6, AD is the only one for which there is no current treatment option to slow disease progression by addressing the underlying disease pathologyCitation7. Even in the absence of a disease-modifying therapy, early diagnosis of AD is important to allow patients and families time to access life-planning advice, financial support, and nonpharmacological and pharmacological treatmentsCitation8. Early diagnosis and intervention may lead to higher up-front costs, which may be offset by subsequent savings, primarily in institutionalization costsCitation9.
AD-related costs increase as a patient’s cognitive and functional skills declineCitation10, which leads to greater dependence on the long-term care system, informal caregivers, and social careCitation1. Patients with AD and their families pay a substantial amount for out-of-pocket care-related costs. A retrospective cohort study of data collected between 2005 and 2010 in the United StatesCitation11 found that the average out-of-pocket spending for patients with dementia was 81% higher ($61,522) than spending for patients without dementia ($34,068). Data collected between 2004 and 2015 in the Health and Retirement study revealed that cumulatively over the last seven years of life, a higher burden of healthcare costs was attributed to families of patients with dementia compared with families of patients without dementiaCitation12. While these known informal caregiving and out-of-pocket costs are significant, a large proportion of the true societal costs are likely hiddenCitation13.
The impacts of AD to patients and their families are tremendous and go well beyond the direct financial costsCitation14. Only 16% of AD costs are directly paid for by the healthcare system, with the vast majority of this enormous cost burden borne by the patient/caregiver in the form of social care costs (42.3%) and informal care or indirect costs (41.7%)Citation15. Given the significant unmet needs for this population and burdensome societal impacts, there is a keen interest in expanding the therapeutic arsenal. Novel therapeutic approaches aim to slow AD progression by addressing the underlying pathology of the disease, which can appear as early as 20 years prior to clinical manifestationCitation16. The launch of such therapies would represent a transformative change for patients with AD.
As AD impacts many factors pertinent to families and society at large, traditional methods for performing value assessments, while useful, may omit some important value considerations. The question then naturally arises how to value therapies that will impact not only the future course of medical spending but aspects outside of direct medical spending, such as social care factors and costs, caregiver or family burden, quality of life, and related impacts of the disease.
What follows is a discussion of key valuation issues facing a breakthrough AD therapy—from the perspectives of patients, patient advocates, payers, policy makers, health economists, innovative manufacturers, and society at large. The discussion here builds upon the themes raised in a session at the virtual ISPOR (International Society for Pharmacoeconomics and Outcomes Research) 2020 Symposium.
The importance of encouraging innovation
Payers and health systems face the challenge of ensuring patient access to new therapies while concomitantly rewarding effective innovation and serving as judicious stewards of limited financial resources. A thoughtful and balanced approach is needed to ensure that while patients have equitable access to these effective new treatments, manufacturers are incentivized to develop new therapies over the long term. The challenge to manufacturers is embodied by the history of failure and associated financial risks of investing in uncharted therapeutic areas. These desires create an inherent tension—how to support the consumer’s need for lower prices and expanded use while enabling the innovator to make significant investments in research and development (R&D)Citation17.
The interest in providing incentives for innovation is recognized worldwide. According to some estimates, in 2018, the US federal government covered approximately 22.2% of R&D expenses, while pharmaceutical companies covered 66.7%Citation18. Policy makers recognize the risky nature of innovation and hence have developed incentives to encourage R&D to find treatments for diseases and reach patient populations that would otherwise be neglected. The introduction of the 1983 US Orphan Drug Act, for example, increased the development of treatments for rare diseasesCitation19. In addition, the implementation of Medicare Part D both improved prescription drug coverage for older patients, and further resulted in an expansion in the number of clinical trials in diseases affecting this populationCitation20.
Innovation is inherently risky, and the way that regulators and payers measure the value of innovation is vital to the fair and comprehensive assessment of an innovative treatment. Thus, an additional avenue to recognize the value of innovation is to expand the types of meaningful benefits included in value assessment. Currently, there is a lack of innovative treatments for AD. However, the way the value of the first breakthrough treatment (e.g. one that treats the underlying pathology of AD) is assessed will set the course for future innovative therapies.
Challenges with value assessments
Multifaceted value of innovative therapies
Current value assessments often evaluate the overall (incremental) costs per quality-adjusted life year (QALY) to determine if the result is “cost effective”, as benchmarked against the value of the QALY. This framework of value allows payers to assess the value that new treatments bring to the health systemCitation21. Such analyses provide a tool that allows payers to compare the effectiveness of interventions across disease areas to make decisions on resource allocationCitation22. However, many published cost-per-QALY analyses do not generally recognize the burden of disease on family, caregivers and society and therefore do not fully recognize the value of treatments that may help alleviate that burden. Typical cost-effectiveness frameworks include direct patient costs and benefits only and omit the numerous opportunity costs. Furthermore, for a disease such as AD in which the patient becomes dependent on caregiving for everyday functioning, the caregiver is effectively considered a dyad with the patient—their lives are commingled. From this perspective, the impact of disease on the caregiver is critical to include in the evaluation. Thus, value assessment frameworks should become more AD-patient centric and evolve to better reflect long-term health. Furthermore, the frameworks must be adapted to capture the burden of disease on families, caregivers, employers, and society.
Various entities are striving to identify a more comprehensive approach to value assessments. For instance, the ISPOR Special Task Force value “flower”Citation21 () is a framework that incorporates the core elements of value assessments (QALYs gained and net costs), as well as elements inconsistently used in value assessments (productivity and adherence-improving factors), and potential novel value elements not typically captured (reduction in uncertainty, fear of contagion, insurance value, severity of disease, value of hope, real option-value, equity, and scientific spillovers). This framework moves beyond conventional cost-per-QALY analyses and outlines a more comprehensive approach to value assessments that may better capture the broader value that an innovative therapy may bring to society.
Figure 1. Elements of value as a framework for value assessment. Circles represent core elements of value (green), common but inconsistently used elements of value (light blue), and potential novel elements of value (dark blue). Lines represent value element included in traditional payer or health plan perspective (blue) and value element also included in societal perspective (red). Reprinted from Lakdawalla DN, et al. Value Health. 2018;21(2):131–139, Copyright 2018, with permission from Elsevier.
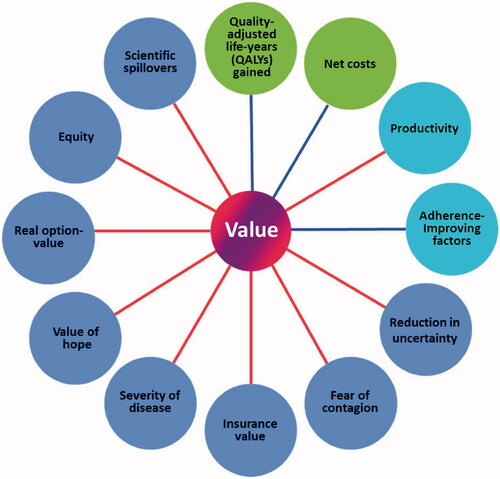
Two of the novel elements of the ISPOR Task Force’s value “flower” are the real option-value and the value of hope. Real option-value refers to the value created by a health technology that extends life and creates opportunities for the patient to benefit from other future advances in medicineCitation23. Hypothetically, if patients had to choose between two interventions offering the same expected QALY gain, they may prefer the intervention that provides a greater chance to benefit from access to future scientific and clinical advancesCitation21 (although the potential costs of those future advances should also be taken into account). The value of hope refers to the fact that patients may be more willing to take chances on therapies that offer small probabilities of larger gains, rather than focusing solely on the mean gains in QALYs. Many severely ill patients, for example, may be willing to trade off some survival (e.g. undertaking a risky procedure) for the chance of a “cure”Citation21, even if there is a very small probability of success.
The value “flower” approach is in line with traditional recommendations from consensus groupsCitation24, which suggest that cost-per-QALY analyses should be integrated with other considerations such as affordability, budget impact, fairness, feasibility, and any other criteria deemed important in local settings. In addition, the perspective of all relevant stakeholders—patients, caregivers, clinical experts, health system administrators, and society in general—should be considered in the assessment. However, one should recognize that if we build other elements into the QALY, the cost-per-QALY threshold may need adjusting downward because the value of a QALY would have changed. In other words, we need to reconsider what resources society is willing to forego to obtain the “new” QALY. Society’s preference for equity in opportunity to benefit from treatment suggests that more value is placed on innovation where no current therapy exists; this is the case in treatments for rare diseasesCitation25 and is also true for future therapies that target the underlying pathology of AD. According to the Second Panel on Cost Effectiveness in Health and Medicine, cost-effectiveness analyses should include an “Impact Inventory” that presents healthcare system and societal perspectives in distinct reference casesCitation26.
A quantum leap in innovation in treatment for a disease may be valued more than incremental steps that, over time, achieve the same aims. However, in an area of rapid innovation and R&D investment, a new therapy that advances the scientific field can contribute significantly, even when “scientific spillovers” are not considered part of value assessments. Highly active antiretroviral therapy (HAART) for HIV is perhaps the canonical example. In the years prior to HAART’s introduction, AIDS remained one of the most devastating diseases globally with a significant unmet need. The entry of HAART in 1995 significantly improved patient survival; it was estimated that life expectancy for individuals with AIDS was 3 years in 1984, compared with 16.5 years in 2000Citation27. However, HAART was introduced with prices higher than the estimated value. Ultimately, long-term research demonstrated that new drugs to treat HIV/AIDS, including HAART, that first came on the market in the late 1980s dramatically improved survival, which was valued at more than $1 trillion in overall value, with 5% of this distributed among the manufacturersCitation27.
Challenges of applying current value assessment frameworks to new AD therapies
We focus on three questions regarding challenges in the methods and process of value assessments for AD therapies.
Do QALYs undervalue treatments for older patients?
Some cost-per-QALY analyses have reported more favorable cost-effectiveness for interventions targeted to older versus younger populations because the former have higher disease prevalence or more risk factors and, ultimately, more to gain from successful therapiesCitation28. Still, given that older people have a lower remaining life expectancy than younger people, interventions for older people may produce less QALY gain than interventions for younger populationsCitation29. In light of this, and recognizing that approximately 80% of people with AD are >75 years oldCitation1 with an estimated mortality rate that is higher than that of a younger population, the use of the cost-per-QALY methodology raises important ethical questions about how to allocate resources for interventions affecting mostly older populations with potentially multiple comorbiditiesCitation29. Additionally, in 2014, of individuals with AD or a related dementia who were ≥65 years old, one in five to as many as one in three had coexisting conditions such as congestive heart failure, chronic kidney disease, diabetes, or coronary artery diseaseCitation1. Interventions for severe conditions such as AD may also warrant special consideration, e.g. higher cost-effectiveness benchmarks to address the cost-per-QALY limitations relevant to ADCitation30.
Non-QALY metrics can also be presented alongside QALYs. An overarching goal in value/health technology assessment is to assess clinical evidence, costs, and societal and ethical impacts in structured and standardized ways. Value/health technology assessment should be transparent and include high-quality data on aspects of care and outcomes that are important to patients and other stakeholders whenever such data exist. Researchers, value assessors, and the patient community agree that conventional approaches to value assessment often inadequately account for the many dimensions of value.
Should cost-effectiveness analyses capture a societal perspective and account for savings beyond healthcare-related costs?
Capturing value beyond the health system is critical for appropriately measuring the value of all innovations, including public health programs, diagnostics, and procedures, as well as drug therapies, such as those for AD. Direct healthcare costs form a relatively small proportion of the known costs of AD, and many value assessments exclude non–healthcare-related costs. In a study published in 2013, informal care costs for dementia were projected to rise to $255 billion in 2020 (in 2010 USD)Citation31, and this finding may have missed “hidden” costs (e.g. those due to delays in diagnosis). Another study found that 70% of cost-utility analyses for AD and other dementias considered at least some family or caregiver spillover costs, but only 19% considered spillover health effectsCitation32. A recent literature review of multinational studies found that informal care costs represent approximately 60% of total costs for patients with early stages of AD, increasing to 72.5% for patients with severe ADCitation33. Although cognitive decline has been identified as the main driver of societal costs of patients with ADCitation34, both cognitive decline and physical disability were found to be predictors of increased informal care costsCitation35. Moreover, the need for social and informal care is already higher in patients with mild cognitive impairment likely due to AD than age-matched controls and increasing further in patients with AD dementiaCitation33. The providers of informal care are also an important element to consider for cost-effectiveness analyses, as it is estimated that an intervention may no longer be considered cost effective if professional care replaces caregiving by family membersCitation36.
Medicare pays for medical care and only some short-term supportive care. However, low Medicare costs for AD does not mean low health system costs. Medicaid, a secondary payer for people with Medicare, is an important source of funding for AD care. Medicaid pays for most of US nursing home care costs, and in 2014, >60% of nursing home residents had moderate to severe dementiaCitation1. Most states have waivers that expand coverage to home and community-based services and support for Medicaid beneficiaries who are at risk of needing nursing home care. Medicaid also pays the Medicare Part B and Part D premiums and may pay the Medicare cost sharing for dual-eligible beneficiaries.
Medicare by statute would cover effective therapies for individuals that prevent or delay AD and dementia. However, Medicaid, as the primary payer of long-term services and support, such as custodial care in nursing homes and home care programs, is the government program that would benefit the most from disease-modifying therapies. The adverse consequences of poor integration of Medicare and Medicaid have been a long-standing concernCitation37.
Furthermore, patients with AD generally have multiple comorbiditiesCitation1,Citation38, which can exacerbate the disease and increase care requirements. Caregivers report higher levels of stress and emotional difficultiesCitation1, due to the increasing requirements of patients with AD, which in turn can increase the caregivers’ susceptibility to health complications and impact their work opportunities and productivity. Collecting evidence on the possible treatment effects on such broader costs and benefits, and reflecting them in cost-effectiveness analyses, is important because it will likely influence results. Additionally, measures of productivity should be broadened to include productivity that is not traditionally economic. Without addressing these limitations, valuations of breakthrough therapies in AD may be too low.
How can we best capture the value that an AD therapy may bring to society?
The health and quality-of-life impacts of AD are typically captured by proxy respondents, such as caregivers, due to the reduced cognitive ability of the patient. Caregivers are often family members providing informal care until a greater loss of independence leads to formal social care and institutionalization. Therefore, value assessment approaches should consider the input of all stakeholders at the appropriate steps. A wide range of criteria needs to be considered in a holistic way to ensure meaningful insight into the broad impact of AD and the value that an innovation may bring to patients, caregivers, and society at large. Integration of behavioral economics would add the perspective of those actually living with AD and their family caregivers, instead of only healthy consumers or economists who are merely contemplating what living with AD might be like and then making assumptions about what matters to those who have the disease. Furthermore, value assessments should explore the heterogeneity of effects within a patient populationCitation39. For instance, patients and caregivers may have divergent preferences regarding health outcomes and different interventions, which should be investigated.
Recommendations and conclusions
To enable a wider range of value elements to be incorporated into the value assessment, additional data will be required, such as improved data on the disease natural history, costs, and utilities; comorbidities associated with the disease; and societal impact of the disease. This will require consideration of the perspectives of all relevant stakeholders, including patients, caregivers, clinical experts, health system administrators, economists, and policy makers. Simultaneously, value assessments should embrace the collection of real-world evidence to address any incomplete information or any uncertainties, particularly for disease natural history prior to and following diagnosis.
Value assessment should use robust methods and models that can integrate a broader set of value elements. This may require adapting existing models or creating new models to explore the value of a breakthrough. For some diseases, such as AD and other dementias, it would be beneficial if the model starts earlier in the patient’s life (prior to diagnosis) to capture a better representation of the costs and utilities associated with the disease. A variety of techniques and data approaches should be investigated, such as multicriteria decision analysis, patient surveys, and Delphi consensus to capture patient preferences. These methods need to be adaptable to therapeutic advances, evidence standards, societal values, and evolving structure of health systems.
The first approved treatment that slows AD progression will represent a breakthrough step in innovation. Payers’ and health systems’ ability to assess value appropriately for this new treatment will influence future innovation not only in AD but also in other therapy areas. Many healthy individuals paying into the health system today may benefit from innovation in AD as it may address their or their families’ future health needs; thus, it is in society’s interest to continue innovation to progress toward a cure for AD. Better alignment of system-wide incentives with the societal willingness to pay for innovation in AD is needed, along with creative contracting and cost-sharing reforms. Such reforms are needed at multiple levels to address drug pricing and total costs of health care more broadly and extend to societal costs as well as savings. Accounting for societal benefits and considering information alongside traditional cost-effectiveness analyses will be useful to appropriately value a breakthrough therapy in AD.
Transparency
Declaration of funding
DG was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG062277. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding for the virtual ISPOR Symposium session on 18 May 2020, Modeling the Value of Innovative Treatments for Alzheimer’s Disease in the United States, was provided by Biogen.
Declaration of financial/other relationships
CM is a Biogen employee and holds stock. PN has received grant support, honoraria, or consulting income from the following sources: AbbVie, Alkermes, Allergan, Alzheimer’s Association, Arnold Ventures, Amgen, Bill and Melinda Gates Foundation, Biogen, Boston Health Economics, Bristol Myers Squibb, Celgene, Congressional Budget Office, Edwards Lifesciences, Eli Lilly, EMD Serono, Genentech, Gilead Sciences, GSK, Intercept, Johnson & Johnson, Merck, National Cancer Institute, National Institute on Aging, National Institutes of Health, National Pharmaceutical Council, Novartis, Novo Nordisk, Otsuka Pharmaceuticals, Pfizer, PhRMA, Precision Health Economics, Robert Wood Johnson Foundation, Roche, Sage, Sanofi, Sarepta, and Takeda. SP is supported through the Alliance for Aging Research. DG has received research support, speaker fees, travel assistance, or consulting income from the following sources: ACADIA Pharmaceuticals, Amgen, The Aspen Institute, Biogen, Blue Cross Blue Shield of Arizona, Bristol Myers Squibb, Cedars-Sinai Health System, Celgene, Edwards Lifesciences, Gates Ventures, Genentech, Gilead Sciences, GRAIL, Johnson & Johnson, Kaiser Family Foundation, National Institutes of Health, Novartis, Pfizer, Precision Health Economics, Roche, and Walgreens Boots Alliance. He also owns equity (<1%) in Precision Medicine Group.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
All authors contributed to the conception of this work and participated in the drafting and critical review of this article. They have provided approval of the final version to be published.
Acknowledgments
Medical writing support, under direction of the authors, was provided by MediTech Media, and was funded by Biogen.
References
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16(3):391–460.
- Aisen PS, Cummings J, Jack CR, Jr, et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res Ther. 2017;9(1):60.
- Joe E, Ringman JM. Cognitive symptoms of Alzheimer’s disease: clinical management and prevention. BMJ. 2019;367:l6217.
- Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA. 2019;322(16):1589–1599.
- Nichols E, Szoeke CE, Vollset SE, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106.
- Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68(6):1–77.
- Cummings J, Lee G, Ritter A, et al. Alzheimer’s disease drug development pipeline: 2020. Alzheimers Dement. 2020;6(1):e12050.
- Rasmussen J, Langerman H. Alzheimer's disease—why we need early diagnosis. Degener Neurol Neuromuscul Dis. 2019;9:123–130.
- Dubois B, Padovani A, Scheltens P, et al. Timely diagnosis for Alzheimer’s disease: a literature review on benefits and challenges. J Alzheimers Dis. 2016;49(3):617–631.
- Leibson CL, Long KH, Ransom JE, et al. Direct medical costs and source of cost differences across the spectrum of cognitive decline: a population-based study. Alzheimers Dement. 2015;11(8):917–932.
- Kelley AS, McGarry K, Gorges R, et al. The burden of health care costs for patients with dementia in the last 5 years of life. Ann Intern Med. 2015;163(10):729–736.
- Kelley AS, McGarry K, Bollens‐Lund E, et al. Residential setting and the cumulative financial burden of dementia in the 7 years before death. J Am Geriatr Soc. 2020;68(6):1319–1324.
- El-Hayek YH, Wiley RE, Khoury CP, et al. Tip of the iceberg: assessing the global socioeconomic costs of Alzheimer’s disease and related dementias and strategic implications for stakeholders. J Alzheimers Dis. 2019;70(2):323–341.
- Gustavsson A, Pemberton-Ross P, Gomez Montero M, et al. Challenges in demonstrating the value of disease-modifying therapies for Alzheimer’s disease. Expert Rev Pharmacoecon Outcomes Res. 2020;20(6):563–570.
- Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15(5):455–532.
- Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–262.
- Lakdawalla DN, Goldman DP, Michaud P-C, et al. US pharmaceutical policy in a global marketplace: reducing copays tends to be a robust and welfare-improving policy, while imposing price controls risks high costs in the hope of a relatively modest benefit. Health Affairs. 2008;27(Suppl1):w138–w150.
- Research America. U.S. Investments in medical and health research and development 2013–2018. Arlington (VA): Research America; 2019.
- Yin W. Market incentives and pharmaceutical innovation. J Health Econ. 2008;27(4):1060–1077.
- Blume-Kohout ME, Sood N. Market size and innovation: effects of Medicare Part D on pharmaceutical research and development. J Public Econ. 2013;97:327–336.
- Lakdawalla DN, Doshi JA, Garrison LP, Jr, et al. Defining elements of value in health care—a health economics approach: an ISPOR Special Task Force report. Value Health. 2018;21(2):131–139.
- Schwappach DL. Resource allocation, social values and the QALY: a review of the debate and empirical evidence. Health Expect. 2002;5(3):210–222.
- Cook JP, Golec JH, Vernon JA, et al. Real option value and path dependence in oncology innovation. Int J Econ Bus. 2011;18(2):225–238.
- Bertram MY, Lauer JA, De Joncheere K, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925–930.
- Goldman D, Lakdawalla D, Philipson TJ, et al. Valuing health technologies at NICE: recommendations for improved incorporation of treatment value in HTA. Health Econ. 2010;19(10):1109–1116.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103.
- National Bureau of Economic Research. Who benefits from new medical technologies? Estimates of consumer and producer surpluses for HIV/AIDS drugs. Cambridge (MA): National Bureau of Economic Research; [cited 2020 Dec 21]. Available from: https://www.nber.org/system/files/working_papers/w11810/w11810.pdf
- Neumann PJ, Levine BS. Do HEDIS measures reflect cost-effective practices? Am J Prev Med. 2002;23(4):276–289.
- Huter K, Kocot E, Kissimova-Skarbek K, et al. Economic evaluation of health promotion for older people-methodological problems and challenges. BMC Health Serv Res. 2016;16(Suppl 5):328.
- Lakdawalla DN, Phelps CE. Health technology assessment with risk aversion in health. J Health Econ. 2020;72:102346.
- Hurd MD, Martorell P, Delavande A, et al. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334.
- Lin P-J, D’Cruz B, Leech AA, et al. Family and caregiver spillover effects in cost-utility analyses of Alzheimer’s disease Iinterventions. Pharmacoeconomics. 2019;37(4):597–608.
- Kosaner Kließ M, Martins R, Connolly MP. Major cost drivers in assessing the economic burden of Alzheimer’s disease: a structured, rapid review. J Prev Alz Dis. 2021:1–9. DOI:https://doi.org/10.14283/jpad.2021.17
- Robinson RL, Rentz DM, Andrews JS, et al. Costs of early stage Alzheimer’s disease in the United States: cross-sectional analysis of a prospective cohort study (GERAS-US)1. J Alzheimers Dis. 2020;75(2):437–450.
- Costa N, Wubker A, De Mauleon A, et al. Costs of care of agitation associated with dementia in 8 European countries: results from the righttimeplacecare study. J Am Med Dir Assoc. 2018;19(1):95 e1–95 e10.
- Pena-Longobardo LM, Rodriguez-Sanchez B, Oliva-Moreno J, et al. How relevant are social costs in economic evaluations? The case of Alzheimer’s disease. Eur J Health Econ. 2019;20(8):1207–1236.
- Report to the Congress: Medicare and the Health Care Delivery System. Chapter 9: Issues affecting dual-eligible beneficiaries: CMS’s financial alignment demonstration and the Medicare Savings Programs. Washington (DC): Medicare Payment Advisory Committee (US). 2016.
- Bunn F, Burn AM, Goodman C, et al. Comorbidity and dementia: a scoping review of the literature. BMC Med. 2014;12:192.
- Garrison LP, Jr, Neumann PJ, Willke RJ, et al. A health economics approach to US value assessment frameworks–summary and recommendations of the ISPOR Special Task Force Report [7]. Value Health. 2018;21(2):161–165.
