Abstract
Aim
To compare the cost of biweekly regimens of first-line (1L) treatments of cetuximab-folinic acid, fluorouracil, and irinotecan (FOLFIRI) versus panitumumab-folinic acid, fluorouracil, and oxaliplatin (FOLFOX) in patients with Kirsten’s rat sarcoma wild type (KRAS WT) metastatic colorectal cancer (mCRC) in the United States, across varying weights and body surface areas (BSAs).
Materials and methods
Cost-minimization analysis (CMA) was performed to estimate per-patient cost differences of cetuximab-FOLFIRI versus panitumumab-FOLFOX. The CMA estimated the costs of RAS testing, premedication, drug acquisition, treating infusion reactions (IRs), supportive therapy, and biweekly administration of chemotherapy, cetuximab (500 mg/m2), and panitumumab (6 mg/kg) over 43 weeks (median progression-free survival). To calculate dose and cost, weight and height data were gathered from an electronic health record-derived de-identified database (n = 7,669; January 2013–October 2020). Base case analysis utilized mean weight/BSA of the overall cohort (82.04 kg/1.92 m2), and alternate scenarios were based on 88.18 kg/2.03 m2 (men, n = 4,477) and 73.43 kg/1.76 m2 (women, n = 3,192).
Results
For the base case, total treatment costs were $167,853 for cetuximab-FOLFIRI and $168,254 for panitumumab-FOLFOX; cost savings per patient receiving cetuximab-FOLFIRI was $400. Cost savings in alternate scenarios (men, $15,138; women, $15,004) resulted from lower drug acquisition costs for cetuximab (men, $14,833; women $14,854) and administration cost ($440) versus panitumumab. Cost savings of cetuximab-FOLFIRI in treating IR ($353) were similar across all scenarios.
Limitations
With no head-to-head clinical trial data in the 1L setting, assumptions of similarity in efficacy and safety of cetuximab versus panitumumab were based on published network meta-analysis and the ASPECCT trial. This model did not consider a lifetime horizon. Costs of managing all adverse events (except IR) were not included.
Conclusions
Biweekly cetuximab-FOLFIRI offers cost savings compared with panitumumab-FOLFOX for 1L therapy of patients with KRAS WT mCRC in the United States. These cost differences were observed for the overall population and across different BSA and weights for men and women.
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer-related mortality in the United States (US)Citation1 accounting for approximately 11% of all cancer deathsCitation2. Treatment options for metastatic CRC (mCRC) have expanded over the years and include several targeted therapies given as monotherapy or in combination with chemotherapy agentsCitation3,Citation4. Cetuximab and panitumumab are targeted therapies that bind to the human epidermal growth factor receptor (EGFR). Both drugs are approved by the US Food and Drug Administration (FDA) for the treatment of patients with Kirsten’s rat sarcoma (KRAS) wild type (WT) mCRCCitation5. Cetuximab is indicated in combination with folinic acid (leucovorin), fluorouracil (FU), and irinotecan (FOLFIRI) as first-line treatment for KRAS WT mCRCCitation6. Cetuximab is approved for both weekly (400 mg/m2 followed by weekly 250 mg/m2) and biweekly (500 mg/m2) dosingCitation7,Citation8. Panitumumab is approved for biweekly dosing at 6 mg/kg and indicated as first-line therapy in combination with folinic acid, FU, and oxaliplatin (FOLFOX)Citation9.
According to National Comprehensive Cancer Network (NCCN) guidelines, both cetuximab and panitumumab are recommended as Category 2A in combination with FOLFOX or FOLFIRI for the first- and subsequent lines of treatment of patients with KRAS WT mCRC [3]. The ASPECCT trial comparing panitumumab to cetuximab in the second-line setting among patients who were refractory to chemotherapy showed that panitumumab was non-inferior to cetuximab in terms of overall survival (OS) benefitCitation10. The results showed that cetuximab and panitumumab had similar efficacy and incidence of adverse events; infusion reactions (IRs) were a key differentiatorCitation10. Although there is no head-to-head comparison in the first line setting, a recently published network meta-analysis reported no significant difference in either efficacy (hazard ratio 1.01; 95% confidence interval 0.76–1.32) or safety of first-line cetuximab-FOLFIRI compared with panitumumab-FOLFOXCitation11. With comparable efficacy and safety, cost may be an important differentiating factor between these treatments. Cost-minimization analysis (CMA) calculates the difference in treatment-related costs of two or more drugs expected to have similar efficacy and safety outcomesCitation12. The CMA can assess the least costly alternative among the drugs and help guide health care decision makingCitation12.
Assuming similar efficacy and safety, several CMA have compared the costs of cetuximab and panitumumab in the first-line settingCitation13–15; these models have estimated lower costs for patients who received biweekly panitumumab versus weekly cetuximabCitation13. A previous CMA from US payers’ perspective showed a per-patient saving of $23,125 in favour of panitumumab-FOLFOX compared to cetuximab-FOLFIRI over a 69.2-week time horizonCitation13. However, all prior CMAs were based on biweekly dosing of panitumumab and weekly dosing of cetuximabCitation13–15. In April 2021, the US FDA approved biweekly dosing of cetuximab for patients with KRAS WT mCRCCitation7. The approval was based on several studies that compared weekly and biweekly dosing of cetuximab and found both to have similar efficacy and safetyCitation16,Citation17. In a dose-escalation study assessing safety, pharmacokinetics, and pharmacodynamics of cetuximab given biweekly, the safety profiles and trough levels were similarCitation18. Furthermore, a prior review based on a comprehensive literature search of clinical trials and retrospective studies found that cetuximab biweekly dosing showed similar efficacy and did not increase the incidence or severity of adverse events when compared to cetuximab weekly dosingCitation17. Similarly, a meta-analysis by Parikh et al. reported similar efficacy and safety of biweekly versus weekly schedule and noted that biweekly dosing may potentially reduce the burden and cost of administrationCitation19. Also, patients with mCRC receiving biweekly cetuximab achieved similar OS to those administered weekly cetuximabCitation16,Citation20,Citation21. Therefore, given that prior CMAs assessed the cost of weekly dosing, similar safety, and efficacy of weekly and biweekly cetuximab, and with recent approval of biweekly dosing for cetuximab in mCRC [7], there is a need to assess the cost difference of biweekly dosing regimens of cetuximab and panitumumab.
To address this knowledge gap, CMA was performed to compare the direct medical care cost for cetuximab and FOLFIRI (cetuximab-FOLFIRI) and panitumumab and FOLFOX (panitumumab-FOLFOX) in the first-line setting when administered biweekly to patients with KRAS WT mCRC. Additionally, cost outcomes for different scenarios based on real world patient values of weight and body surface area (BSA) were evaluated.
Methods
This CMA compared the treatment-related per-patient costs associated with cetuximab-FOLFIRI and panitumumab-FOLFOX. The model was developed using the process recommended by the International Society for Pharmacoeconomics and Outcomes ResearchCitation22 and cost data to model the direct costs of cetuximab and panitumumab. The model was developed from a US third-party payer perspective. The time horizon of 43 weeks was based on the median progression-free survival (mPFS) in the CRYSTAL (weekly cetuximab; 9.9 months) and PRIME trials (biweekly panitumumab; 10.0 months)Citation23,Citation24.
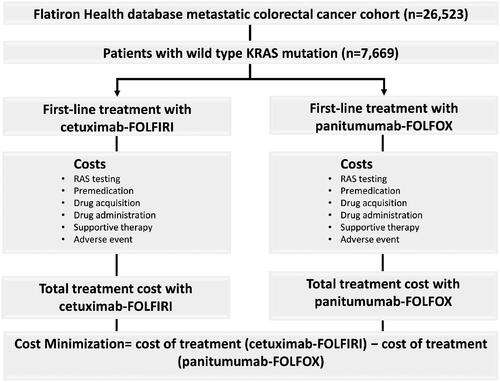
The model inputs included costs for RAS testing, premedication, drug acquisition, treatment administration, supportive therapy, and management of IRs. The respective combination chemotherapies used with cetuximab and panitumumab in the CMA were selected in accordance with their approved prescribing information in the USCitation8,Citation25. The structural flow of the model is depicted in .
Figure 1. Structural flow of cost-minimization model. Patients with KRAS WT metastatic colorectal cancer with data on height and weight were identified from the Flatiron Health database. Patients were eligible for first-line treatment, and it was assumed they would receive either cetuximab-FOLFIRI or panitumumab-FOLFOX. FOLFIRI, folinic acid, fluorouracil, and irinotecan; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; KRAS, Kirsten’s rat sarcoma; WT, wild type.
Model inputs
Patient population
To calculate cost, average weight and BSA values were obtained from real-world patients. The patient population for this CMA was derived from nationwide Flatiron Health electronic health record-derived de-identified databaseCitation25. The Flatiron Health database is a real world, longitudinal database, consisting of de-identified patient-level structured and unstructured data, curated via technology-enabled abstraction. The de-identified data originated from approximately 280 US cancer clinics (approximately 800 sites of care), the majority of which were derived from community oncology settings. The Flatiron Health database mCRC cohort comprised 26,523 patients aged ≥18 years, diagnosed with mCRC from January 2013 to October 2020. Of these, 8,108 patients were confirmed to have KRAS WT, and 7,669 had both weight and height values available, which were used to input body weight and BSA data. The patients included were eligible to receive either cetuximab-FOLFIRI or panitumumab-FOLFOX in the first-line setting. The mean weight and BSA of the overall population (base case), and different scenarios for various weight/BSA combinations were used to calculate dose. BSA was calculated using the Dubois formula (m2=0.007184 × height [cm]0.725 × weight [kg]0.425)Citation26,Citation27. The scenario analyses for men and women were informed by the fact that cetuximab and panitumumab are weight-based regimens, and weight and height values for men and women differ in the general populationCitation28. The population characteristics, mean and median weight, and BSA values are summarized in .
Table 1. Demographic and health characteristics of patients with KRAS WT mCRC in Flatiron Health database.
Drug dose, acquisition, and administration
Intravenous infusions of 500 mg/m2 (cetuximab) or 6 mg/kg (panitumumab) every 2 weeks for 22 cycles were considered for the estimation of costCitation8,Citation29. The administration schedule and dosing utilized for mFOLFOX6 and FOLFIRI were based on NCCN guidelines for first-line treatment of KRAS WT mCRC and are summarized in . Drug acquisition costs were obtained from the wholesale acquisition costs based on Red Book (2021)Citation30. Where more than one price was available for the drugs, the cheapest unit price was used. Given that cetuximab and panitumumab are single-use vialsCitation8,Citation29, we modelled cost on the number of vials used. Therefore, costs were reflective of the rounded-up number of vials where applicable. Administration costs were calculated using the Centers for Medicare and Medicaid Services (CMS) physician fee schedule 2021 and were based on the dose, type, and infusion time of anti-EGFR, and combination chemotherapyCitation31. Biweekly dosing for cetuximab is infused intravenously over 2 h while panitumumab is infused intravenously over 1 h for doses less than 1,000 mgCitation3,Citation8,Citation29. It was assumed that all patients received 22 cycles of infusion (43 weeks) and no dose modifications or discontinuations occurred.
Table 2. Dose and administration schedules of cetuximab, panitumumab, FOLFIRI and FOLFOX.
Adverse events
Based on the network meta-analysisCitation11 there were no differences in adverse events observed among patients with mCRC receiving cetuximab-FOLFIRI and panitumumab-FOLFOX as first-line treatment. Furthermore, the ASPECCT trial with the head-to-head comparison of cetuximab and panitumumab concluded that the adverse events of any grade were similar except for IR, skin toxicity, and hypomagnesaemiaCitation10. In the absence of a head-to-head trial in first-line therapy and given the clinical relevance and expected cost impact of hypersensitivity IRs, especially in patients receiving cetuximab, the cost for IRs was included. The cost of managing Grade 1–2 and 3–4 IRs were included and it was assumed that the patients would require outpatient services and hospitalization, respectively. The incidence rates for Grade 3–4 IRs were based on the US prescribing information (2.2% and 1.0% for cetuximab and panitumumab, respectively)Citation8,Citation29. Since neither the PRIME nor CRYSTAL trial provided incidence rates of Grade 1–2 IRs, the incidence rates from the ASPECCT trial were used assuming similar rates in the first-line and second-line therapy: cetuximab 13% and panitumumab 3%Citation10. According to the cetuximab prescribing information premedication is required prior to administrationCitation8. As a majority of severe allergic reactions are known to be associated with the first dose, it was assumed premedication was needed for the loading dose for each patient receiving cetuximabCitation8 but not for panitumumab. The proportion of maintenance infusions needing premedication was based on the incidence rate of IRs in the ASPECCT trial (13% cetuximab, 3% panitumumab)Citation13. Based on NCCN treatment guidelines, ondansetron (8 mg per-oral [PO], daily, three doses every 8 h on day 1, and two doses every 12 h on day 2) and metoclopramide (10 mg PO) are recommended to prevent nausea and vomiting caused by concomitant chemotherapy-FOLFIRI or FOLFOXCitation32.
Lastly, the model incorporated RAS testing as a one-time cost of $682 for both treatments, as per the 2021 Clinical Laboratory Fee ScheduleCitation33. summarizes all model inputs including weight/BSA, premedication, drug acquisition, administration, supportive care, and adverse events. The dosing, administration rates, and unit costs for all drugs are provided where applicable.
Table 3. Model input parameters.
Base case, sensitivity, and scenario analyses
In the base case analysis, total treatment-related costs per patient were calculated over 43 weeks (22 infusions). Costs for each input parameter were compared between cetuximab and panitumumab. In the base case scenario, mean weight and BSA of the overall population were considered to calculate dosing. A one-way sensitivity analysis was performed to assess the impact of various parameters on the modelled results. The parameters included: BSA, body weight, cost of cetuximab, cost of panitumumab, time horizon, cost of concomitant chemotherapy, cost of premedication, cost of administration, and incidence rate of IR. All parameters were subjected to ±20% variation individually while holding other parameters at their input values, and assuming they were all independent of each other. Scenario analyses were performed to assess cost outcomes with varying weight and BSA values from the overall patient population. The mean and median values of weight and BSA for men and women were modelled to assess their impact on cost outcomes for patients diagnosed with KRAS WT mCRC.
Results
Base case analysis
Biweekly cetuximab-FOLFIRI was associated with a lower cost of treatment over 43 weeks compared with biweekly panitumumab-FOLFOX with a cost savings of $400 per patient. Incremental costs for cetuximab-FOLFIRI resulted from drug cost of cetuximab (+$102) and management of IRs (+$353), while for panitumumab-FOLFOX, incremental costs resulted from drug cost of chemotherapy (FOLFOX) (+$418) and drug administration (+$440). The base case results are shown in . Results of the sensitivity analyses demonstrated that the BSA, cost of cetuximab, body weight, and cost of panitumumab had a comparable impact on the CMA results. The sensitivity analysis further demonstrated that cetuximab-FOLFIRI remained a cost saving option compared to panitumumab-FOLFOX when BSA and cost of cetuximab were decreased by 20%, and when body weight and cost of panitumumab were increased by 20% ().
Figure 2. Tornado diagram depicting the results of one-way sensitivity analyses. BSA, body surface area.
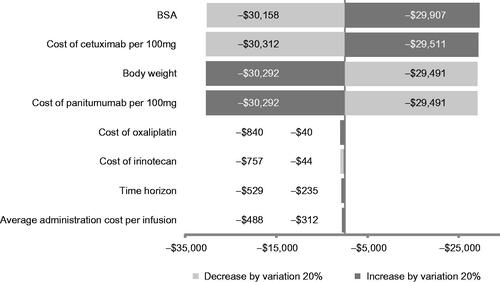
Table 4. Cost per treated patient: biweekly cetuximab-FOLFIRI and panitumumab-FOLFOX as first-line therapy for base case and case scenarios over 43 weeks.
Scenario analyses
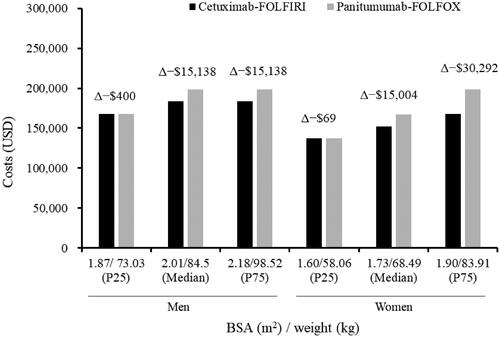
In the scenario analysis where mean BSA/body weight was 2.03 m2/88.18 kg for men and 1.76 m2/73.43 kg for women receiving cetuximab-FOLFIRI, a total per patient cost saving was $15,138 and $15,004, respectively (). The cost of cetuximab acquisition compared with panitumumab was lower by $14,833 and $14,854 for men and women, respectively. The cost savings were similar, $15,138 and $15,004, for cetuximab-FOLFIRI based on median values of 2.01 m2/84.5 kg and 1.73 m2/68.49 kg for men and women, respectively (). The cost saving for cetuximab-FOLFIRI versus panitumumab-FOLFOX was minimal in patients with BSA/weight below the 25th percentile which included 1.87 m2/73.03 kg and 1.73 m2/68.49 kg for men and women, respectively.
Figure 3. Costs of biweekly cetuximab-FOLFIRI and panitumumab-FOLFOX treatment for median and percentile values of BSA and weight over 43 weeks for men and women. Δ depicts the cost savings per patient (cetuximab-FOLFIRI − panitumumab-FOLFOX). P25 and P75 represent 25th and 75th quartile, respectively. BSA, body surface area; kg, kilogram; P25, 25th percentile; P75, 75th percentile; USD, United States dollars.
Discussion
This CMA evaluated the economic outcome of first-line treatment with cetuximab-FOLFIRI versus panitumumab-FOLFOX among patients with KRAS WT mCRC from a U.S. payer’s perspective over a 43-week time horizon. Overall, the results demonstrated that cetuximab-FOLFIRI administered biweekly may provide cost savings compared with biweekly panitumumab-FOLFOX. For the base case analysis based on overall population estimates (mean BSA/body weight 1.92 m2/82.04 kg), a cost reduction of $400 per patient was observed with cetuximab-FOLFIRI compared with panitumumab-FOLFOX. Cost savings for cetuximab-FOLFIRI resulted from lower drug cost of combination chemotherapy (FOLFIRI versus FOLFOX) and cost of administration. Cetuximab-FOLFIRI showed cost savings compared with panitumumab-FOLFOX in all scenarios. A cost saving of >$15,000 per patient was observed with cetuximab-FOLFIRI for mean, median, and upper limits of BSA and weight inputs for both men and women.
With FDA approval, effective from April 2021[7], of biweekly dosing for cetuximab in KRAS WT mCRC, we believe this is the first study to compare economic outcomes of biweekly dosing of cetuximab-FOLFIRI and panitumumab-FOLFOX in the first-line treatment of KRAS WT mCRC. Earlier CMAs which compared costs of weekly cetuximab (alone or in combination with chemotherapy) versus panitumumab have reported panitumumab to be a potential cost saving option for treatment of mCRCCitation13–15. Graham et al. reported a CMA from a third-party payer perspective in the US and reported per-patient saving of $23,125 (11.4%) for first-line treatment of mCRC over 69.27 weeks in favour of panitumumab-FOLFOX relative to weekly cetuximab-FOLFIRI[13]. Similarly, Gravalos Castro et al. reported that cost of first-line treatment with panitumumab-FOLFOX was 17.2% lower versus weekly cetuximab-FOLFIRI over 22 weeks;Citation15 costs estimated by Fragoulakis et al. over 20 weeks also followed a similar trendCitation14.
In contrast, this analysis showed cost savings in favour of biweekly cetuximab-FOLFIRI. The difference in results of the current analyses from those of earlier studies may be attributed to the change in weekly versus biweekly treatment regimen of cetuximab, increase in drug acquisition price over the years, and differences in time horizon. The base case values of weight and BSA in the current analyses (82.04 kg/1.92 m2) were similar to those reported by Graham et al. (81.59 kg/1.91 m2). However, time horizon was shorter compared to Graham et al. (43 versus 69.27 weeks)Citation13. This CMA considered mPFS of the phase 3 CRYSTAL and PRIME clinical trials while Graham et al. conducted a parametric survival analyses on the PFS of patients from PRIME study to estimate the time horizonCitation13. The time horizon for the current CMA is longer than that reported by Gravalos Castro et al. and Fragoulakis et al. (20 and 22 weeks, respectively)Citation14,Citation15.
Weight and BSA are key parameters in calculating dose for cetuximab, panitumumab, and chemotherapy drugs. For simplicity, given the weight and height differences in men and women in the general populationCitation28, cost outcomes based on the mean and median values of men and women were explored. Of importance, the mean height and mean weight of men and women in this CMA derived from the Flatiron Health database and general population are comparableCitation28. Using mean and median weights and BSA, the cost savings for cetuximab-FOLFIRI compared with panitumumab-FOLFOX were observed. An average man or woman with KRAS WT mCRC receiving cetuximab-FOLFIRI is expected to incur less cost (potential savings of >$15,000) over 43 weeks, and the difference is a result of reduced drug acquisition costs of cetuximab compared to panitumumab. In this study, while the costs for cetuximab-FOLFIRI and panitumumab-FOLFOX were similar in the base case, the savings were pronounced in gender-specific results. This may be explained by the number of vials utilized which is dependent on the weight/BSA of patients. In our study, we used the parameter mean approach to calculate the number of vials (i.e. number of vials was rounded off to the nearest whole vial). The overall average patient with weight/BSA 82.04 kg/1.92 m2 required 10 vials (100 mg) of cetuximab or 5 vials (100 mg) of panitumumab. The average female patient (73.43 kg/1.76 m2) required 9 vials of cetuximab or 5 vials of panitumumab, which is one less vial for cetuximab but the same number of vials for panitumumab in comparison to the base case (hence impacting the differential). On the other hand, the average male (88.18 kg/2.03 m2) required 11 and 6 vials of cetuximab and panitumumab, respectively. To our knowledge, no other study has explored scenarios with different weight and BSA values. The scenario analyses underscore that one size does not fit all and assessing weight and height by gender may impact total treatment-related cost.
The results of this study should be interpreted in consideration of the limitations. A key limitation of this CMA stems from the absence of head-to-head data comparing cetuximab and panitumumab efficacy and safety in first-line setting. Additionally, this model does not consider a lifetime horizon but rather a 43-week time horizon. With this assumption, costs beyond the mPFS are not captured, and this underestimates the total cost of care of the patient throughout their treatment beyond first line. Patients receiving cetuximab-FOLFIRI or panitumumab-FOLFOX in first-line of therapy may receive other treatment regimens in later lines of therapy as variability in the regimens used appears to increase by line of therapyCitation40. There is no specific single treatment regimen being used in second line of therapy for patients diagnosed with KRAS WT mCRC who received an anti-EGFR in the first-line of therapyCitation41. Furthermore, the NCCN guidelines for colon cancer recommend FOLFOX or FOLFIRI as interchangeable in combination with anti-EGFRCitation3. However, this CMA was performed in line with FDA-approved indication for KRAS WT mCRC in the first-line setting, specifically cetuximab in combination with FOLFIRI and panitumumab in combination with FOLFOXCitation24,Citation42. Furthermore, this model only used wholesale acquisition cost pricing and did not consider discounts or reimbursements. This model did not include costs of managing all adverse events (except IRs), and all supportive care therapy for patients with KRAS WT mCRC who received cetuximab-FOLFIRI or panitumumab-FOLFOX; therefore, the costs of treatment may be underestimated. According to cetuximab prescribing information, a patient experiencing a Grade 3-4 IR is expected to immediately discontinue the treatmentCitation8. Since this may occur at any stage during the 43-week time horizon, the costs of treatment were not adjusted for timing of Grade 3–4 IRs. In addition, the model assumed that all components of FOLFIRI and FOLFOX were continued throughout the 43-week time horizon, and there was no clinical evidence of neurotoxicity requiring fewer treatment cycles for oxaliplatin. In our analysis, we used the mFOLFOX6, as recommended in the NCNN guidelinesCitation3. However, we note that there are various FOLFOX regimens (FOLFOX-4, mFOLFOX7), which may result in different costs because of different dosing and schedule of 5-FUCitation43. While this CMA showed cost of cetuximab-FOLFIRI and panitumumab-FOLFOX for different weight and BSA scenarios, a more comprehensive analysis of cost based on log-normal distribution of weight and BSA of patients in the real world would be insightful to demonstrate cost across a variety of weight and BSA.
Conclusions
The results of this CMA demonstrated that biweekly cetuximab-FOLFIRI may have cost savings compared with panitumumab-FOLFOX, for first-line treatment of patients with KRAS WT mCRC from the US payer’s perspective. These savings were observed for the base case and across scenario analyses of varying BSA and weight for men and women.
Transparency
Declaration of funding
This work was funded by Eli Lilly and Company.
Declaration of financial/other interests
Authors: WGGM, HS, MGA, and TK are employees of Eli Lilly and Company.
MGA and WGGM own Eli Lilly stocks. DB is a former employee of Eli Lilly and Company.
Journal peer reviewer: Journal peer reviewers on this manuscript have received an honorarium from PGM for their review work.
A journal reviewer on this manuscript has disclosed that he worked on cetuximab health economic topics when he was employed at Merck Serono from 2005 to 2011, the marketing authorization holder for Cexutimab outside the US and Canada. They do not own any stocks for Merck or Eli Lilly. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
WGGM contributed to study design, interpretation of data, revision, and final approval of the manuscript.
HS contributed to analyses and interpretation of the data, revision, and final approval of the manuscript.
MGA contributed to study design, revision, and final approval of the manuscript.
DB contributed to collection and interpretation of the data, revision, and final approval of the manuscript.
TK contributed to study design, revision, and final approval of the manuscript.
Acknowledgements
The authors would like to acknowledge Swati Bhandari (Eli Lilly Services India Private Limited) for medical writing assistance with the preparation of this manuscript.
Data availability statement
The patient data that support the findings of this study have been originated by Flatiron Health, Inc. These de-identified data may be made available upon request and are subject to a license agreement with Flatiron Health; interested researchers may contact <[email protected]> to determine licensing terms.
References
- Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA A Cancer J Clin. 2020;70(3):145–164.
- American Cancer Society [Internet]. Key statistics for colorectal cancer. 2021. [cited 2021 February 19]. Available from: https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html
- Benson AB, Venook AP, Al-Hawary MM, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(3):329–359.
- Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16(4):359–369.
- Jean GW, Shah SR. Epidermal growth factor receptor monoclonal antibodies for the treatment of metastatic colorectal cancer. Pharmacotherapy. 2008;28(6):742–754.
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–345.
- US Food and Drug Administration [Internet]. FDA approves new dosing regimen for cetuximab. 2021 April 7. [cited 2021 April 20]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-new-dosing-regimen-cetuximab
- Erbitux (cetuximab) [Internet] US prescribing information 2020 November. [cited 2021 April 20]. Available from: https://uspl.lilly.com/erbitux/erbitux.html
- Giusti RM, Cohen MH, Keegan P, et al. FDA review of a panitumumab (vectibix) clinical trial for first-line treatment of metastatic colorectal cancer. Oncologist. 2009;14(3):284–290.
- Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15(6):569–579.
- Hoang T, Kim J. Combining correlated outcomes and surrogate endpoints in a network Meta-analysis of colorectal cancer treatments. Cancers (Basel). 2020;12(9):2663.
- Rai M, Goyal R. Chapter 33 - Pharmacoeconomics in healthcare. In: Vohora D, Singh G, editors. Pharmaceutical medicine and translational clinical research. Boston (MA): Academic Press; 2018. p. 465–472.
- Graham CN, Hechmati G, Fakih MG, et al. Cost-minimization analysis of panitumumab compared with cetuximab for first-line treatment of patients with wild-type RAS metastatic colorectal cancer. J Med Econ. 2015;18(8):619–628.
- Fragoulakis V, Papagiannopoulou V, Kourlaba G, et al. Cost-minimization analysis of the treatment of patients with metastatic colorectal cancer in Greece. Clin Ther. 2012;34(10):2132–2142.
- Gravalos Castro C, Pérez-Alcantara F, Gasquet Espuna JA, et al. Study of minimization of panitumumab versus cetuximab costs in combination with first-line and second-line chemotherapy in native KRAS metastatic colorectal cancer in Spain. Pharmacoecon Spanish Res Article. 2014;11(4):135–145.
- Matsuda A, Yamada T, Jamjittrong S, et al. Comparison between biweekly and weekly cetuximab in patients with metastatic colorectal cancer: a Meta-analysis. Anticancer Res. 2020;40(6):3469–3476.
- Hubbard JM, Alberts SR. Alternate dosing of cetuximab for patients with metastatic colorectal cancer. Gastrointest Cancer Res. 2013;6(2):47–55.
- Tabernero J, Ciardiello F, Rivera F, et al. Cetuximab administered once every second week to patients with metastatic colorectal cancer: a two-part pharmacokinetic/pharmacodynamic phase I dose-escalation study. Ann Oncol. 2010;21(7):1537–1545.
- Parikh A, Gugel EG, Smolyakova N, et al. 455P a Meta-analysis of efficacy and safety of cetuximab with biweekly vs. weekly dosing. Ann Oncol. 2020;31:S435.
- Agg H, Han Y, Cui ZL. Real-world data on overall survival associated with biweekly versus weekly cetuximab among metastatic colorectal cancer (mCRC) patients in the United States. J Clin Oncol. 2021;39(3):33–33. (
- Kasper S, Foch C, Messinger D, et al. Noninferiority of cetuximab every-2-weeks versus standard once-weekly administration schedule for the first-line treatment of RAS wild-type metastatic colorectal cancer. Eur J Cancer. 2021;144:291–301.
- International Society for Pharmacoeconomics and Outcomes Research. Good practices. [accessed on March 2021]. Available from: https://www.ispor.org/heor-resources/good-practices/report-other-report-types/-in-category/index-types/ispor-good-practices-for-outcomes-research
- Cutsem EV, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as First-Line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–2019.
- Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25(7):1346–1355.
- Ma X, Long L, Moon S, et al. Comparison of population characteristics in Real-World clinical oncology databases in the US: flatiron health. SEER and NPCR. medRxiv. 2020.
- Sacco JJ, Botten J, Macbeth F, et al. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLoS One. 2010;5(1):e8933.
- Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1989;5(5):303–311.
- Fryar CD, Kruszon-Moran D, Gu Q, et al. Mean body weight, height, waist circumference, and body mass index among adults: United States, 1999–2000 through 2015–2016. Natl Health Stat Rep. 2018;(122):1–16.
- Vectibix (Panitumumab). [Internet] US prescribing information [updated]. 2017. [Jun cited 2021 Mar 23]. Available from: https://www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/vectibix/vectibix_pi.pdf
- IBM [Internet]. IBM Micromedex. Red book [cited 2021 Mar 23]. In Truven health analytics/IBM Watson health. 2021. Available from https://www.ibm.com/products/micromedex-red-book
- CMS [Internet]. Physician fee schedule. 2021. [updated 2021 Jan 20; cited 2021 Feb 11]. Available from https://www.cms.gov/medicare/physician-fee-schedule/search. [Internet]. [cited accessed 11 February 2021]. Available from: https://www.cms.gov/medicare/physician-fee-schedule/search
- NCCN: Antiemesis: NCCN clinical practice guidelines in oncology. Version 12021-December 23, 2020. [accessed on April 20].
- CMS CfMMS. Clinical laboratory fee schedule-2021. Baltimore (MD): Centers for Medicare & Medicaid Services; 2021. [Internet].
- Johns Hopkins Health System and Office of Johns Hopkins Physicians. Injection and infusion services coding in. 2019. [accessed 11 February 2021]. Available from https://www.hopkinsmedicine.org/compliance/forms/infusion-guideline-092020.pdf
- Solutions SM. Coding rules for chemotherapy administration and non-chemotherapy injections and infusion services. 2021. [accessed 11 February 2021]. Available from https://www.choptx.org/wp-content/uploads/2017/12/Coding-for-Oncology-Presentation-Final.v2pptx.pdf
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–4705.
- Foley KA, Wang PF, Barber BL, et al. Clinical and economic impact of infusion reactions in patients with colorectal cancer treated with cetuximab. Ann Oncol. 2010;21(7):1455–1461.
- Lacouture ME. Prevention and treatment of acneiform rash caused by EGFR inhibitors. The Asco Post. 2021. [accessed 11 February 2021]. Available from https://ascopost.com/issues/may-15-2013/prevention-and-treatment-of-acneiform-rash-caused-by-egfr-inhibitors/
- U.S. Bureau of Labour Statistics CPI Inflation Calculator [Internet]. Available from https://www.bls.gov/data/inflation_calculator.htm
- Hess LM, Cui ZL, Mytelka DS, et al. Treatment patterns and survival outcomes for patients receiving second-line treatment for metastatic colorectal cancer in the USA. Int J Colorectal Dis. 2019;34(4):581–588.
- Lee JJ, Sun W. Options for second-line treatment in metastatic colorectal cancer. Clin Adv Hematol Oncol. 2016;14(1):46–54.
- Cutsem EV, Lang I, Folprecht G, et al. Cetuximab plus FOLFIRI: final data from the CRYSTAL study on the association of KRAS and BRAF biomarker status with treatment outcome. J Clin Oncol. 2010;28(15):3570–3570.
- Akdeniz N, Kaplan MA, Uncu D, et al. The comparison of FOLFOX regimens with different doses of 5-FU for the adjuvant treatment of colorectal cancer: a multicenter study. Int J Colorectal Dis. 2021;36(6):1311–1319.
