Abstract
Aims
Dynamic changes in the payer landscape have resulted in increasing out-of-pocket costs (OOPCs). Little is known about OOPC for patients undergoing biopsy for suspicious pulmonary nodules in the United States. This study seeks to describe the spectrum of OOPC for diagnostic tissue sampling for suspicious pulmonary nodule with an ultimate diagnosis of lung cancer.
Methods
Retrospective cohort study of adult patients with a primary lung cancer diagnosis and treatment who underwent diagnostic biopsy for suspicious pulmonary nodule utilizing IBM Marketscan Databases (2013–2017). Claims data included both total hospital and physician billed costs, insurer reimbursement and OOPC. OOPCs were further stratified by type of biopsy, whether the patient underwent a single or multiple biopsies, and year of biopsy.
Results
A total of 22,870 patients aged 18–95 who underwent diagnostic lung biopsy were identified. The gender ratio was 49:51 for female:male and 50% of patients were aged 65 or above. 78% of patients had a co-morbidity. The median OOPC for a patient receiving a single biopsy (any type) was $600, two biopsies: $706, three biopsies: $811, and four biopsies: $1,177. By biopsy type, the median OOPC for a patient requiring a single biopsy was $604 for percutaneous biopsy, $316 for surgical biopsy, $674 for bronchoscopic biopsy, and $545 for mediastinoscopic biopsy.
Limitations
Under-estimation of OOP expenses from costs of transportation, job loss, and loss of productivity. Over-estimation of OOPC from lack of individual claims adjudication.
Conclusions
The median OOPC for lung cancer patient requiring a single diagnostic lung biopsy is $600. Prior research indicates that almost 50% of the lung cancer patient population undergoes multiple biopsies increasing costs anywhere between 20% and 100% resulting in further patient financial burden for each episodic biopsy attempt. Further cost-effectiveness research is needed to differentiate various diagnostic technologies for lung biopsy.
Background
Lung cancer remains the leading cause of cancer-related mortality worldwide. Despite the development of lung cancer screening regimens for high-risk patients, the vast majority of lung cancers worldwide are diagnosed at late stageCitation1. Though imaging modalities can discriminate high-risk lesions from low-risk lesions, tissue biopsy is required not only for definitive diagnosis but is also used for molecular testing for further prognostic stratification and determination of treatment with targeted biologics. A new area of study is the financial burden of lung cancer screening and diagnosis on patients. Though some recent studies have examined the financial toxicity to patients from diagnosis through an initial period of treatment or for a specific treatment regimen, virtually nothing is known about the financial burden patients face in their journey to obtain a definitive tissue diagnosis. Financial toxicity or financial stress related to treatment has been correlated with patient non-compliance with recommended treatment, a lower quality of life, and more pain in cancer patientsCitation2–4. The purpose of this study is to quantify and characterize patient out-of-pocket costs (OOPCs) for lung cancer patients from the date of initial suspicious imaging to definitive diagnosis of lung cancer.
Methods
This retrospective observational cohort study uses data from the IBM Marketscan Commercial Claims and Encounter and Medicare Supplemental DatabasesCitation5. This database aggregates inpatient, outpatient, and prescription drug claims from a large sample of US-based employer-sponsored plans. The database contains data from 245 million unique patients since 1995, with 41.1 million patients in the most recent full data year. The database is fully de-identified and compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996. This study was institutional review board exempt.
Study population
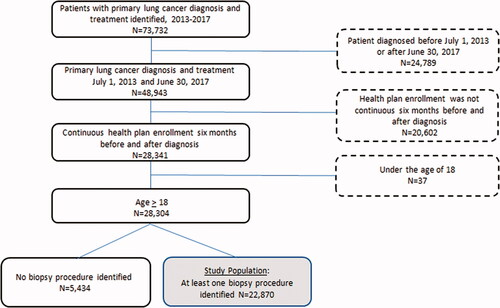
The cohort in MarketScan was selected by including patients ages 18+, who carried a primary International Classification of Diseases, Ninth Revision (ICD 9) and/or International Classification of Diseases, Tenth Revision (ICD 10) diagnosis of lung cancer between July 2013 and June 2017 and received at least one biopsy procedure in the 6 months prior to the diagnosis. Biopsy procedures were defined using both Current Procedural Terminology (CPT) codes and/or ICD 9/10 codes. Patients without continuous health plan enrolment 6 months prior and 6 months after the diagnosis of lung cancer and those who did not receive any lung cancer treatment after diagnosis were excluded from the study population ().
Study variables
Within the selected cohort, the following variables were extracted and analysed: year of diagnosis, type and number of procedures, cancer history, comorbidity, age, gender, and type of insurance plan. Co-morbidity burden was calculated using the Charlson comorbidity index. Chest imaging scans were identified with CPT codes: 71,250, 71,260, and/or 71,270. Four types of lung biopsies were sorted using CPT, ICD-9 CM, and ICD-10 CM procedure codes: bronchoscopy-based biopsy, computer tomography (CT) guided percutaneous biopsy, surgical biopsy, and mediastinoscopic biopsy. Multiple biopsy CPT codes from the same day were counted as a single biopsy session for the analysis.
Out-of-pocket costs (OOPC)
OOPC and total costs were estimated from the date of the first suspicious chest imaging study to the date of initial treatment. OOPC included the sum of co-insurance, co-payments, and deductible amounts. Costs included both professional fees and facility fees and were further categorized as inpatient and outpatient costs. All costs were normalized to 2017 US dollars using the Bureau of Labour Statistics’ Consumer Price Index Medical Care Component. Outlier costs, which were defined as those lower than the 1st or higher than the 99th percentile, were winsorized. Mean differences in groups by age and by plan type were evaluated using Satterthwaite two-sample T-tests. Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
In the IBM Marketscan database, we identified 73,732 patients with a primary diagnosis and evidence of treatment for lung cancer between 1 January 2013 and 31 December 2017. Of these, 24,789 patients had an initial diagnosis date outside of 1 July 2013 or after 30 June 2017 and were excluded. In addition, there were 20,502 patients who did not have evidence of continuous enrolment 6 months prior to diagnosis and 6 months following diagnosis; these patients were also excluded. Finally, 37 paediatric patients under age 18 and 5,434 patients who did not have any biopsy claims were excluded ().
The final analysed cohort consisted of 22,870 patients. The female to male ratio was 51:49. Seventy-eight percent of patients had a co-morbidity. This was the first cancer diagnosis for 76.5% of patients. The majority of this population, 50.6%, had a Preferred Provider Organization (PPO) plan ().
Table 1. Demographics of lung cancer patient who received at least one biopsy (N = 22,870).
Fifty-eight percent (58%) of patients underwent a single biopsy session in the study period with a median OOPC of $600 (IQR $206 to $1,473). Forty-two percent (42%) of patients underwent multiple biopsy sessions, with median OOPC of $706, $811 and $1,177 for two, three, and four biopsy sessions, respectively (). Over the 5 year study period, OOPC remained similar year to year ().
Table 2. Patient out-of-pocket costs based on number of biopsy sessionsa.
Table 3. Patient out-of-pocket costs by yeara.
For the subset of patients undergoing only one biopsy (N = 13,264), 48% had a percutaneous imaged guided biopsy with a median OOPC of $604 (IQR $219–$1,422), 41% had a bronchoscopic biopsy with a median OOPC of $674 (IQR $238–$1,682), 9% had a surgical biopsy with a median OOPC of $316 (IQR $75–$831), and 1% had a mediastinoscopic biopsy with a median OOPC of $545 (IQR $178–$1,496) ().
Table 4. Patient out-of-pocket costs for patients undergoing single biopsy by biopsy typea.
Patients who were under 65 faced higher OOPC with mean spending of $1,813 than patients over 65 with a mean of $658 (p>.001). Patients who had PPO plans had higher spending with a mean of $1,418 compared to other plans with a mean of $1,050 (p<.001) ( and ).
Table 5. Patient out-of pocket-costs by age groupa.
Table 6. Patient out-of-pocket costs based on number of biopsy sessions by insurance typea.
Discussion
Previous studies of financial toxicity in cancer patients have focused on treatment costs. Lung cancer patients face OOPCs for the first 6 months of treatment estimated at a median of $2,150 (range $0–$50,000) Citation2. Increased financial toxicity contributes to a poorer quality of life and puts patients at lifelong risk of medical debt and medical bankruptcyCitation6,Citation7. However, current studies on financial burden likely underestimate the problem as most studies focus on the financial toxicity of a specific treatment modality or a specific treatment regimen and not the entire patient journey from work-up to diagnosisCitation7.
Little is known about diagnostic costs prior to the initiation of treatment. Recent studies have sought to quantify total spending from time of first suspicious nodule to initiation of treatment including the costs of repeat biopsies and complicationsCitation8,Citation9. Total costs for a single biopsy ranged from $1,029 to $3,740 for commercially insured patients depending on the primary biopsy modality. OOPC for diagnostic tests may already deplete financial resources for patients prior to treatment. However, if diagnosis and treatment fall within the same plan year, OOPC applied to diagnostic workup will contribute to deductibles and out-of-pocket maximums which may mitigate OOPC for treatment. This is the first paper to quantify OOPCs for patients in the journey to a lung cancer diagnosis. The median individual weekly earnings in the US is $994Citation10. An OOPC of $600 for a single biopsy is a considerable financial burden for many Americans.
An interesting finding of this study was that surgical biopsy was less costly than other modalities despite its more invasive nature. Bronchoscopic, mediastinoscopic, and CT-guided biopsy tend to be outpatient procedures whereas surgical biopsy is more likely to be an inpatient procedure. This may be due to the construction of many commercial insurance plans where cost-sharing is typically higher for outpatient than inpatient procedures.
This study also demonstrates 42% of patients require more than one biopsy to obtain a definitive diagnosis and treatment plan each of which incrementally and predictably increases OOPCs. Diagnostic algorithms and technologies must be streamlined to minimize non-diagnostic and inadequate biopsy to minimize both cost to the insurer and the patient.
Additional factors associated with higher costs were age <65 and PPO-type plans. Patients who are included in this dataset and are over 65 likely have Medicare and employer-sponsored plans as secondary insurance. It is perhaps intuitive that their OOPC is lower as a result of this additional coverage. PPO plans are usually associated with higher premiums and higher cost-sharing in exchange for greater patient choice in clinicians and facilities and the findings in this study are consistent with design of those plans.
This study has several limitations. It is a retrospective study of a commercially insured population and may not be generalizable to the US population with other primary insurance types. It does not reflect the financial burden of patients with state-sponsored or military-sponsored plans or uninsured patients. However, this dataset is an excellent representation of commercially available plans across the United States. The majority of patients in this dataset (>75%) have a co-morbid condition that can inflate the OOPC estimate attributable to lung cancer diagnostic procedures. However, this approach is pragmatic in that lung cancer costs do not exist in a vacuum; they exist in the context of a patient’s other medical conditions and the financial burden should be taken in its totality. Finally, this study likely underestimates the total financial burden in that it does not account for job loss, loss of productivity, or travel costs.
In a recent study of the impact of cost on treatment choices for lung cancer, 27.7% of patients made financial adjustments to pay for treatment but only 4.5% of patients refused treatment based on cost considerationsCitation11. This study excludes patients who ultimately did not seek treatment for lung cancer under their existing plan and whether OOPC was a deterrent to completing diagnostic workup and initiating treatment. Qualitative studies are needed to understand why patients stall in their diagnostic journey and to what degree cost is a contributing factor.
Patients with lower socio-economic status are more likely to be smokers and of smokers, they are more likely to be heavy smokersCitation12. Lung cancer tends to impact patients of lower socioeconomic status disproportionately despite controlling for smoking statusCitation13. Though this database has some demographic variables, it does not have financially relevant socio-economic variables such full employment vs. underemployment, income, educational attainment, and baseline financial security which would add greater context to these findings and should be an area of future study.
Conclusion
Diagnostic workup for lung cancer is a costly process often requiring repeat biopsies; increasing OOPCs and can be a substantial financial burden to lung cancer patients, even before the initiation of treatment. Diagnostic algorithms and technologies that provide a definitive tissue diagnosis upon initial biopsy procedure attempt are necessary to reduce the financial toxicity of escalating OOPC as it relates to a lung cancer patient’s journey.
Transparency
Declaration of funding
This study was funded by Intuitive Surgical.
Declaration of financial/other relationships
FZ was previously a consultant for Johnson and Johnson and is an employee of Intuitive Surgical.
MS is a consultant for Intuitive Surgical.
JL is an employee of Intuitive Surgical.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors made substantial contributions to the conception, design, analysis, interpretation, and writing of this manuscript.
Previous presentations
This work was presented as a poster at the International Society for Pharmacoeconomics and Outcomes Research meeting in April 2021.
Acknowledgements
None reported.
References
- SEER Cancer Stat Facts: Lung and bronchus cancer. National Cancer Institute B, MD. [accessed on 15 May 2021]. Available from: https://seer.cancer.gov/statfacts/html/lungb.html
- Hazell SZ, Fu W, Hu C, et al. Financial toxicity in lung cancer: an assessment of magnitude, perception, and impact on quality of life. Ann Oncol. 2020;31(1):96–102.
- Febbo J, Little B, Fischl-Lanzoni N, et al. Analysis of out-of-Pocket cost of lung cancer screening for uninsured patients among ACR-Accredited imaging centers. J Am Coll Radiol. 2020;17(9):1108–1115.
- Seetasith A, Wong W, Tse J, et al. The impact of copay assistance on patient out-of-pocket costs and treatment rates with ALK inhibitors. J Med Econ. 2019;22(5):414–420.
- Putting Research Data into Your Hands with the MarketScan Databases. 2016. Available from: http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases.
- Lathan CS, Cronin A, Tucker-Seeley R, et al. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34(15):1732–1740.
- Klein J, Bodner W, Garg M, et al. Pretreatment financial toxicity predicts progression-free survival following concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer. Future Oncol. 2019;15(15):1697–1705.
- Chiu YW, Kao YH, Simoff MJ, et al. Costs of biopsy and complications in patients with lung cancer. Clinicoecon Outcomes Res. 2021;13:191–200.
- Lokhandwala T, Bittoni MA, Dann RA, et al. Costs of diagnostic assessment for lung cancer: a medicare claims analysis. Clin Lung Cancer. 2017;18(1):e27–e34.
- US Bureau of Labor Statistics. 2021. [accessed 20 January 2021]. Available from: https://www.bls.gov/bls/blswage.htm
- Friedes C, Hazell SZ, Fu W, et al. Longitudinal trends of financial toxicity in patients with lung cancer: a prospective cohort study. J Clin Oncol Oncol Pract. 2021;17(8):e1094–e1109. Op2000721.
- Tian J, Gall S, Patterson K, et al. Socioeconomic position over the life course from childhood and smoking status in mid-adulthood: results from a 25-year follow-up study. BMC Public Health. 2019;19(1):169.
- Hovanec J, Siemiatycki J, Conway DI, et al. Lung cancer and socioeconomic status in a pooled analysis of case-control studies. PLoS One. 2018;13(2):e0192999.