Abstract
Aim
This study aimed to model the financial impact of caplacizumab with therapeutic plasma exchange (TPE) + immunosuppression for patients experiencing an acute acquired thrombotic thrombocytopenic purpura (aTTP) episode versus TPE + immunosuppression, from a US hospital’s perspective.
Methods and materials
We developed an economic model to estimate the impact of caplacizumab on a US hospital’s budget. Cost offsets from caplacizumab utilization targeted inpatient general ward days, intensive care unit (ICU) days, and TPE utilization. Costs and event probabilities were estimated from primary data analyses of the phase 3 HERCULES trial and peer-reviewed literature or other public sources. Plan reimbursement was obtained from 2019 Medicare Fee Schedules and adjusted to represent reimbursement from different US payers. Cost of ICU and general ward utilization were estimated from Medicare Provider Analysis and Review data analyses capturing hospital discharges.
Results
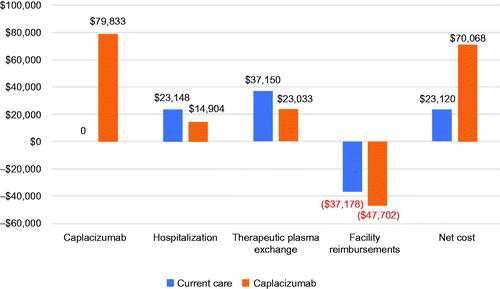
The model results indicate that caplacizumab leads to hospitalization cost savings of over $8,000 ($23,148 versus $14,904) along with TPE cost savings of over $14,000 ($37,150 versus $23,033) per patient. When the cost of caplacizumab and plan reimbursement are incorporated into the results, the per-patient cost of TPE + immunosuppression is $23,120 versus $70,068 for caplacizumab with TPE + immunosuppression, an incremental cost of $46,948. The model was robust to several scenario analyses; however, when limited to Medicare fee-for-service (FFS), the incremental cost of caplacizumab per patient was reduced to $4,852 due to add-on payments.
Conclusions
Caplacizumab with TPE + immunosuppression is associated with an increase in costs; however, the increase is nominal among payers who provide an add-on payment consistent with that of Medicare FFS.
Introduction
Acquired thrombotic thrombocytopenic purpura (aTTP), also known as autoimmune thrombotic thrombocytopenic purpura, is an ultra-rare, autoimmune hematological disorder that causes significant mortality and long-term morbidityCitation1. The reported incidence for this condition is 1.2–11 cases per million (4–5 cases per million in the US)Citation2–5, and it mainly affects young and otherwise healthy adults aged 30–50 yearsCitation6. It is caused by inhibitory autoantibodies to von Willebrand factor-cleaving enzyme ADAMTS13Citation7–9; severely decreased ADAMTS13 activity leads to the extensive uncontrolled formation of microvascular thrombi, which can result in tissue ischemia, organ dysfunction, and/or deathCitation10–16. aTTP has been treated for many years with therapeutic plasma exchange (TPE) and immunosuppressive therapy, and a considerable proportion of patients are refractory to therapy and/or experience multiple exacerbations/relapsesCitation11,Citation17–20.
Caplacizumab, an orphan drug approved by the US Food and Drug Administration (FDA) in combination with TPE and immunosuppressive therapy in 2019 for the treatment of aTTP, is an innovative, novel nanobody with a unique mechanism of action that specifically targets platelet adhesionCitation21,Citation22. In the phase 3 HERCULES trial (NCT02553317), caplacizumab with TPE and immunosuppressive therapy significantly reduced the time to platelet count normalization (p < .001) versus TPE with immunosuppressive therapyCitation22. In addition, fewer patients treated with caplacizumab experienced recurrence of aTTP (67% lower) or refractory TTP (no patients treated with caplacizumab versus three treated with TPE + immunosuppression) in this randomized controlled trial. Moreover, patients receiving caplacizumab also achieved a faster resolution of the acute aTTP episode, as demonstrated by reduced use of TPE and a shorter length of hospitalization and intensive care unit (ICU) stayCitation22. More importantly, based on an integrated analysis of data from phase 3 HERCULES and the phase 2 TITAN study, results show that caplacizumab has also produced clinically meaningful and statistically significant reductions in mortality during treatment, refractoriness to therapy, and use of TPE among the pooled study populationCitation23–25. Caplacizumab, along with TPE and immunosuppression, has also illustrated positive outcomes in a real-world setting; the triple regimen resulted in 2.2% of patients with aTTP experiencing refractoriness or death within 30 days of diagnosis versus 12.2% in patients treated with TPE and immunosuppression (p = .01)Citation26.
Real-world data from the UK and Germany also confirm the positive outcomes in patients treated with caplacizumabCitation27,Citation28. These data demonstrate that caplacizumab offers clinical value when added to TPE and immunosuppressive therapy in patients with aTTP. Consequently, the 2020 International Society of Thrombosis and Haemostasis (ISTH) guidelines recommend caplacizumab as part of the treatment regimen for patients with aTTP. Therefore, this study aims to estimate the health service use and financial impact of caplacizumab in addition to TPE and immunosuppression compared to TPE and immunosuppressive therapy alone through a cost analysis from the perspective of a US hospital.
Methods
An economic model simulating one hypothetical patient with US Medicare, Medicaid, or commercial insurance and an aTTP diagnosis was developed from the perspective of a US hospitalCitation29. This model is designed to illustrate the per-patient financial impact of inpatient utilization of caplacizumab in addition to TPE and immunosuppression, compared to TPE and immunosuppression alone, throughout one index episode of inpatient care (including exacerbations, which are recurrences of an index event requiring a restarting of daily inpatient TPE within 30 days of the last daily TPE).
The model was developed to be used at any given hospital. And given the rarity of the disease, the model describes a single patient moving through the healthcare system to better reflect reality at the hospital level, rather than model a larger population of patients with aTTP.
The model considers 100% adoption of caplacizumab into clinical practice and the subsequent improved clinical and health service use outcomes, while modeling costs related to the drug along with TPE and hospitalizations (inpatient and ICU days) related to an aTTP event. The input parameters () were derived from the published literature, caplacizumab phase 3 clinical trial data, an analysis of Medicare claims, and plan reimbursement rates.
Table 1a. HERCULES clinical and healthcare utilization base case model inputs.
Table 1b. Healthcare cost base case model inputs.
Table 1c. Health plan reimbursement base case model inputs.
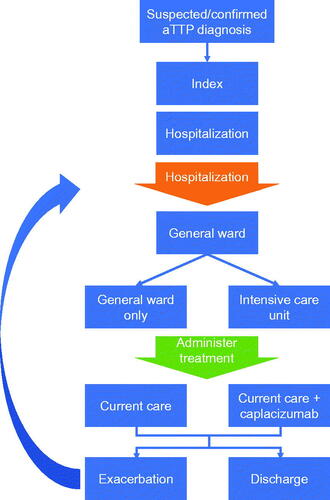
contains the details of potential clinical pathways a patient could go through in the model. Specifically, the base case model follows a hypothetical patient, aligned with the phase 3 HERCULES trial, with an acute index hospitalization for aTTP through discharge. The model can capture potential exacerbations. Patients with a suspected or confirmed case of aTTP are admitted to the hospital, spending a portion of the hospitalization in the ICU or spending the entire hospital stay in the general ward.
Treatment of caplacizumab is initiated through an intravenous loading dose followed by subcutaneous injection during each day hospitalized. Following clinical remission, the patient is discharged and will return to the hospital only in case of an exacerbation (i.e. symptoms re-emerge within 30 days). Of note, an index event could be either a newly diagnosed patient or a patient experiencing a relapse, which is considered a new aTTP episode and defined as recurrences outside of the 30 days following cessation of daily TPE treatment. Because the model was developed from the US hospital perspective, it only considers the direct costs associated with providing treatments for aTTP; therefore, indirect costs (e.g. facility workflow, etc.) were not included in the model. Additionally, the costs to treat comorbidities were not incorporated into the model; however, these should be similar among patients with both treatment options.
To evaluate the cost of healthcare resource utilization (i.e. general ward and ICU utilization) from the hospital perspective, the 2018–2019 Medicare Provider Analysis and Review (MEDPAR) file was used to obtain Medicare payments and charges for aTTP casesCitation30. MEDPAR data contained information on Medicare beneficiaries utilizing inpatient services, including information about admission, discharge, diagnosis and procedure codes, as well as total charges, Medicare reimbursement, and total length of stay, among others. Because there is no International Classification of Diseases (ICD)-10 code for aTTP, an algorithm was developed to identify patients with an expected aTTP diagnosis: (1) patients with an ICD-10 code for thrombotic microangiopathy (TMA) were captured; (2) TMA patients were limited to those with a procedure code for TPE; and (3) patients with the hemolytic uremic syndrome were excluded. Cost-to-charge ratios were also obtained from the 2018 to 2019 MEDPAR data. These ratios were employed to convert charges that hospitals set for treating the patient to the costs that they incur, to generate an estimated cost for general ward and ICU days from the hospital perspective. The hospital costs of TPE were based on Heatwole et al.Citation31.
Reimbursement rates paid to treat facilities varied by health plan type (i.e. Medicare Fee-for-Service [FFS], Medicaid Advantage [MA], and Medicaid versus commercial) but were anchored to the FFS reimbursement rates captured through the 2019 Medicare Fee Schedules and adjusted to 2020 US dollars using the medical care component of the US Consumer Price Index. The reimbursement for commercial plans was inflated by 89% over FFS ratesCitation32, while other plan types were set equal to FFS rates. FFS reimbursement rates reflect the mean base Diagnosis-Related Group (DRG) and add-on payments to hospitals. The DRG payment system provides hospitals with a fixed payment for services rendered to a patient, regardless of the actual cost of treating the patient. Additionally, given the relatively low reimbursement rates and the high likelihood of facilities’ financial losses from the treatment of aTTP, the model includes FFS provision of “add-on payments” (i.e. caplacizumab was granted New Technology Add-on Payment [NTAP] by Medicare in 2020) to facilities to account for these financial losses. Two add-on payments were modeled: (1) the New Technology Add-on Payment [NTAP] for caplacizumab granted by Medicare in 2020 ($33,215); (2) outlier payments to both model comparators derived from the Medicare Claims Processing ManualCitation33.
Scenario analyses
To test the robustness of the model to the source of clinical and health service utilization data, a series of scenario analyses were performed to: (1) limit the model population to only patients with Medicare FFS; (2) reflect clinical and resource use outcomes from only HERCULES participants enrolled in the US; and (3) reflect clinical and resource use outcomes derived from US real-world data. Among other aims, these analyses were conducted to assess variation among US HERCULES patients versus global HERCULES patients, as well as variation between the tightly controlled clinical trial setting and a setting more reflective of routine clinical practice in the US.
The input parameters associated with each scenario are presented in . Of note, the input parameter estimates for the Medicare FFS scenario were equivalent to the base case estimates (), with the only differences being: (1) the elimination of commercial reimbursement (189% of Medicare FFS reimbursement), and (2) provision of an add-on payment (i.e. NTAP and incremental outlier payment) provided to the facility for all patient episodes treated.
Table 2. Scenario analyses – clinical and healthcare utilization model inputs.
Results
Base case model
Using a payer mix of 20% FFS, 8% MA, 10% Medicaid, and 62% commercial plans, reflecting enrolment in the caplacizumab Patient Services Hub from January 2020 to August 2020, the base case model results, leveraging clinical data from HERCULES, indicate that utilization of caplacizumab in the inpatient setting is expected to reduce total hospital days by 31% (14.4 versus 9.9 days with the addition of caplacizumab), days in the ICU by 65% (9.7 versus 3.4 days with the addition of caplacizumab), and days with TPE by 38% (9.4 versus 5.8 days with the addition of caplacizumab)Citation22. Furthermore, the facility realizes hospitalization cost savings of over $8,000 ($23,148 versus $14,904 [36% decrease]) with TPE cost savings of over $14,000 ($37,150 versus $23,033 [38% decrease]). When the cost of caplacizumab and health plan reimbursement, including add-on payments for FFS patients, are incorporated into the results, the net cost of care for patients on TPE + immunosuppression is $23,120 versus $70,068 for caplacizumab with TPE + immunosuppression, which translates to an incremental cost of $46,948 ().
Sensitivity analyses
The results from most of the scenario analyses (Supplemental Table 1) suggest that the base case model outcomes are robust with little variation in the relative cost and resource use reductions across scenarios, though absolute values among US patients enrolled in HERCULES were lower than those in the overall HERCULES population and the real-world data. Of note, the impact of caplacizumab on the reduction of TPE in the US HERCULES population was lower than the base case and real-world data scenarios (32% versus 38%, respectively).
The scenario analysis that limits the base case population to those covered by Medicare FFS uncovered a departure from the base case results. The hypothetical hospital receives a NTAP (only for patients taking caplacizumab) and an outlier payment (for all patients and based on a formula that employs a fixed cost loss threshold) from Medicare FFS for each covered patient treated, which reduces the financial impact hospitals face when providing intensive care to patients with an aTTP episode. However, the model assumes that the other payer types do not provide add-on payments to supplement DRG-based reimbursement rates paid to facilities, though commercial rates vary and are negotiated between hospitals and payers. Consequently, limiting the population to patients for whom the hypothetical hospital receives an add-on payment decreases the incremental cost for treating these patients with caplacizumab: the net cost of patients receiving caplacizumab is $33,525 versus $28,673 for patients receiving TPE and immunosuppression alone (incremental cost of $4,852).
Discussion
aTTP imposes a significant burden on patients and healthcare systems with a substantial unmet treatment need. For many years, the mainstay of treatment for this disease included TPE and immunosuppressive therapy, typically involving complications and requiring lengthy hospitalization, with many at risk for exacerbations and relapsesCitation10,Citation17–20. A significant proportion of patients did not respond adequately to current therapiesCitation35,Citation36. Recently, caplacizumab, a novel therapy, was FDA-approved for the treatment of aTTP and soon after, it was recommended by the ISTH to be used in patients with acute aTTP episodeCitation37. We thus analyzed the financial impact of the addition of caplacizumab in the treatment of aTTP. For each patient, the results of this economic model indicate that caplacizumab is accompanied by a $46,948 increase in the total cost of care from the hospital perspective. The general ward, ICU, and TPE cost reductions lead to an offset of the cost of caplacizumab of over 41% ($79,833 [cost of caplacizumab] versus $46,948 [difference between the net costs of caplacizumab and TPE + immunosuppression]). Additionally, the net costs may be overestimated as health plans other than FFS are likely to provide add-on payments to hospitals to compensate them for financial losses from treating patients in either treatment arm. If the other health plans supplement their DRG-based reimbursement rates with add-on payments, the results of the base case model would more resemble the scenario analysis that limits the population to Medicare FFS, with an offset to the cost of caplacizumab of almost 94%. In this case, most costs of caplacizumab for aTTP can be recouped through reduction in healthcare service use along with insurer reimbursement.
This model evaluated the incremental costs that arise as a result of the routine use of caplacizumab + TPE + immunosuppression versus plasma exchange + immunosuppression alone. Therefore, there is no financial impact for treatments, irrespective of their costs, which are given in both treatment arms such as rituximab. This model assumed that all complications, and most importantly those that would require utilization of the health care system, would be captured from an economic point of view. For instance, if a patient has a major bleeding episode, all else held equal, we would expect to see that clinical bleeding episode manifest into longer hospital lengths of stay, or entry into an ICU unit, etc., which will impact the financial outcome of the drug (i.e. the incremental cost outcome in the model). Because the clinical data were drawn almost exclusively from HERCULES, we believe that the healthcare resource use captured in the trial includes treatment for the disease itself, but also any unforeseen adverse events that may have presented themselves during the episode of care.
Our cost analysis has several limitations, nonetheless. First, the model was based on several and parameter assumptions (). However, the parameter sources were varied through scenario analyses to assess the model’s robustness to these assumptions, and the directionality of the results was consistent across scenarios. Also, the model only focused on costs from the inpatient perspective and did not include exposure to outpatient or other healthcare services. The model utilized reimbursement rates based on the Medicare Fee Schedules to standardize the costs, as because other health plans reimburse health services at varying rates, the model may have under or overestimated total costs for either treatment arm. Lastly, the model did not evaluate the impact of utilizing caplacizumab in the treatment of aTTP from a societal perspective, which may include complex modeling of effectiveness outcomes, such as loss of productivity due to the disease and its comorbidities. Thus, the results may not be extrapolated to fully assessing the national impact of this medication.
Table 3. Key model assumptions.
Conclusion
When considering the impact on reducing healthcare resource use, the addition of caplacizumab, an orphan drug approved by the FDA in 2019, to TPE and immunosuppression is associated with an increased budget spend from the perspective of US hospitals; however, the increase is nominal among payers who provide an add-on payment consistent with that of Medicare FFS. However, caplacizumab offers a new standard of care, accompanied by improved clinical outcomes compared to TPE and immunosuppression, while addressing key patient concerns around time to platelet normalization, refractoriness to TPE, survival, frequency of TPE, and reduced hospital stays. Thus, clinical improvements with a manageable health economic impact support the routine use of caplacizumab in the treatment of patients with aTTP.
Transparency
Declaration of funding
This study was sponsored by Sanofi.
Declaration of financial/other interests
LP and MF were a Sanofi employee and shareholder at the time when this study was conducted. HPP was a consultant for Sanofi and speaker for Alexion. BL was an employee of Avalere Health at the time when this study was conducted and received consulting fees from Sanofi for this project.
Author contributions
LP, BL, and MF contributed towards the study design, analysis, interpretation of results and manuscript writing. HPP contributed towards the analysis, interpretation of results and manuscript writing.
Supplemental Material
Download MS Word (26 KB)Acknowledgements
Avalere participated in the interpretation of data, review, and approval of publication. Anne Murunga, Avalere Health, provided manuscript writing support for the study. Jianyi Lee, Sanofi, provided support during manuscript development.
References
- Elverdi T, Eskazan AE. Caplacizumab as an emerging treatment option for acquired thrombotic thrombocytopenic purpura. Drug Des Devel Ther. 2019;13:1251–1258.
- Terrell DR, Williams LA, Vesely SK, et al. The incidence of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS-13 deficiency. J Thromb Haemost. 2005;3(7):1432–1436.
- Miller DP, Kaye JA, Shea K, et al. Incidence of thrombotic thrombocytopenic purpura/hemolytic uremic syndrome. Epidemiology. 2004;15(2):208–215.
- Kremer Hovinga JA, Coppo P, Lämmle B, et al. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3(1):17020.
- Reese JA, Muthurajah DS, Kremer Hovinga JA, et al. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired ADAMTS13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer. 2013;60(10):1676–1682.
- Bennett CL, Djulbegovic B. Thrombotic thrombocytopenic purpura: gaining knowledge. Lancet Haematol. 2016;3(5):e210–1–e211.
- Sadler JE. Von willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112(1):11–18.
- Tsai H-M, Lian EC. Antibodies to von willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339(22):1585–1594.
- Furlan M, Robles R, Galbusera M, et al. Von willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339(22):1578–1584.
- Scully M, Hunt BJ, Benjamin S, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158(3):323–335.
- Benhamou Y, Assie C, Boelle PY, et al. Development and validation of a predictive model for death in acquired severe ADAMTS13 deficiency-associated idiopathic thrombotic thrombocytopenic purpura: the French TMA reference center experience. Haematologica. 2012;97(8):1181–1186.
- Thejeel B, Garg AX, Clark WF, et al. Long-term outcomes of thrombotic microangiopathy treated with plasma exchange: a systematic review. Am J Hematol. 2016;91(6):623–630.
- Kremer Hovinga JA, Vesely SK, Terrell DR, et al. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(8):1500–1511.
- Chaturvedi S, Carcioppolo D, Zhang L, et al. Management and outcomes for patients with TTP: analysis of 100 cases at a single institution. Am J Hematol. 2013;88(7):560–565.
- Goel R, King KE, Takemoto CM, et al. Prognostic risk-stratified score for predicting mortality in hospitalized patients with thrombotic thrombocytopenic purpura: nationally representative data from 2007 to 2012. Transfusion. 2016;56(6):1451–1458.
- Vesely SK. Life after acquired thrombotic thrombocytopenic purpura: morbidity, mortality, and risks during pregnancy. J Thromb Haemost. 2015;13(Suppl. 1):S216–S222.
- Goel R, Ness PM, Takemoto CM, et al. Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Blood. 2015;125(9):1470–1476.
- Lee W, Perimbeti S, Vazquez Martinez M, et al. Higher incidence of TTP in african americans and females: an analysis of demographics, cost and length of stay in teaching and nonteaching hospitals for thrombotic thrombocytopenic purpura between 1999 and 2013. Blood. 2016;128(22):4735–4735.
- Page EE, Kremer Hovinga JA, Terrell DR, et al. Thrombotic thrombocytopenic purpura: diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv. 2017;1(10):590–600.
- Sarode R, Bandarenko N, Brecher ME, et al. Thrombotic thrombocytopenic purpura: 2012 American Society for Apheresis (ASFA) consensus conference on classification, diagnosis, management, and future research. J Clin Apher. 2014;29(3):148–167.
- FDA Prescribing Information for Caplacizumab [Internet]. FDA; [cited 2021 May 16]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761112s000lbl.pdf
- Scully M, Cataland SR, Peyvandi F, et al. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335–346.
- Peyvandi F, Cataland S, Scully M, et al. Caplacizumab prevents refractoriness and mortality in acquired thrombotic thrombocytopenic purpura: integrated analysis. Blood Adv. 2021;5(8):2137–2141.
- Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511–522.
- Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2017;15(7):1448–1452.
- Coppo P, Bubenheim M, Azoulay E, et al. A regimen with caplacizumab, immunosuppression and plasma exchange prevents unfavorable outcomes in immune-mediated TTP. Blood. 2021;137(6):733–742.
- Dutt T, Shaw RJ, Stubbs MJ, et al. Real-world evidence of caplacizumab use in the management of acute TTP. Blood. 2021;137(13):1731–1740.
- Völker LA, Kaufeld J, Miesbach W, et al. Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura. Blood Adv. 2020;4(13):3085–3092.
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14.
- 2018-2019 Medicare Provider Analysis and Review (MEDPAR) data files [Internet]. Baltimore: Centers for Medicare & Medicaid Services; [cited 2021 May 16]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/MEDPARLDSHospitalNational.
- Heatwole C, Johnson N, Holloway R, et al. Plasma exchange versus intravenous immunoglobulin for myasthenia gravis crisis: an acute hospital cost comparison study. J Clin Neuromuscul Dis. 2011;13(2):85–94.
- Maeda JLK, Nelson L. How do the hospital prices paid by medicare advantage plans and commercial plans compare with medicare fee-for-service prices. Inquiry. 2018;55:469508018779654.
- Medicare Claims Processing Manual. Chapter 3 – Impatient Hospital Billing [Internet]. Centers for Medicare & Medicaid Services; [cited 2021 May 16]. Available from: https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c03.pdf.
- Pollissard L, Shah A, Punekar RS, et al. Burden of illness among medicare and Non-Medicare US populations with acquired thrombotic thrombocytopenic purpura. J Med Econ. 2021;24(1):706–716.
- Benhamou Y, Boelle PY, Baudin B, et al. Cardiac troponin-I on diagnosis predicts early death and refractoriness in acquired thrombotic thrombocytopenic purpura. Experience of the French thrombotic microangiopathies reference center. J Thromb Haemost. 2015;13(2):293–302.
- Chemnitz JM, Uener J, Hallek M, et al. Long-term follow-up of idiopathic thrombotic thrombocytopenic purpura treated with rituximab. Ann Hematol. 2010;89(10):1029–1033.
- Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2496–2502.