Abstract
Background
DiviTum TKa, a blood-based biomarker assay developed to monitor and predict treatment response in hormone receptor positive metastatic breast cancer (HR + mBC), may decrease traditional disease monitoring assessments and avoid prolongation of futile treatments.
Objective
To estimate the diagnostic and treatment budget impact of the assay on a postmenopausal HR + HER2- mBC population in a one-million-member U.S. health plan.
Methods
We developed a budget impact model comparing inclusion and exclusion of DiviTum TKa to standard care under which DiviTum TKa (1) reduces the frequency of traditional mBC monitoring tools, and (2) predicts treatment futility in advance of radiological disease progression. Traditional disease monitoring assessment schedules were based on guidelines and expert opinions. DiviTum TKa’s impact on therapy utilization was based on published literature and expert opinion. Modeled costs included DiviTum TKa, NCCN-recommended treatments, imaging, biomarker testing, and adverse events. We calculated total and per-member per-month (PMPM) costs with a 3-year time horizon.
Results
The inclusion of 416 DiviTum TKa assays ($166,400) was largely offset by 193 fewer CT scans, 88 fewer bone scans, and 55 fewer biomarker tests over 3 years, a savings of −$128,400, resulting in a PMPM of $0.001. In scenario analyses, adding DiviTum TKa resulted in additional treatment-related cost-savings (−$465,600), resulting in a PMPM cost-savings of −$0.013, or an average savings of −$7,400 per each patient initiating first-line cyclin-dependent kinase 4/6 inhibitor plus aromatase inhibitor therapy. Expected savings approached 3× the spend on the new test. Results were most sensitive to DiviTum TKa cost, population parameters, and treatment costs.
Conclusions
Clinical use of the DiviTum TKa assay is expected to decrease traditional imaging and monitoring and may reduce the overall cost of managing mBC if it leads to clinical decisions to avoid futile therapy. Post-coverage, real-world monitoring of palliative therapies among post-menopausal mBC populations is needed to better categorize cost savings over time.
PLAIN LANGUAGE SUMMARY
What is already known about this subject
Current monitoring of therapy for hormone receptor positive metastatic breast cancer involves a combination of tumor marker testing, imaging, and other tools. Monitoring is variable in practice, and therefore relatively insensitive to evidence of tumor progression.
What this study adds
DiviTum TKa is a novel biomarker that may offer a more convenient and sensitive alternative to traditional monitoring of metastatic breast cancer. Factoring in cost offsets due to reduced monitoring and earlier discontinuation of futile therapies may be cost-saving to health plans that cover this technology.
Introduction
Metastatic breast cancer (mBC) represents a significant human and economic burden in the United States. Breast cancer is the second leading cause of cancer death among women in the United States with nearly 44,000 deaths annuallyCitation1. Among women age 50–64 and over 65 with mBC, five-year relative survival is 30.2% and 28.3%, respectivelyCitation2, while the total costs of mBC across all age groups and phases of care were $63.4 billion in 2015 and are expected to increase to $152.4 billion in 2030Citation3. Recent economic studies estimate that cumulative per patient total costs for patients with mBC range from $200,000 to more than $400,000 in the initial years following diagnosisCitation4,Citation5.
Advances in therapy—specifically cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) and fulvestrant—have improved progression-free and overall survival for women with mBCCitation6. The benefits of new therapy options have increased the role of monitoring in disease managementCitation7. Current recommendations, which include a combination of carcinoembryonic antigen (CEA), cancer antigen (CA) 15-3, and CA 27.29 tumor markers and imaging, reflect the need to detect disease activity and response to treatment so that therapies can be adjusted accordinglyCitation8–11. Monitoring itself can be burdensome and costlyCitation12; furthermore, there is documented variability in both the type and schedule of monitoring among patients, likely reflecting multiple factors, including clinical uncertainty regarding disease progression, interpretation of test results, and patient and physician preferences regarding monitoring and therapy selectionCitation12,Citation13.
Circulating thymidine kinase activity (TKa) is a potential prognostic and monitoring marker in HR + mBC patients treated with endocrine therapy alone or in combination with a CDK4/6iCitation14,Citation15. DiviTum TKaFootnotei is a blood-based biomarker assay for TKa that has been developed to monitor and predict treatment response in HR + HER2- mBC, a subtype that accounts for approximately 70% of breast cancer cases in the USCitation16. Assessments of DiviTum TKa in early clinical studies suggest that it may decrease the need for traditional disease monitoring assessments on the basis of identifying good prognosis patients. Clinical studies also suggest that the test may enable identification of poor prognosis patients, and avoid prolongation of futile treatmentsCitation15,Citation17,Citation18. Accordingly, the purpose of this study (previously presented at ISPOR 2021Citation19) is to estimate the projected budget impact of including DiviTum TKa on a U.S. payer formulary as a treatment option for postmenopausal women with HR + HER2- mBC.
Methods
Model overview
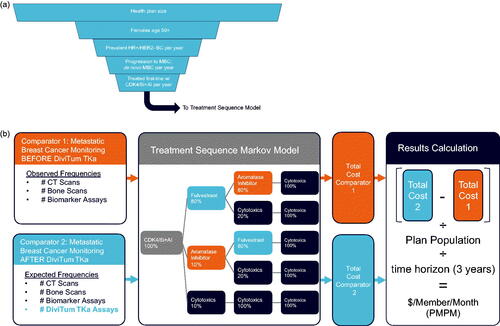
We developed a budget impact model in Excel (Microsoft, Redmond, WA) to estimate the costs (in 2021 US Dollars) associated with mBC disease monitoring and treatment in the United States. The model compared (1) disease monitoring before the introduction of DiviTum TKa, wherein the frequencies of CT scans, bone scans, and biomarker assays were based on current standards of care, versus (2) disease monitoring after the introduction of DiviTum TKa, with consequently reduced frequencies of CT scans, bone scans, and biomarker assays. We used a population model to estimate successive monthly patient cohorts who then entered a treatment sequence model that tracked disease progression and overall survival from first through fourth line therapy along with monitoring, treatment, and adverse event costs over a three-year time horizon. The model considered only direct medical expenditures. The estimated budget impact of DiviTum TKa was estimated as the cost of monitoring with DiviTum TKa minus the cost of monitoring without DiviTiumTKa, divided by the plan population, and divided by 36 months to derive the total cost per plan member per month (PMPM).
Population model
The population model was used to estimate the monthly number of postmenopausal women with HR + HER2- mBC who receive first-line treatment with a CDK4/6i plus an aromatase inhibitor (AI) (). We assumed a one-million-member U.S. health plan and then applied U.S.-based population data for post-menopausal women age 50–64 and 65 and older ()Citation20. The proportion of women over age 65 enrolled in the health plan through Medicare Advantage was 30%Citation21.
Table 1. Model parameters.
We then estimated the number of prevalent cases of nonmetastatic HR+/HER2- (Luminal A) breast cancer in the two age groups using data from the Surveillance, Epidemiology, and End Results (SEER) ProgramCitation22; the proportion of these nonmetastatic cases that progressed to mBC (18.5%) was derived from Malmgren et al., who conducted a retrospective cohort study of BC patients from 1990 to 2010 with follow up through 2015 of de novo mBC patients (stage IV at diagnosis) and recurrent mBC patients with subsequent distant metastatic recurrence (stage I-III initial diagnosis)Citation23. The age-based incidence of de novo mBC cases was also obtained from SEERCitation24. Lastly, we estimated the total number of mBC cases who go on to receive first-line treatment with CDK4/6i + AI (the target population) from Goldschmidt et al., who examined 64 community oncologists’ medical records to describe contemporary treatment patterns for mBC among postmenopausal women in a real-world setting.
Cancer monitoring
Monitoring frequencies before the introduction of DiviTum TKa were based on NCCN guidelines and expert opinionCitation9. These included CT scans for all patients administered at diagnosis, 12 weeks, then quarterly for three years; bone scans administered to 50% of patients at diagnosis and 2 weeks, and then quarterly for three years; and biomarker testing administered to 50% of patients at diagnosis, at 4 weeks, then monthly for six months and quarterly for 1.5 years ().
The DiviTum TKa testing schedule was based on the manufacturer’s recommendation and clinical opinionCitation25. This schedule assumed all patients received DiviTum TKa at mBC diagnosis and two weeks after diagnosis, then monthly for six months, then quarterly for 1.5 years. Reductions in the NCCN guideline-based disease monitoring frequencies after the introduction of DiviTum TKa were based on clinical opinion, as follows: CT scans administered at diagnosis, 12 weeks, then semi-annually for 1.5 years; bone scans administered at diagnosis and 2 weeks, and then semi-annually for 1.5 years; and biomarker testing (including annual declines in the number of tested patients) administered at diagnosis, at 4 weeks, then monthly for 3 months and quarterly for 9 months, with this last estimate representing an average across variable standards of care.
Treatment
Treatment choices were modeled on NCCN-recommended treatments for HR + HER2- mBC patientsCitation9. All patients derived from the population model entered the treatment sequence model via first-line treatment with CDK4/6i + AI. Patients who progressed after first-line treatment could then receive second-line treatment with either fulvestrant, aromatase inhibitors, or cytotoxic therapy. In the third line, cytotoxic-naïve patients could receive treatment among fulvestrant or aromatase inhibitors (if naïve), or additional cytotoxic therapy. Once patients began cytotoxic therapy, they were assumed to be ineligible for anything other than additional cytotoxic therapy.
We modeled treatment-associated progression-free and overall survival using transition probabilities derived from an exponential fit to the respective median survivals, obtained from the treatment regimens’ clinical trials. The transition probabilities were used in a Markov model framework to move each monthly cohort entering the model from first-line treatment to fourth-line treatment and/or death.
Costs
We based the unit cost of DiviTum TKa, CT scans, bone scans, and biomarker testing on internal estimates for DiviTum TKa cost and Centers for Medicare & Medicaid Services (CMS) Healthcare Common Procedural Coding System (HCPCS) codes ()Citation26. We used the wholesale acquisition cost per drug, obtained from Analy$ource.com, an online drug pricing databaseCitation31. The cost per adverse event was derived from the 2021 CMS Final Rule and Correction Notice TablesCitation32.
Sensitivity and scenario analyses
We evaluated the uncertainty of all model inputs on the PMPM using one-way sensitivity analysis, in which one parameter at a time is varied to its low and high value while keeping all other parameters constant; larger resultant PMPM ranges indicate parameters with the greatest impact on results. In a scenario analysis, we explored DiviTum TKa’s impact on therapy utilization based on published literature and expert opinionCitation17,Citation25; in this scenario, DiviTum TKa predicted treatment futility and led to therapy changes an average of one month in advance of radiological disease progression-driven changes in 15% of patients at first follow-up, and these patients immediately transitioned from first-line treatment to second-line treatment.
Results
Population model
In a health plan with 1,000,000 members, we estimated 228 prevalent (i.e. previously diagnosed) HR+/HER2- (Luminal A) BC patients per year. Of this cohort, 42 progressed to mBC, and 17 initiated treatment with CDK4/6i + AI. We further estimated 270 incident (newly diagnosed) women with HR+/HER2- (Luminal A) BC per year, of whom 11 had metastatic disease at diagnosis and 4 initiated treatment with CDK4/6i + AI. In total, approximately 21 women per year (1.7 per month) would initiate treatment with CDK4/6i + AI ().
Table 2. Population model results.
Before DiviTum TKa approval
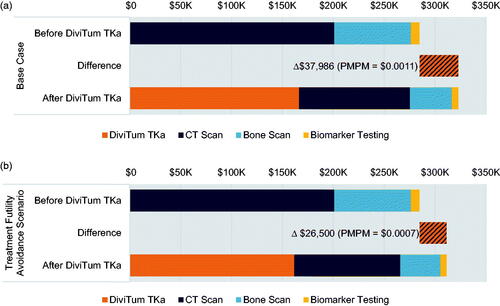
Total treatment and adverse event costs were $13.7 million (including $13.3 million for first-line treatment with CDK4/6i + AI) and $530,500, respectively (). Before the addition of DiviTum TKa, patients received 423 CT scans, 194 bone scans, and 208 biomarker tests over three years, for $201,000, $75,400, and $8,300, respectively. In one year, on average, individual patients received 4.8 CT scans, 2.4 bone scans, and 4.3 biomarker tests. The total three-year cost of monitoring before the addition of DiviTum TKa was $284,700. The overall three-year cost of monitoring, treatment, and adverse events before the addition of DiviTum TKa was $14.5 million.
Table 3. Budget impact model results.
After DiviTum TKa approval
Treatment and adverse event costs were equivalent after the addition of 416 DiviTum TKa assays, however, we estimated this led to 193 fewer CT scans, 88 fewer bone scans, and 55 fewer biomarker tests over three years. In one year, on average, individual patients received 8.5 DiviTum TKa assays, 2.8 CT scans, 1.4 bone scans, and 3.0 biomarker assays. The total cost of DiviTum TKa testing over three years was $166,400, while the costs of CT scans, bone scans, and biomarker tests were $109,100 (Δ -$91,900), $41,200 (Δ -$34,300), and $6,100, (Δ -$2,200), respectively (). The total cost of monitoring after the addition of DiviTum TKa was $322,700 (Δ $38,000), resulting in an overall three-year PMPM of $0.001.
Scenario analysis
In the scenario analysis accounting for reduced time spent on futile first-line therapy after the addition of DiviTum TKa, meantime on treatment with CDK4/6i + AI therapy was 28 months without DiviTum TKa testing; with DiviTum TKa, 27 months; this led to treatment-related cost savings due to avoidance of time on futile therapy. Treatment costs decreased to $13.2 million (Δ -$494,600) and adverse event costs increased to $533,000 (Δ $2,600, due to increased use of cytotoxic therapy in later lines of treatment). The total cost of DiviTum TKa testing over three years was $161,700, while the costs of CT scans, bone scans, and biomarker tests were $104,300 (Δ -$96,700), $39,300, (Δ -$36,200), and $5,900, (Δ -$2,400), respectively. The total cost of monitoring after the addition of DiviTum TKa was $311,200 (Δ $26,500; ). The overall three-year cost of monitoring plus treatment and adverse events, factoring in reduced time on futile first-line therapy after the addition of DiviTum TKa, was $14.1 million (Δ -$465,600), resulting in a PMPM cost-savings of -$0.013 ().
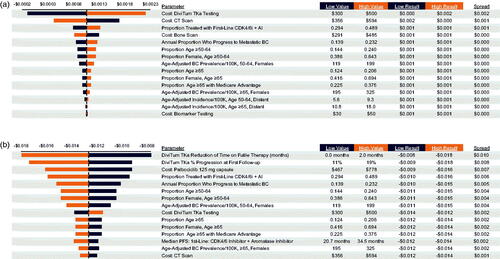
Sensitivity analysis
In one-way sensitivity analysis for the base case, the PMPM was most sensitive to the cost of DiviTum TKa testing, the cost of CT scans, the proportion treated with first-line CDK4/6i + AI, and the annual proportion of prevalent BC patients who progress to mBC (). For the reduced time on futile therapy scenario, PMPM results were most sensitive to the reduction of time on futile therapy, the proportion of patients predicted by DiviTum TKa to progress early, the cost of the CDK4/6i palbociclib, the proportion treated with first-line CDK4/6i + AI, and the annual proportion of prevalent BC patients who progress to mBC ().
Discussion
In this budget impact analysis, the use of DiviTum TKa reduced the use of a substantial proportion of traditional monitoring tools such that the budget impact on a health plan with 1,000,000 members was minimal ($0.001 PMPM). The more impactful potential clinical value of the DiviTum TKa assay, however, is identifying women for whom lack of response to a costly CDK 4/6i was detected and acted upon earlier than standard monitoring. In such situations, the DiviTum TKa can be cost-saving to plans under adherence scenarios that are expected by clinical experts.
We focus the base estimate on monitoring only because it is a more likely—and therefore more conservative—estimate of the costs of management with the novel assay. While it is possible that DiviTum TKa will prove useful in detecting treatment failure/futility earlier than traditional monitoring, it is likely that such savings will be realized after clinicians gain experience with the assay over time. It is also possible that early changes to therapy in patients with tumors resistant to CDK 4/6i + AI therapy could lead to improved clinical outcomes, which is a hypothesis that should be tested in future clinical studies.
In the monitoring-only scenario, the cost of CT scans was an important factor influencing the budget impact. Reimbursements for CT scans can be somewhat higher for commercial insurers versus Medicare. Thus, a plan serving primarily commercially-insured patients may realize modest cost savings with changes to monitoring alone. Furthermore, the addition of DiviTum TKa may help refine current clinical guidelines’ nonspecificity as to the recommended frequency of imaging exams. When considering the expanded scenario that includes futile therapies, the price of CDK4/6is is the most influential factor impacting the overall budget. The average sales price of albociclib, ribociclib, and abemaciclib have increased 5–10% per year since they were introducedCitation33. Further price increases will increase the potential savings afforded by DiviTum TKa.
Limitations
We note the limitations of our analysis. Because clinicians have not yet had experience with DiviTum TKa beyond clinical trials, the assumptions made in this model must be viewed as preliminary. Also, to our knowledge, there are no universal fixed patterns of mBC monitoring, therefore the cost offsets estimated in this analysis are specific to the assumptions about real-world use. Lastly, we restricted the available treatment pathway in our model due to limited pathway data and to focus our findings on the impacts of HR + HER2- mBC monitoring. Further, the focus was on a single treatment pathway only: those initiating first-line treatment with CDK4/6i + AI therapy, which amounts to 21 patients per year within a 1 million-member plan. Following approval and commercial introduction of DiviTum TKa, future research on practice patterns and costs in this population will be necessary to validate the analysis.
Conclusions
The inclusion of DiviTum TKa reduced the use of a substantial proportion of traditional monitoring. If the use of DiviTum TKa can also predict lack of benefit from costly CDK4/6i therapy and clinicians act on that information in a timely fashion, this can result in substantial cost savings to patients and health plans. In this scenario, net savings on adding a new test to care could be three times the cost of the new test itself. Following approval and commercial introduction of DiviTum TKa, future research on practice patterns will be necessary to validate our analysis.
Transparency
Declaration of funding
This work was funded by Biovica International AB.
Declaration of financial/other interests
JJC, GFG, and SDR are paid consultants of Biovica International AB; RAD is a full time employee of Biovica International AB. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
JJC, GFG, RAD, and SDR all contributed to analysis and manuscript writing.
Acknowledgements
None stated
Previous presentations
This work was previously presented as a digital poster at ISPOR 2021.
Notes
i DiviTum TKa is a registered trademark of Biovica AB, Uppsala, Sweden.
References
- American Cancer Society. Cancer Facts & Figures. 2021. [cited 2021 June 21]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
- Surveillance Epidemiology, and End Results (SEER) Program. HR+/HER2- (Luminal A) Breast Cancer (Female only) SEER 5-Year Relative Survival Rates, 2011–2017 Female By Age, All Races (includes Hispanic), Distant 2021. [cited 2021 Nov 2]. Available from: https://seer.cancer.gov/explorer/application.html?site=622&data_type=4&graph_type=5&compareBy=age_range&chk_age_range_141=141&chk_age_range_157=157&series=9&hdn_sex=3&race=1&stage=106&advopt_precision=1&advopt_show_ci=on
- Gogate A, Wheeler SB, Reeder-Hayes KE, et al. Projecting the prevalence and costs of metastatic breast cancer from 2015 through 2030. JNCI Cancer Spectr. 2021;5(4):pkab063.
- Sussell JA, Sheinson D, Wu N, et al. HER2-Positive metastatic breast cancer: a retrospective cohort study of healthcare costs in the targeted-therapy age. Adv Ther. 2020;37(4):1632–1645.
- Trogdon JG, Baggett CD, Gogate A, et al. Medical costs associated with metastatic breast cancer in younger, midlife, and older women. Breast Cancer Res Treat. 2020;181(3):653–665.
- Li J, Huo X, Zhao F, et al. Association of cyclin-dependent kinases 4 and 6 inhibitors with survival in patients with hormone receptor-positive metastatic breast cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(10):e2020312.
- Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res Treat. 2017;166(1):41–54.
- Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Annals Oncol. 2020;31(12):1623–1649.
- Gradishar WJ, Moran MS, Abraham J, et al. Breast cancer, version 2.2021, NCCN clinical practice guidelines in oncology 2021. [cited 2020 Dec 13].
- Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J Clin Oncol. 2016;34(25):3069–3103.
- Van Poznak C, Somerfield MR, Bast RC, et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2015;33(24):2695–2704.
- Accordino MK, Wright JD, Vasan S, et al. Use and costs of disease monitoring in women with metastatic breast cancer. J Clin Oncol. 2016;34(24):2820–2826.
- Goldschmidt D, Dalal AA, Romdhani H, et al. Current treatment patterns among postmenopausal women with HR+/HER2- metastatic breast cancer in US community oncology practices: an observational study. Adv Ther. 2018;35(4):482–493.
- Bonechi M, Galardi F, Biagioni C, et al. Plasma thymidine kinase-1 activity predicts outcome in patients with hormone receptor positive and HER2 negative metastatic breast cancer treated with endocrine therapy. Oncotarget. 2018;9(23):16389–16399.
- McCartney A, Bonechi M, De Luca F, et al. Plasma thymidine kinase activity as a biomarker in patients with luminal metastatic breast cancer treated with palbociclib within the TREnd trial. Clin Cancer Res. 2020;26(9):2131–2139.
- Surveillance Epidemiology, and End Results (SEER) Program. Breast Recent Trends in SEER Age-Adjusted Incidence Rates, 2000-2018 Observed SEER Incidence Rate By Subtype, Female, All Races (includes Hispanic), All Ages, All Stages 2021. [cited 2021 Nov 2]. Available from: https://seer.cancer.gov/explorer/application.html?site=55&data_type=1&graph_type=2&compareBy=subtype&chk_subtype_55=55&chk_subtype_622=622&chk_subtype_623=623&chk_subtype_620=620&chk_subtype_621=621&hdn_rate_type=1&sex=3&race=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&advopt_display=2
- Malorni L, Tyekucheva S, Hilbers FS, et al. Serum thymidine kinase activity in patients with luminal metastatic breast cancer treated with palbociclib and fulvestrant within the PYTHIA trial [abstract]. In: Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium; 2020 Dec 8-11; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2021;81(4 Suppl):Abstract no. PS5-05.
- Paoletti C, Barlow WE, Bergqvist M, et al. Evaluating serum thymidine kinase 1 in hormone receptor positive metastatic breast cancer patients receiving first line endocrine therapy in the SWOG S0226 trial [abstract]. In: Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium; 2020 Dec 8-11; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2021;81(4 Suppl):Abstract no. PS2-04.
- Guzauskas G, Carlson J, Dann R, et al. PCN190 budget impact of the DiviTum®TKa assay in postmenopausal women with hormone receptor positive metastatic breast cancer. Value Health. 2021;24:S55.
- Statista I. Resident population of the United States by sex and age as of July 1, 2019 2020. [cited 2020 Dec 19]. Available from: https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age/
- Kaiser Family Foundation. An Overview of Medicare 2019. [cited 2020 Dec 2]. Available from: https://www.kff.org/medicare/issue-brief/an-overview-of-medicare/
- Surveillance Epidemiology, and End Results (SEER) Program. HR+/HER2- (Luminal A) Breast Cancer (Female only) People Alive with Cancer (U.S. Prevalence) on January 1, 2017 2020 [cited 2020 Dec 13]. Available from: https://seer.cancer.gov/explorer/application.html?site=622&data_type=5&graph_type=12&compareBy=age_range&chk_age_range_1=1&chk_age_range_9=9&chk_age_range_141=141&chk_age_range_157=157&series=9&hdn_sex=3&race=1&prev_duration=3&advopt_limprev_y_axis_var=2&advopt_display=2
- Malmgren JA, Mayer M, Atwood MK, et al. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990-2010. Breast Cancer Res Treat. 2018;167(2):579–590.
- Epidemiology S, and End Results (SEER) Program. HR+/HER2- (Luminal A) Breast Cancer (Female only) Recent Trends in SEER Age-Adjusted Incidence Rates, 2010–2017 2020 [cited 2020 Dec 13]. Available from: https://seer.cancer.gov/explorer/application.html?site=622&data_type=1&graph_type=2&compareBy=race&chk_race_1=1&hdn_sex=3&age_range=141&stage=101&hdn_rate_type=1&advopt_precision=1&advopt_display=2
- Biovica International AB. Data on file. 2020.
- Centers for Medicare and Medicaid Services (CMS). Physician Fee Schedule Search. 2020. [cited 2020 Dec 13]. Available from: www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx
- Food and Drug Administration (FDA). Fulvestrant Package Insert. 2019. [cited 2020 Dec 27]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021344Orig1s039lbl.pdf
- Food and Drug Administration (FDA). Anastrozole Package Insert. 2014. [cited 2020 Dec 27]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
- Perez EA, Vogel CL, Irwin DH, et al. Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2001;19(22):4216–4223.
- Finn RS, Boer K, Bondarenko I, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res Treat. 2020;183(2):419–428.
- AnalySource.com. Active NDCs. 2020. [cited 2020 Dec 15]. Available from: https://www.analysource.com/products/active/
- Centers for Medicare and Medicaid Services (CMS). FY 2020 Final Rule and Correction Notice Tables. 2020. [cited 2020 Dec 22]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2020-IPPS-Final-Rule-Home-Page-Items/FY2020-IPPS-Final-Rule-Tables
- Martin J. U.S. Drug Prices to Rise in 2020 as Companies Prepare to Charge More for Ibrance, Xeljanz, 200 Others: Newsweek Digital LLC; 2019. [cited July 12]. Available from: https://www.newsweek.com/us-drug-prices-rise-2020-companies-prepare-charge-more-ibrance-xeljanz-200-others-1479939