Abstract
Objective
This study aimed to understand the impact of different efficacy endpoints on reimbursement decisions made by health technology assessment (HTA) bodies.
Materials and methods
European Medicines Agency (EMA) oncology product marketing authorizations were screened to identify products that completed review by 3 HTA bodies during 2016–2019: United Kingdom’s National Institute for Health and Care Excellence, Germany’s Gemeinsamer Bundesausschuss, and France’s Haute Autorité de Santé. Each decision’s endpoint information, including overall survival (OS) and progression-free survival (PFS), was extracted. Each endpoint’s influence on added benefits rating (the degree of added benefit as judged by the HTA agency) and full reimbursement (i.e. reimbursed population to label) decisions was tested using bivariate analyses.
Results
An increasing trend was observed toward HTA submissions with immature OS data (36.8% and 71.4% in 2016 and 2019, respectively), which was a predictor of limited added benefit (p < .001). Regarding data availability, 63% of submissions provided OS, 2% provided PFS without OS; and 35% provided neither. OS availability significantly influenced added benefit (p < .001) but not full reimbursement (p > .05) decisions, whereas PFS without OS had no significant impact compared with either OS or PFS data for either outcome (p = .99).
Conclusions
The trend toward fewer products filing mature OS data over time suggests sponsors may be increasingly confident achieving reimbursement with surrogate endpoint data, although mature OS data provided the strongest correlation to positive reimbursement decisions. Notably, in some locally advanced settings, OS data maturity will take a long time to obtain. To expedite patient access to new medicines, payers should consider the acceptance of surrogate endpoints predictive of clinical benefit.
Keywords:
Introduction
Marketing authorization (MA) from the European Medicines Agency (EMA) is the necessary first step in bringing a new oncology medicine to patients in Europe. Usually, EMA approval is based on data from rigorously designed and conducted pivotal clinical trials. Historically, the goal for any new oncology treatment is to demonstrate overall survival (OS) improvement as an objective and clinically meaningful measure. Surrogate outcomes such as progression-free survival (PFS), overall response rate, and disease-free survival are substitute quantitative measures expected to predict ‘hard’ objective outcomes such as OS.
Recent analyses suggest that manufacturers’ traditional focus on OS may be in decline due to the growing acceptance of surrogate outcomes as a basis for EMA regulatory approvalCitation1,Citation2. A recent analysis of approved EMA MA applications revealed that the use of surrogate endpoints is increasingly commonCitation2. This changing emphasis may reflect the fact that OS can be time-consuming to measure and difficult to interpret, particularly in locally advanced and first-line metastatic settings (where patients are more likely to receive post-progression treatment or when trial designs incorporate cross-over to subsequent treatmentsCitation3,Citation4.
Regulatory approval is only a first step toward patient access to new medicines. Whereas regulators focus on benefit-risk profiles, health technology assessment (HTA) agencies also need to promote an equitable, efficient, and high-quality health systemCitation5,Citation6. HTA processes and timelines vary among countries and are heterogeneous and complex. Some agencies are more restrictiveCitation7; each imposes varying restrictions and draws conclusions that reflect different weights given to data sources (e.g. clinical, economic), resulting in potentially delayed patient accessCitation8,Citation9. Cost and the inclusion of formal cost-effectiveness assessments are key differences between agencies. The United Kingdom’s National Institute for Health and Care Excellence (NICE) focuses on cost-effectiveness, whereas Germany’s Gemeinsamer Bundesausschuss (G-BA) and France’s Haute Autorité de Santé (HAS) focus on comparative effectiveness.
Increasingly, HTA decision-makers face situations where OS data are not available or feasibly expectedCitation10. Previously, HTA agencies were cautious about the reliability of surrogate endpoints such as PFSCitation11, and concerns remain that surrogate endpoints represent a shortcut to MACitation12. The impact of growing regulatory acceptance of surrogate endpoints for HTA assessment in oncology has yet to be fully evaluated. A 2014 analysis suggested that in oncology trials, positive HTA decisions from G-BA, HAS, and NICE were more likely when OS was the primary endpointCitation13. The present study aimed to understand the impact of different efficacy endpoints on recent reimbursement decisions made by European HTA bodies and to specifically address whether the nature and timing of recent HTA recommendations for new oncology treatments were affected by the type, maturity, and significance of efficacy outcomes available at initial appraisal. These factors were investigated in terms of treatment features (such as first indication, a novel mechanism of action), clinical trial design (comparative or single arm), efficacy endpoints (OS and PFS data maturity and significance) and HRQOL availability.
Methods
Identification of appraisals
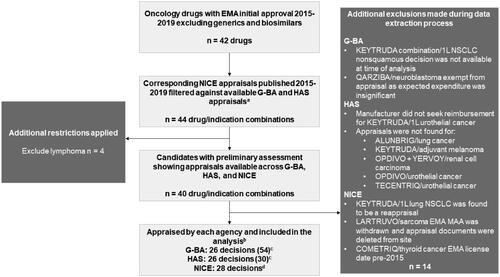
G-BA, HAS, and NICE was selected for this analysis because they publish detailed descriptions of the rationale for reimbursement decisions and also provide a diverse approach to HTA appraisals, from cost-effectiveness (NICE) to relative clinical effectiveness (G-BA). Using agency websites, medicines for solid tumors that were granted an EMA marketing authorization between 2015 and 2019 were cross-referenced with published NICE appraisals for the same drug and indication (). Corresponding appraisals by G-BA and HAS were then identified. Medicines for hematologic malignancies were excluded, as were generic, biosimilar, and supportive treatments (e.g. antiemetics). Reappraisals or subsequent submissions for the same drug or indication were excluded because they were likely to be based on more mature trial data.
Figure 1. Identification Process for Eligible Appraisals for Analysis. Abbreviations. 1L, first line; EMA, European Medicines Agency; G-BA, Gemeinsamer Bundesausschuss; HAS, Haute Autorité de Santé; MAA, marketing authorization application; NICE, National Institute for Health and Care Excellence; NSCLC, non-small cell lung cancer. aReflects both first-time approvals and license extensions for additional cancer treatment indications. bIncludes breast cancer, colorectal cancer, hepatocellular carcinoma, head and neck cancer, melanoma, Merkel cell carcinoma, NSCLC, renal cell carcinoma, urothelial carcinoma, and ovarian cancer. cTotal number of subpopulations assessed across appraisals. dBased on 2 products’ populations being split across 2 appraisals.
Data extraction
To collect relevant information from each appraisal, a data extraction template was developed. To focus the assessment on the HTA agency’s perspective rather than the content of the manufacturer’s submission, data sources were restricted to specific document sections. For G-BA, we extracted the following sections: early benefits assessment, executive summary of added benefit assessment, and extent and probability of added benefit. For HAS, we extracted clinical added value, amélioration du service médical rendu (ASMR), transparency committee conclusions, and the summary and discussion section of the data analysis. For NICE, we extracted evidence for clinical effectiveness, as well as the summary of the appraisal committee’s key conclusions.
Product (indication, treatment line, tumor type), licensing (first indication, first mechanism of action [MOA] to market, EMA approval date), trial design (comparative vs single arm, pivotal, type of control [i.e. placebo or active comparator], additional trials submitted), and efficacy endpoints reported (including OS/PFS, data maturity and significance, and HRQOL availability and significance) were tabulated. PFS and HRQoL were selected as they were the markers most frequently reported across all products. Two HTA final outcomes were captured: (1) whether the product received ‘full reimbursement’ and (2) the ‘added benefit’ for the product. Full reimbursement was defined as a positive recommendation of the treatment to a patient population aligned with that designated by the EMA authorization; however, full reimbursement was not assessed for Germany, where all products are reimbursed according to the EMA license. Final ratings from HAS and G-BA were also captured, defining high ‘added benefit’ reimbursement from HAS as service médical rendu (SMR) important and amélioration du service médical rendu (ASMR) IV or higher, and from G-BA, a designation better than ‘no added benefit.’ An added benefit was not assessed for the United Kingdom, because NICE does not report a final rating. Data were extracted by 1 reviewer, and a random sample of 10% was checked independently by a second reviewer.
Statistical analyses
Descriptive analyses were planned to assess tumor type, distribution of year of EMA approval, distribution of year of appraisal decision, the average time from EMA approval to HTA decision, and the proportion of decisions with high ‘added benefit’ based on different data packages. Bivariate analyses were also performed to understand the impact of different data packages on the probability of a product receiving high ‘added benefit’ reimbursement.
Results
Overall, 42 oncology drugs with an initial EMA approval between 2015 and 2019 were identified. After excluding drugs not appraised by all 3 HTA agencies during that time, we had 40 drug/indication combinations (). Fourteen combinations were subsequently excluded for the following reasons: 9 had no corresponding HAS appraisals, 1 predated the 2015 threshold, 1 had final decision pending at assessment (n = 1), 1 was exempt from G-BA appraisal due to level of anticipated expenditure (n = 1), 1 had a manufacturer that did not seek reimbursement (n = 1), and 1 MAA (marketing authorization application) was withdrawn.
26 drug/indication combinations per agency were included (; NICE was found to have 28 decisions associated with the same drug/indications because 2 decisions were subdivided into 2 separate appraisals). Most agency decisions occurred during 2018 (25), followed by 2017 (20), 2016 (19), 2019 (14), and 2015 (2). The median time from EMA approval to HTA agency decision was 213 days (mean, 304 days).
Trends in data packages over time
Tabulated data were analyzed to identify changes in the submitted data packages over time. The analysis focused on studying changes in aspects that are considered markers of robust data packages (e.g. comparative trial design, OS/PFS data maturity). The proportion of comparative studies (such as RCTs) vs. single-arm studies in the data packages submitted by manufacturers to HTA bodies (excluding data from 2015 because only 2 decisions were identified) with no significant change over time: in 2016, 84.2% (n = 16); in 2017, 95% (n = 19); in 2018, 80.0% (n = 20); and in 2019, 92.9% (n = 13). On the other hand, a trend was observed toward an increasing percentage (n) of products with immature OS data submitted to HTA bodies: in 2016, 36.8% (7); in 2017, 45.0% (9); in 2018, 44.0% (11); and in 2019, 71.4% (10). In contrast, the percentage (n) of products with immature PFS data submitted to HTA bodies has remained relatively constant over time: in 2016, 68.4% (13); in 2017, 75.0% (15); in 2018, 68.0% (17); and in 2019, 64.3% (9). Data are summarized in .
Table 1. Features of submitted data packages over time.
Predictors of reimbursement
In the 84 decisions (including all subpopulation decisions) for products approved between 2015 and 2018, 40.4% (34 of 84) received a positive reimbursement decision across G-BA and HAS. In univariate analyses, a significant negative effect on the added benefit was observed when a product was reviewed for reimbursement in its first indication (p = .0341; ). In contrast, we observed a significant positive effect of having comparative data (such as from a controlled clinical trial) available (p < .001; ). Having OS data was a significant predictor of positive added benefit vs having neither OS nor PFS data; however, availability of PFS data was not significant compared with no availability of either OS or PFS data (p = .99). This finding is likely due to the small number of events because we identified only 2 decisions with PFS and not OS, as opposed to 54 decisions with OS and 28 with neither OS nor PFS (). When OS maturity and OS statistical significance were analyzed individually, a significant positive effect on added benefit was observed for each (p < .001; ). When PFS was analyzed, only maturity had a statistically significant positive effect (p < .001; ), whether or not a submission reported data, where PFS results were statistically significant, showed only a positive trend and not a significant result (p = .11; ). Finally, the presence of HRQOL data also had a significant positive effect on approval (p < .001; ).
Table 2. Factors predicting reimbursement.
Predictors of full reimbursement
Over time, the proportion of decisions receiving full reimbursement from NICE and/or HAS stayed fairly constant (57.1% in 2015; 50.0% in 2018). Compared with submissions without any OS or PFS data, having PFS only (without OS) was not a significant predictor of full reimbursement (p = .991). The presence of mature PFS data was a significant predictor of full reimbursement from NICE and HAS (p = .017; ). Notably, the presence of statistically significant OS data trended toward positivity with full reimbursement decisions (p = .051; ). In addition, the availability of HRQOL data had a significant positive impact on full reimbursement (p = .044; ). These data suggest that the decision to provide full reimbursement per EMA label did not depend heavily on endpoint selection; however other considerations (such as patient selection criteria and subgroup analyses) were not examined. Given that this analysis was restricted to NICE and HAS, the positive influence of PFS may reflect the importance of PFS maturity for increasing certainty in extrapolations of cost-effectiveness models, reducing uncertainty for NICE around incremental cost-effectiveness ratio (ICER) estimates, and the meaningfulness to HAS of PFS as an endpointCitation14. It is likely that the availability of HRQOL, particularly of EQ-5D data, would also reduce uncertainty in economic models submitted to NICE.
Discussion
This study found there is an increasing trend over time towards HTA submissions with immature OS data, which is negatively associated with HTA reimbursement decisions. Specifically, our results showed that for added benefit ratings, the mature OS remains the most important endpoint to HTA agencies, although the presence of PFS data and its maturity or statistical significance were also positively associated with a full reimbursement. These findings are consistent with prior analyses demonstrating that OS was the gold standard for achieving a positive HTA decision in Germany, France, and the United KingdomCitation13.
In principle, the acceptability of surrogate endpoints to G-BA, HAS, and NICE is heavily dependent on demonstrable correlation (or strong association) between the surrogate and with a hard clinical endpoint such as that demonstrated between PFS and OSCitation15. In practice, this correlation has been achieved in only a handful of solid tumor typesCitation15–17. Evaluating the validity of surrogate endpoints in terms of how accurately they predict the magnitude of benefit should be an ongoing priority for all concerned be they regulators, HTA agencies, or pharmaceutical companies.
The observed trend toward a lower proportion of products having OS data over time suggests that sponsors are now more confident in submitting to HTA bodies based on surrogate endpoint data (including endpoints other than PFS) when OS data are immature. This trend is likely to continue, if not accelerate, as more companies bring new drugs to market without mature OS available at filing. In addition, this trend highlights the dichotomy in how regulatory bodies such as EMA are incentivizing innovation through rapid EMA authorization for new products based on limited data, while HTA bodies place a lower value based on limited data (e.g. immature OS) in terms of reimbursement decisionsCitation1,Citation2.
These findings highlight the need to align requirements, given the different evidence expectations between EMA and HTA bodies. This alignment can also be accomplished via HTA bodies looking for ways to implement coverage with evidence development, highlighting the need to identify ways in which developers and governments can align on consistent and cost-effective means of providing additional real-world data to support applications post-launch. But this alignment cannot be at the expense of patient safety. Expedited patient access accompanied by the promise of future real-world data dose not negate the primary importance of the benefit/risk profile.
Globally, the burden of cancer is increasing, and ensuring universal access to cancer care is required for health equity and to fulfill global commitments to disease controlCitation18. Furthermore, evaluating a new oncology treatment earlier in the disease pathway can exacerbate existing challenges between HTA requirements and data availability. Lower mortality rates, particularly in locally advanced settings, make achieving OS maturity and statistical significance even more difficult, leading to extended timelines and delays to patient access. The appropriate use of other outcomes affords opportunities to remove barriers to early patient access and also to design more efficient clinical trialsCitation19,Citation20. Maximizing these opportunities will require regulatory and HTA policy adaptation and a dynamic life-cycle approachCitation21,Citation22. Simply demanding more evidence has an ethical cost in terms of depriving patients of potential benefits of reduced mortality and morbidityCitation20.
This analysis has several potential limitations. Firstly, by restricting selection to products reviewed by all 3 agencies, this study was likely to have selected decisions underpinned by stronger data, and these findings might be clearer in a broader data set. Secondly, appraisals are necessarily complex and other factors driving these decisions, such as unmet need, patient selection criteria, subgroup analyses, and effect size on examined endpoints were not examined. Some factors such as the level of unmet need and effect size for examined endpoints would likely affect only the magnitude of importance of these endpoints rather than their intrinsic importance. However, the impact of other factors on reimbursement decisions may warrant further investigation. Thirdly, analyses were not stratified by line of therapy yet the relative importance OS and PFS may differ between settings; 57% of appraisals reviewed herein were set in the first line (in patients with previously untreated advanced or metastatic disease) where OS is potentially greater importance. Fourthly, this analysis considered only EMA approvals that led to HTA decisions from 2015 onwards; a broader span might emphasize these findings, given the more recent regulatory use of surrogate endpoints. Finally, this analysis looked only at timings of and outcomes from HTA decisions, not at pricing negotiation outcomes. It is likely that positive outcomes in HTA decisions would be predictive of more constructive pricing negotiations.
Conclusions
The trend over time toward fewer products filing mature OS data suggests that manufacturer sponsors may be increasingly confident in submitting reimbursement applications to HTA bodies based on surrogate endpoint data in the absence of mature OS data. However, regarding added benefits and reimbursement, mature OS endpoint data had the strongest correlation to positive decisions. Notably, in some locally advanced settings, OS data maturity will take a long time to achieve. To expedite patient access to new medicines, when balanced carefully against patient risks, acceptance of surrogate endpoints predictive of clinical benefit should be considered.
Transparency
Declaration of funding
This study was funded by EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA (CrossRef Funder ID: 10.13039/100004755).
Declaration of financial/other interests
NS and DM received consulting fees from EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, for this work. ACF and VP are employees of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA. TF is an employee of EMD Serono Research & Development Institute, Inc., Rockland, MA, USA, an affiliate of Merck KGaA. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed equally to this work.
Previous presentation
These data were previously presented at the Health Technology Assessment International 2020 Virtual Annual Meeting.
Acknowledgements
The authors would like to acknowledge Krzysztof Lach and Jodie Worrall for manuscript writing support, which was funded by Merck (CrossRef Funder ID: 10.13039/100009945) in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3)
References
- Kemp R, Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 2017;15(1):134.
- Kordecka A, Walkiewicz-Zarek E, Lapa J, et al. Selection of endpoints in clinical trials: trends in European Marketing authorization practice in oncological indications. Value Health. 2019;22(8):884–890.
- Cawston H, Genestier V, Li N, et al. Adjustment for subsequent therapies received: the impact on outcomes of an economic model of nivolumab + ipilimumab in first line metastatic renal cell carcinoma.Barcelona, Spain: ISPOR Europe; 2018.
- Kim H, Goodall S, Liew D. Health technology assessment challenges in oncology: 20 years of value in health. Value Health. 2019;22(5):593–600.
- McKendrick J, Malcolm B, Sheahan K, et al. The difference between regulatory and market access decisions on treatment availability for new drugs in six common cancers across Australia, Canada, and Europe. Value in Health. 2017;20(9):A467.
- O'Rourke B, Oortwijn W, Schuller T. Announcing the new definition of health technology assessment. Value Health. 2020;23(6):824–825.
- Ivandic V. Requirements for benefit assessment in Germany and England – overview and comparison. Health Econ Rev. 2014;4(1):12.
- Akehurst RL, Abadie E, Renaudin N, et al. Variation in health technology assessment and reimbursement processes in Europe. Value Health. 2017;20(1):67–76.
- Leyens L, Brand A. Early patient access to medicines: health technology assessment bodies need to catch up with new marketing authorization methods. Public Health Genomics. 2016;19(3):187–191.
- Sola-Morales O, Volmer T, Mantovani L. Perspectives to mitigate payer uncertainty in health technology assessment of novel oncology drugs. J Mark Access Health Policy. 2019;7(1):1562861.
- Mangiapane S, Velasco Garrido M. Use of surrogate end points in HTA. GMS Health Technol Assess. 2009;5:Doc12.
- Satherley A. Understanding Payer Sensitivities when Considering the Use of Surrogate Endpoints to Substantiate Clinical Value Propositions. [cited 2019 Nov]. Available form: https://www.evidera.com/wp-content/uploads/2017/05/Surrogate-Endpoints-Used-in-Health-Technology-Assessments.pdf
- Droeschel D, de Paz B, Houzelot D, et al. A comparison of market access evaluations for new oncology therapies in France, Germany and the UK: an analysis using the prismaccess database. Value Health. 2014;17(7):A654.
- Chouaid C, Borget I, Braun E, et al. French health technology assessment of antineoplastic drugs indicated in the treatment of solid tumours: perspective for future trends. Target Oncol. 2016;11(4):515–534.
- Savina M, Gourgou S, Italiano A, et al. Meta-analyses evaluating surrogate endpoints for overall survival in cancer randomized trials: a critical review. Crit Rev Oncol Hematol. 2018;123:21–41.
- Ciani O, Davis S, Tappenden P, et al. Validation of surrogate endpoints in advanced solid tumors: systematic review of statistical methods, results, and implications for policy makers. Int J Technol Assess Health Care. 2014;30(3):312–324.
- Michiels S, Saad ED, Buyse M. Progression-Free survival as a surrogate for overall survival in clinical trials of targeted therapy in advanced solid tumors. Drugs. 2017;77(7):713–719.
- Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4(11):1553–1568.
- Ciani O, Buyse M, Drummond M, et al. Time to review the role of surrogate end points in health policy: state of the art and the way forward. Value Health. 2017;20(3):487–495.
- Sandman L, Liliemark J. From evidence-based to hope-based medicine? Ethical aspects on conditional market authorization of and early access to new cancer drugs. Semin Cancer Biol. 2017;45:58–63.
- Eichler H-G, Pignatti F, Flamion B, et al. Balancing early market access to new drugs with the need for benefit/risk data: a mounting dilemma. Nat Rev Drug Discov. 2008;7(10):818–826.
- Pauwels K, Huys I, Casteels M, et al. Industry perspectives on market access of innovative drugs: the relevance for oncology drugs. Front Pharmacol. 2016;7(144):144.
