?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims
To develop a model to evaluate the cost-effectiveness of 4 mg dibotermin alfa/absorbable collagen sponge (ACS) versus iliac crest bone graft (ICBG) in patients with lumbar degenerative disc disease in the United Kingdom.
Materials & methods
A Markov decision-analytic model was constructed to calculate costs and quality-adjusted life-years over a 4-year time horizon in each treatment group, from a United Kingdom National Health Service perspective. An individual patient data meta-analysis was undertaken to synthesize data from four randomized controlled trials and two single-arm studies concerning health-related quality of life and procedural resource use. Current cost data from the United Kingdom were then applied to determine the overall mean cost per patient in each group. One-way and probabilistic sensitivity analyses were undertaken to explore the impact of parameter uncertainty.
Results
The model predicted 4-year discounted cost savings of £192 per patient treated with dibotermin alfa/ACS, compared with ICBG, and a gain of 0.0114 QALYs per patient over the same time period. Sensitivity analyses indicated that the results were most sensitive to variability in the differences in health-related quality of life and secondary surgery rate, with dibotermin alfa/ACS having a 60% probability of being cost-effective at a willingness-to-pay threshold of £20,000 per QALY gained.
Limitations
There is uncertainty in the difference in cost and QALYs between the two groups. However, comprehensive sensitivity analyses were undertaken to explore this and present the results in a transparent manner.
Conclusions
Our results provide an economic case for the use of 4 mg dibotermin alfa/ACS versus iliac crest bone graft, with additional health benefits predicted at reduced overall cost.
Introduction
Degenerative changes in the discs of the spine are a normal part of ageing. The pathological process leads to lumbar degenerative disc disease (LDDD) in which the water content becomes depleted. The degenerative process can cause instability, height loss, spinal stenosis, degenerative spondylolisthesis, herniated discs and end-plate changes. The development of LDDD can be related to biomechanical, traumatic, environmental and genetic factors. Symptoms as a result of the degenerative process include lower back pain, and if the nerve roots are compressed can cause neurological symptoms that can lead to leg pain, paresthesia, weakness and reduced mobility.
The annual incidence of LDDD in Europe has been reported at 8,586 new cases per 100,000 populationCitation1, while prevalence has been reported to range from 40% to 90% and increases with ageCitation2,Citation3. LDDD imposes a significant detrimental effect on various dimensions of quality of life, including a 40% decrement in physical functioning compared with an age- and the gender-matched general populationCitation4. Low back pain resulting from LDDD is also associated with a substantial economic burden. In the United States, the cost of elective lumbar fusion surgery in 2015 was estimated at more than $10 billion, while work absenteeism and reduced productivity due to low back pain related to lumbar disc disorders also lead to a substantial societal costCitation5. A UK study concluded that the direct cost of back pain care in 1998 was £1.6 billion, with informal care and productivity losses related to back pain totaling more than £10 billionCitation6.
Management of pain resulting from LDDD may in some cases progress to surgical intervention if conservative approaches failCitation7,Citation8. The current standard of care for eligible patients with LDDD is spinal fusion using an iliac crest bone graft (ICBG)Citation9, which aims to immobilize the painful segment and fuse two or more vertebrae together using bone graft harvested from the patient. However, complication rates of up to 20% have been reported after ICBG harvesting, including donor site pain, sensory loss, deep vein thrombosis and infectionCitation10,Citation11. An alternative to ICBG is dibotermin alfa/absorbable collagen sponge (ACS), a powder containing the active substance recombinant human bone morphogenetic protein-2 (rhBMP-2). It aims to help new bone to develop when used in conjunction with an approved medical device for spinal fusion in LDDDCitation12. One vial contains 4 mg or 12 mg dibotermin alfa and the required volume is determined by the intervertebral disc space and the size, shape and internal volume of the lumbar interbody fusion device(s) used. The efficacy of dibotermin alfa/ACS is supported by three meta-analyses of randomized controlled trials (RCTs), offering clinical outcomes similar to those of ICBG and a positive risk-benefit profileCitation13–16. A recent UK health-economic study concluded that the 12 mg pack (with a dose of 4–8 mg delivered) was cost-effective compared with ICBG, with an incremental cost-effectiveness ratio of £13,523 per quality-adjusted life-year (QALY) gainedCitation17.
To date, however, a specific economic analysis of the recently launched 4 mg pack, the dose most frequently used in licensing studies, has not been undertaken. We, therefore, undertook an individual patient data (IPD) meta-analysis of RCTs and single-arm studies for the 4 mg pack and used the results to update the economic model and determine the cost-effectiveness of the dibotermin alfa/ACS 4 mg pack versus ICBG in patients with LDDD undergoing lumbar interbody spine fusion from a United Kingdom payer perspective. The objective was to develop evidence to allow payers and clinicians to make decisions regarding the management of patients with LDDD. This study was reported in accordance with the Consolidated Health Economics Evaluation Reporting Standards (CHEERS)Citation18.
Methods
Economic model description
The model structure and overall modelling approach have previously been described in detailCitation17. Briefly, a two-state Markov structure was developed in Microsoft Excel to calculate costs and QALYs of dibotermin alfa/ACS versus ICBG from a UK National Health Service (NHS) and Personal Social Service perspective over a four-year period with a six-month cycle length and is therefore intended to be applicable to the UK setting. The two health states were used ‘surgical success’ (after one or more surgeries) and ‘surgical failure’. The patient population considered patients who had at least six months of non-operative treatment and who required a lumbar fusion procedure. Operative time and post-operative length of stay in the hospital, a failure-related second surgery and the proportion of patients returning to work were all derived from the IPD meta-analysis and were used to determine the incremental cost in the dibotermin alfa/ACS group (versus ICBG). The main health outcome measure was QALYs (thus capturing both survival time and quality of life), with the improvements in health utility for the first two years based on data collected using the Short-Form-36 (SF-36) and mapped to SF-6D utilitiesCitation19, with mean differences between the treatment groups estimated from the mixed-effects model. For the remaining two years of the model, the utility for patients treated with dibotermin alfa/ACS was assumed to remain constant; the utility difference between dibotermin alfa/ACS and ICBG was assumed to decline to zero over this latter two-year period (‘utility offset’).
Cost analysis included the acquisition of dibotermin alfa/ACS, the cost per day in the hospital and per hour of operating time, repeat surgery cost, and mean salary per six months (societal perspective only). Since the model was built around the mean differences derived from the meta-analysis, the model outputs consisted of the difference in costs and QALYs between the two treatment groups. Thus, the results presented below are the incremental values rather than treatment-specific values. Costs and QALYs were discounted at 3.5% per year, in accordance with recommendations from the National Institute for Health and Care Excellence (NICE)Citation20.
Model inputs
A subset of analyses from the IPD meta-analysis was used as the basis for populating specific model inputs, including failure-related secondary surgery rates, utility weights over time, operative parameters (procedure duration and in-hospital length of stay), and the proportion of patients returning to work (which was used for sensitivity analysis). These parameter values are described in the following sections. Full details of the meta-analytic methods, covering endpoint definitions, included studies and statistical methods, are provided in the Supplement. Briefly, individual patient data were taken from four RCTs and two single-arm studies and meta-analyzed using multi-level, mixed-effects regression models to determine to mean differences (for continuous variables) and odds ratios (for binary variables) for the model inputs described above. All model inputs are shown in and are described in more detail in the following sections.
Table 1. Model input parameters and values.
Failure-related secondary surgery rates
shows the odds ratio for the difference in the proportion of patients requiring failure-related secondary surgery up to 24 months post-surgery, derived from the meta-analysis of included studies. Further details on the underlying number of patients in each treatment group are given in the Supplement (Table S3). Thus, a higher proportion of patients in the ICBG group underwent a second surgery and incurred the corresponding cost.
Quality of life parameters
also provides the mean between-group difference in the change from baseline in mean SF-6D utility, based on the meta-analysis and for several time points during trial follow-up. All utility weights were calculated by mapping the SF-36 to SF-6D utilities using a published algorithmCitation19. Further details of the treatment-specific utility weights are given in the Supplement (Table S4).
Over 24 months, these mean differences led to a QALY gain of 0.0041 (95% CI: −0.049, 0.058) for patients receiving dibotermin alfa/ACS. In the subsequent two years of the model, this QALY gain was assumed to decline to zero in a linear fashion.
Procedural resource use parameters
shows the model inputs relating to procedure duration and post-operative length of hospital stay, each of which was based on the meta-analysis of individual patient data. For each outcome, the mean difference is presented together with the 95% confidence interval and p-value. Patients receiving dibotermin alfa/ACS were therefore modelled to spend less time in the operating room and to have a shorter stay in the hospital. Further details of these parameters are given in Supplement (Table S5).
Return-to-work parameters
Detailed data regarding return-to-work parameters are provided in the Supplement (Table S6).
Unit cost parameters
The unit cost of each element of resource use applied in the model is shown in (with further details provided in Table S7 of the Supplement). The cost of dibotermin alfa/ACS was based on the average selling price (ASP) of the 4 mg pack in the UK per data extracted in June 2020. The cost per hospital bed day (to determine cost savings associated with a reduced length of stay) and the cost of failure-related second surgery were based on NHS Reference CostsCitation21, using a weighted mean of payments for inpatient spinal reconstruction procedures. The cost per hour of an operating theatre was based on data from a study by NHS ImprovementCitation22. Patient mean salary per 6 months was based on employee earnings data from the UK in 2019Citation23. All costs were based on 2019 prices.
Data analyses
The base-case deterministic analysis was performed using a four-year time horizon to calculate mean costs and QALYs in the dibotermin alfa/ACS and ICBG groups. This horizon was considered sufficiently long to capture relevant costs and health outcomes, including secondary surgery and post-recovery quality of life. The incremental cost-effectiveness ratio (ICER) was calculated using the following formula:
A series of one-way sensitivity analyses (OWSA) was then conducted, varying individual input parameters within plausible ranges (or using alternative model settings) to identify inputs whose uncertainty had the most impact on the ICER. Finally, a full probabilistic sensitivity analysis (PSA) was performed to explore the impact on the ICER of the joint uncertainty in all model inputs. Statistical distributions were chosen for each input to be appropriate to the type of data (for example, gamma and beta distributions for cost and probability inputs, respectively). Ten thousand sets of input parameters were sampled with the resulting incremental costs and QALYs used to generate cost-effectiveness scatter plot and acceptability curve. Full details of the distributions used for the PSA are given in the Supplement (Table S8).
Patient and public involvement
Neither patients nor the public was involved in our study. Our analysis simulated a hypothetical cohort of adult patients.
Results
Base-case analysis
shows the incremental mean discounted cost and QALYs per patient over the 4-year horizon. As all clinical and safety parameters were estimated using a mixed-effects approach, the model calculated the incremental QALYs based on the mean between-group differences for each parameter rather than directly calculating total costs and QALYs in each treatment group. Thus, the table reports only the incremental values. Dibotermin alfa/ACS was predicted to dominate ICBG in the deterministic analysis, with cost savings of £192 and a QALY gain of 0.0114 per patient.
Table 2. Deterministic cost-effectiveness results (discounted).
Sensitivity analyses
The results of the OWSA are shown in in the form of a tornado diagram (tabulated results are provided in the Supplement [Table S9]). The parameters whose uncertainty has the most influence on the ICER are shown towards the top of the diagram. To avoid the issue of computing negative ICERs, the results of each scenario are instead presented in the form of the incremental net benefit (INB) Citation24, for which positive and negative values indicate cost-effectiveness and cost-ineffectiveness of dibotermin alfa/ACS, respectively, at a threshold of £20,000 per QALY gained. The solid vertical line represents the INB for the base-case analysis (£420), and the values at the ends of each bar represent the range of values tested for the corresponding parameter. The dotted vertical line indicates an INB of £0 to separate cost-effective and cost-ineffective results.
Figure 1. Tornado diagram of one-way sensitivity analysis results. Abbreviations. ACS, absorbable collagen sponge; ICBG, iliac crest bone graft; QALY, quality-adjusted life-years.
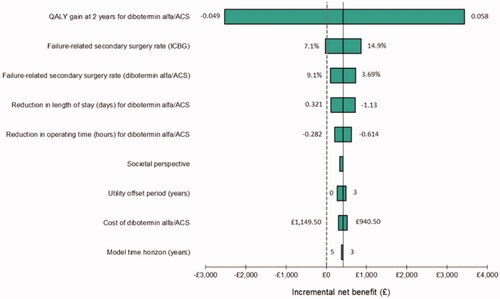
The tornado diagram shows that dibotermin alfa/ACS remains cost-effective (a positive INB, with the horizontal bars positioned to the right of the dashed vertical line) in all but two scenarios, using the confidence interval for each parameter from the meta-analysis as the basis for the range used in each case. When the lower confidence interval value for the QALY gain at 2 years (−0.049) was used, the model predicted that dibotermin alfa/ACS was not cost-effective at a £20,000 per QALY threshold. Similarly, applying the lower limit of the confidence interval around the rate of failure-related secondary surgery on ICBG (7.1%) also led to a scenario in which dibotermin alfa/ACS was marginally cost-ineffective (denoted by an INB below zero).
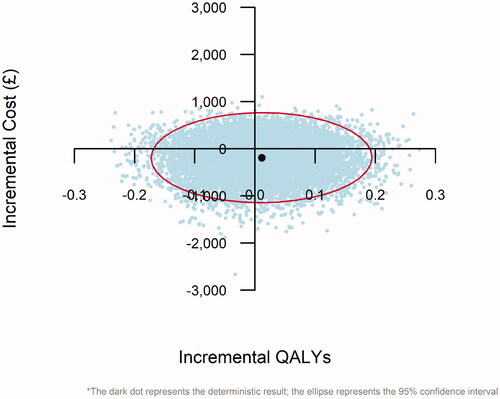
shows the scatter plot derived from the PSA, with incremental QALYs (x-axis), plotted against incremental costs (y-axis). Each light-coloured dot represents the incremental cost and QALYs predicted by one set of sampled input parameter values, while the dark dot denotes the deterministic result described previously. The 95% confidence interval around the ICER is shown via the ellipse. Dibotermin alfa/ACS was predicted to be less costly than ICBG in 66.4% of model replications (the proportion of dots below the x-axis) and more effective (in terms of QALYs) in 55.6% of replications (the proportion of dots to the right of the y-axis).
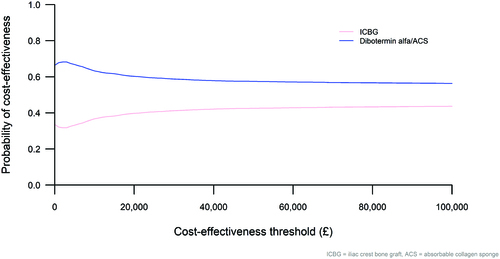
shows the corresponding cost-effectiveness acceptability curve (CEAC), which presents the probability of each intervention being cost-effective across a range of willingness-to-pay thresholds. At the current NICE threshold of £20,000 per QALY gained, the model predicted a 60% chance of dibotermin alfa/ACS being cost-effective compared with ICBG. The dibotermin alfa/ACS line intersects the y-axis at 66.4%, denoting the proportion of replications in which it was predicted to save money, and tends to a value of 55.6% which is the proportion of replications associated with QALY gains.
Discussion
This study sought to update an existing decision-analytic model to determine the cost-effectiveness of the 4 mg dose of dibotermin alfa/ACS compared with ICBG from a UK NHS perspective. Model inputs were updated using a revised meta-analysis of studies evaluating the 4 mg dose, including procedure duration, failure-related secondary surgery rate, QALY gain at 2 years and patient length of stay. The updated cost model-predicted mean cost savings of £192 per patient and a mean QALY gain of 0.0114 for patients on dibotermin alfa/ACS, and a 60% probability of cost-effectiveness at the current willingness-to-pay threshold.
A key strength of our analysis is that it combines data from multiple studies to inform the model inputs, thus providing more robust evidence than relying on data from a single study. The meta-analytic approach allows us to incorporate variation in surgical practice and capture different levels of surgeon skill. The 4 mg dose of dibotermin alfa/ACS is the most commonly used dose in licensing studies and therefore represents the most appropriate formulation to compare against ICBG.
Our analysis has some limitations which warrant discussion. Firstly, the meta-analysis concluded that many of the clinical benefits of dibotermin alfa/ACS were not statistically significant, leading to uncertainty in the model projections. Nevertheless, the deterministic results show cost savings and marginal QALY gains for patients receiving dibotermin alfa/ACS when using the meta-analysis results as calculated, and the probabilistic analysis enables the reader to understand the impact of the uncertainty. Secondly, the model did not explicitly include procedure-only-related adverse events; however, previous studies have reported similar complication rates for the two interventionsCitation25, and many complications are related to surgical technique rather than the product usedCitation26. Thirdly, the model conservatively included only failure-related repeat surgeries, and thus some re-operations (such as those for harvest graft site reasons) were excluded. Finally, the analysis excluded the costs of pre-surgery visits for repeat surgeries; as re-operation was more common in the ICBG group, however, this assumption was conservative from the perspective of dibotermin alfa/ACS.
The most relevant results against which to compare our own are those from Svedbom et al.Citation17, upon which our analysis is based but which did not focus on a particular dose of dibotermin alfa/ACS. The two studies reached similar overall conclusions, with the previous analysis predicting incremental costs and QALYs of £737 and 0.055, respectively, and an ICER of £13,253 per QALY gained. That study performed a meta-analysis covering a broader range of doses and gave an overall estimate of the cost-effectiveness of dibotermin alfa/ACS. As a result of the wider set of studies included and greater sample size, there was more certainty in the meta-analysis outputs and thus in the cost-effectiveness results.
One aspect not accounted for in the model was a surgeon and patient preference. Most surgeons will prefer to use a technique that reduces time, stress and associated morbidity, and the use of ICBG has been reduced due to these factors. Dibotermin alfa/ACS provides a simpler, single procedure that avoids the bone graft procedure and the associated risks and potential for patient morbidityCitation10,Citation11. Further, the procedure time saved by using dibotermin alfa/ACS gives surgeons more available theatre time, allowing them to improve efficiency, and the reduced length of stay may also reduce overall bed capacity.
Further research would be beneficial to have greater certainty around the quality of life benefit of dibotermin alfa/ACS and the rate of failure-related secondary surgery in each group. The model used a relatively short time horizon (4 years), and so long-term data on quality of life and other outcomes would be helpful to confirm that the cost-effectiveness profile does not change with a longer time horizon.
Conclusions
In summary, this analysis indicates that the 4 mg dose of dibotermin alfa/ACS is cost-effective compared with ICBG and supports its wider uptake in spinal fusion procedures, consistent with previous research.
Transparency
Declaration of funding
This study was funded by Medtronic International Trading Sàrl.
Declaration of financial/other interests
MZ, AW and SE are employees of Medtronic and hold stock in Medtronic. DC, FS and RT are all paid consultants for Medtronic. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
DC, RT, FS and MZ led the study. FS undertook the meta-analysis of clinical studies, with input from DC, RT, AW and MZ. MZ and SE populated and ran the decision-analytic model, with methods and data informed by DC and RT. SE drafted the manuscript, and all authors reviewed and provided input. All authors approved the submitted version of the manuscript and agree to be accountable for all aspects of the work.
Supplemental Material
Download PDF (366.5 KB)Acknowledgements
The authors thank Farai Goromonzi for his assistance in sourcing UK cost data for the model and for validating data and assumptions.
References
- Ravindra VM, Senglaub SS, Rattani A, et al. Degenerative lumbar spine disease: estimating global incidence and worldwide volume. Global Spine J. 2018;8(8):784–794.
- Takatalo J, Karppinen J, Niinimäki J, et al. Prevalence of degenerative imaging findings in lumbar magnetic resonance imaging among young adults. Spine. 2009;34(16):1716–1721.
- Cheung KMC, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine. 2009;34(9):934–940.
- Pahl MA, Brislin B, Boden S, et al. The impact of four common lumbar spine diagnoses upon overall health status. Spine J. 2006;6(2):125–130.
- Katz J. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Jt Surg. 2006;88(suppl_2):21–24.
- Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84(1):95–103.
- Phillips FM, Slosar PJ, Youssef JA, et al. Lumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic review. Spine. 2013;38(7):E409–422.
- Awad J, Moskovich R. Lumbar disc herniations: surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443:183–197.
- Lee YC, Zotti MGT, Osti OL. Operative management of lumbar degenerative disc disease. Asian Spine J. 2016;10(4):801–819.
- Dimitriou R, Mataliotakis GI, Angoules AG, et al. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(Suppl. 2):S3–S15.
- Tuchman A, Brodke DS, Youssef JA, et al. Iliac crest bone graft versus local autograft or allograft for lumbar spinal fusion: a systematic review. Global Spine J. 2016;6(6):592–606.
- European Public Assessment Report for InductOs: EPAR summary for the public. European Medicines Agency, 2015 [cited 2021 Oct 31]. Available from: https://www.ema.europa.eu/en/documents/overview/inductos-epar-summary-public_en.pdf
- Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25(3):376–381.
- Noshchenko A, Lindley EM, Burger EL, et al. What is the clinical relevance of radiographic nonunion after single-level lumbar interbody arthrodesis in degenerative disc disease?: A meta-analysis of the YODA project database. Spine. 2016;41(1):9–17.
- Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15(5):337–349.
- Haid RW, Branch CL, Alexander JT, et al. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527–538.
- Svedbom A, Paech D, Leonard C, et al. Is dibotermin alfa a cost-effective substitute for autologous iliac crest bone graft in single level lumbar interbody spine fusion? Curr Med Res Opin. 2015;31(11):2145–2156.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health. 2013;16(2):e1–e5.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. London, UK; 2013.
- NHS England. National Schedule of Reference Costs, 2017-2018; 2018. p. 1–16. [cited Aug 2020]. Available from: https://improvement.nhs.uk/resources/reference-costs/
- NHS Improvement. Operating theatres: opportunities to reduce waiting lists; 2019. (February):1–38. [cited Aug 2020]. Available from: https://improvement.nhs.uk/documents/3711/Theatre_productivity_report__Final.pdf
- Office for National Statistics. Employee earnings in the UK: 2019. 2019;(October):1–19. [cited Aug 2020]. Available from: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2019#employee-earnings-data
- Paulden M. Calculating and interpreting ICERs and net benefit. Pharmacoeconomics. 2020;38(8):785–807.
- Bodalia PN, Balaji V, Kaila R, et al. Effectiveness and safety of recombinant human bone morphogenetic protein-2 for adults with lumbar spine pseudarthrosis following spinal fusion surgery: a systematic review. Bone Joint Res. 2016;5(4):145–152.
- Crandall DG, Revella J, Patterson J, et al. Transforaminal lumbar interbody fusion with rhBMP-2 in spinal deformity, spondylolisthesis, and degenerative disease-part 2: BMP dosage-related complications and long-term outcomes in 509 patients. Spine. 2013;38(13):1137–1145.