Abstract
Aims
Chemotherapy-induced myelosuppression, which commonly exhibits as neutropenia, anemia, or thrombocytopenia, represents a substantial burden for patients with cancer that affects health-related quality of life and increases healthcare resource utilization (HCRU). We evaluated the burden of myelosuppression among chemotherapy-treated patients with small cell lung cancer (SCLC) using real-world data from community cancer care providers in the Western United States.
Materials and methods
This was a retrospective, observational analysis of electronic medical records (EMRs) from Providence St. Joseph Health hospital-associated oncology clinics between January 2016 and December 2019. Patient demographics were assessed from the date of first SCLC diagnosis in adult patients with chemotherapy-induced grade ≥3 myelosuppression in first-line (1L) or second-line-and-beyond (2L+) treatment settings. Myelosuppressive adverse events (AEs), treatment patterns, and HCRU were assessed from the date of chemotherapy initiation (index date) until 12 months, date of the last visit, date of death, or study end, whichever occurred earliest.
Results
Of 347 eligible patients with SCLC who had received chemotherapy (mean age 66; 49% female), all had received at least 1L treatment, and 103 (29.7%) had a 2L + treatment recorded within the EMR during the study period. Of 338 evaluable patients with longitudinal laboratory data, 206 (60.9%) experienced grade ≥3 myelosuppressive AEs, most commonly neutropenia, anemia, and thrombocytopenia (44.9, 41.1, and 25.4 per 100 patients, respectively). Rates of granulocyte colony-stimulating factor use and red blood cell transfusions were 47.0 and 41.7 per 100 patients, respectively. There was a trend toward increasing the use of supportive care interventions and visits to inpatient and outpatient facilities in patients with myelosuppressive AEs in more than one cell lineage.
Conclusions
Chemotherapy-induced myelosuppression places a substantial real-world burden on patients with SCLC in the community cancer care setting. Innovations to protect bone marrow from chemotherapy-induced damage have the potential to reduce this burden.
PLAIN LANGUAGE SUMMARY
This study looked at the medical records of people with a particular type of lung cancer known as small cell lung cancer. When treated with chemotherapy, people with this cancer may develop a condition called myelosuppression. This causes people to have fewer blood cells, which can lead to tiredness, or increase the risk of infection or bleeding. The study looked at what types of chemotherapy people with small cell lung cancer were given, what the side effects of myelosuppression were, how often the side effects were reported, and what treatments were given to manage these side effects. The study also looked at whether people with side effects from myelosuppression needed more visits to the doctor or hospital. Around 3 out of 5 people in the study experienced serious side effects resulting in reduced numbers of white blood cells (which fight infection), red blood cells (which carry oxygen), or platelets (which help the blood to clot), and many needed drugs or blood transfusions to treat these side effects. On average, people with side effects from myelosuppression had more visits to healthcare facilities than those people without these side effects. The findings suggest that myelosuppression places a large burden on people with small cell lung cancer who are treated with chemotherapy.
Introduction
Small cell lung cancer (SCLC) accounts for ∼13% of all lung cancer cases in the United States, with most patients diagnosed at an advanced stageCitation1,Citation2. Prognosis is poor, with a 5-year survival rate of 6%, decreasing to 3% among patients with distant metastasisCitation1.
Unlike other solid tumor types, for which various hormonal and molecular targeted therapies have been developed, very few advances have been made in the treatment of SCLC, and chemotherapy remains a major component of treatment for both limited-stage (LS-) and extensive-stage (ES-) diseaseCitation3. In the United States, systemic chemotherapy agents commonly used for the treatment of patients with SCLC include cisplatin and carboplatin (platinum agents), etoposide, irinotecan, paclitaxel, and topotecanCitation3,Citation4. Until recently, the first-line (1L) standard treatment for SCLC (including LS- and ES-SCLC) in the United States has been etoposide plus platinum, with a preference for carboplatin over cisplatin owing to its comparable efficacy and favorable toxicity profileCitation3,Citation4. In March 2019, combination therapy with the immune checkpoint inhibitor atezolizumab plus etoposide and carboplatin was approved by the United States Food and Drug Administration (FDA) for 1L treatment of ES-SCLCCitation5,Citation6. In March 2020, the combination of durvalumab plus etoposide and cisplatin or carboplatin was additionally approved for this indicationCitation7. These recently approved immunotherapies in combination with platinum plus etoposide chemotherapy regimens are recommended (category 1) for 1L systemic treatment of ES-SCLC in the National Comprehensive Cancer Network® Clinical Practice Guidelines in Oncology (NCCN Guidelines®)Citation3. For the past 20 years, topotecan has been the only preferred regimen for subsequent systemic therapyCitation3; however, in June 2020, lurbinectedin was approved by the FDA for second-line (2L) systemic treatment of SCLC after the failure of platinum-based therapyCitation8.
Although effective in prolonging survival, chemotherapy (and chemotherapy plus immunotherapy combination) regimens for SCLC present a treatment challenge due to the resulting damage to hematopoietic stem and progenitor cells in the bone marrow. In turn, this causes clinically significant, multilineage myelosuppression that manifests as a range of cytopenias (including anemia, neutropenia, and thrombocytopenia)Citation9. The burden associated with chemotherapy-induced myelosuppression for patients with cancer is substantial, contributing to increased fatigue, time spent receiving additional treatment for myelosuppressive adverse events (AEs), and reduced health-related quality of lifeCitation10,Citation11. Additionally, hematologic toxicities may potentially lead to poor treatment outcomes related to dose reductions (e.g. shorter duration of response, earlier disease recurrence), treatment delays, and treatment discontinuationCitation12–14. Serious and life-threatening complications, such as infections and bleeding complications from neutropenia and thrombocytopenia, respectively, can also occurCitation9,Citation12,Citation13. Patients with SCLC are often older and have comorbid conditions, which may further impact their prognosis and tolerance of cancer treatmentsCitation15,Citation16.
In addition to dose modifications, current supportive interventions recommended in clinical practice guidelines to manage myelosuppression include granulocyte colony-stimulating factor (G-CSF) agents and red blood cell (RBC) transfusions; erythropoiesis-stimulating agents (ESAs) are used less frequentlyCitation3,Citation12,Citation17. Management strategies include primary prophylaxis, such as administration of G-CSF to patients at risk of developing neutropenia, or secondary prophylaxis to treat occurrences of myelosuppressionCitation12,Citation17–20.
Myelosuppression has been associated with higher healthcare resource utilization (HCRU), particularly hospitalizations, and higher healthcare-related costsCitation13,Citation21–23. However, most previous studies have included patients with cancers across multiple tumor sites (e.g. breast, lung, or colon) and there are limited data specific to SCLC, despite treatments for this diagnosis being particularly notable for their degree of myelotoxicity. In addition, previous studies used data from insurance claims or national inpatient databases. Although it is estimated that up to 65% of patients with cancer are treated at community cancer centersCitation24–26, there are very few data on the real-world burden of myelosuppression among chemotherapy-treated patients with SCLC in this setting. For these reasons, we conducted a study to describe the burden of myelosuppression in patients with SCLC using data from electronic medical records (EMRs) from a community cancer care provider network in the Western United States.
The study objectives were to describe the incidence of myelosuppressive AEs and associated treatment patterns among patients diagnosed with SCLC and to characterize HCRU associated with myelosuppression among patients receiving chemotherapy. A better understanding of the incidence of myelosuppression and associated treatment patterns and HCRU may help clinicians to better design and use treatment regimens that maximize patient benefit and minimize potential damage to healthy cells.
Methods
Data source
This retrospective observational study utilized EMR data from the Providence St. Joseph Health (PSJH primary EMR [Epic Systems, Inc.]) and the Providence Cancer Reporting Registry. PSJH is the third-largest non-profit health system in the United States, formed by the merger of St Joseph Health of Irvine, California, and Providence Health and Services of Renton, Washington in 2016Citation27. The dataset was obtained from 40 oncology clinics associated with community hospitals across seven states in the United States. Data curation and analysis were performed by members of the PSJH Health Insights analytics group in Renton, Washington.
This study was approved by the PSJH institutional review board (IRB 2019000565). Only minimally required protected health information was accessed for this retrospective study, and study databases and analyses utilized anonymized data. As such, the IRB waived the requirement for informed consent. All investigators and research staff were trained in compliance and data-handling practices, and no protected health information is included in this publication.
Patient selection and study design
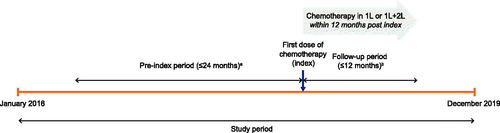
Adult patients with SCLC considered for the analysis were identified by ≥1 clinical encounter with a code for SCLC (International Classification of Diseases, Tenth Revision, Clinical Modification C34*, and International Classification of Diseases for Oncology histology between 8041 and 8045) between January 2016 and December 2019 in the PSJH primary EMR. Patients were required to have received chemotherapy in 1L or both 1L and 2L-and-beyond (2L+) treatment settings between January 2016 and December 2018. Patients with prior stem cell transplants or preexisting disorders of the bone marrow were excluded. The date of the first chemotherapy dose was considered the index date. Myelosuppressive AEs, treatment patterns, and HCRU were assessed for the follow-up period of 12 months from the index date, or until the date of the last visit, date of death, or the end of the study period (December 2019), whichever occurred earliest ().
Figure 1. Study design. aThe pre-index period was the period from study start (January 2016) to index, or the 24-month period prior to index, whichever was shorter. bPatients were followed for 12 months post-index date, or until death, loss to follow-up, or end of the study period (December 2019), whichever occurred sooner. 1L: first line; 2L: second line; SCLC: small cell lung cancer.
Study measures
The date of the first diagnosis of SCLC was used to assess patient demographics and other clinical characteristics. Patients’ baseline characteristics and Charlson-definedCitation28 comorbidities were evaluated during a pre-index period of 24 months before the index date, or between study start (January 2016) and index, whichever was shorter. Patient baseline characteristics included age, sex, race, smoking history, Eastern Cooperative Oncology Group performance status, radiation treatment, and payer type. For the analysis of treatment patterns, percentages of patients receiving 1L and/or 2L + treatment were reported.
Myelosuppressive AEs were identified based on laboratory values from EMR data according to the Common Toxicity Criteria definition of grade 3 or above AEsCitation29. Myelosuppressive AEs were defined as follows: anemia, hemoglobin <8.0 g/dL; neutropenia, absolute neutrophil count <1,000 mm3; and thrombocytopenia, platelet count <50,000 mm3. Patients could have experienced multiple AEs depending on whether their laboratory values fell within the defined parameters. Time to myelosuppressive AE was reported. Treatment of myelosuppressive AEs were evaluated in terms of transfusions (RBC or platelet), G-CSF administration, and ESA use. Both prophylactic (received before documented low absolute neutrophil count) and therapeutic (received after documented low absolute neutrophil count) administration of G-CSF were reported.
For the analysis of myelosuppressive AE–related HCRU, patients were stratified into four separate groups according to the number of grades ≥3 myelosuppressive AEs by lineage (i.e. neutropenia, anemia, and/or thrombocytopenia) that they experienced (i.e. no grade ≥3 AEs, grade ≥3 AE in one lineage, grade ≥3 AEs in two lineages, or grade ≥3 AEs across all three lineages). Patients who had more than one episode of the same lineage event were counted only once when reporting the percentage of patients with that type of AE. Healthcare resources assessed were outpatient visits (as recorded within the EMR), emergency department (ED) visits, inpatient visits (including inpatient service, and patients who were treated in the ED and then admitted for inpatient services), and admissions to an intensive care unit. Chemotherapy and supportive care regimens administered at PSJH were also reported.
Statistical analyses
Descriptive statistics were used to describe patient characteristics and outcomes. Continuous variables were summarized with means and standard deviations, and median and range values. Frequency counts and the percentage of patients within each category were reported for categorical variables. For the rates of myelosuppressive AEs, time to AE, and treatment of myelosuppressive AEs (transfusions, G-CSF administration, and ESA use), stratified analyses were conducted by the line of therapy (1L: AEs that occurred after initiation of 1L therapy and before 2L therapy start date; 2L: AEs that occurred after initiation of 2L therapy).
Results
Patient characteristics
A total of 347 patients diagnosed with SCLC who had received chemotherapy were eligible for the analysis. Patients’ baseline demographic and clinical characteristics are shown in . The median (range) age of patients was 65 (35–93) years, 48.7% were female, and 88.8% were White. Almost two-thirds of patients (61.1%) presented with stage IV (ES) disease at diagnosis. Overall, 36.9% were reported as current smokers and 26.5% as past smokers; 6.1% were reported as having never smoked, and 30.5% as not documented or not asked. Among patients with documented Charlson comorbid conditionsCitation28 (n = 338), the most prevalent conditions (present in >10% of patients) included chronic obstructive pulmonary disease (51.8%), diabetes (23.4%), and peripheral vascular disease (19.5%). At baseline, 42.1% of patients had received radiation therapy. During follow-up, 226 patients out of 338 patients with longitudinal laboratory data (66.9%) died.
Table 1. Patient baseline demographic and clinical characteristics.
Treatment patterns
Among study patients (N = 347), all had received at least 1L treatment, and 29.7% (n = 103) had a documented 2L + treatment recorded within the EMR during the study period. Over 70% of patients received platinum plus etoposide as 1L treatment (carboplatin plus etoposide: n = 192, 55.3%; cisplatin plus etoposide: n = 67, 19.3%). Overall, 7.5% (n = 26) of patients received an immune checkpoint inhibitor (atezolizumab [n = 19], nivolumab [n = 4], pembrolizumab [n = 2], or durvalumab [n = 1]) as part of their 1L treatment regimen (including treatments received as part of a clinical trial). The most common 2L + treatment regimens were topotecan (n = 21 of 103, 20.4%), combination therapy with ipilimumab plus nivolumab or pembrolizumab (n = 20 of 103, 19.4%), carboplatin (alone or in combination with irinotecan or etoposide; n = 15 of 103, 14.6%), and paclitaxel (n = 11 of 103, 10.7%). Immune checkpoint inhibitors were prescribed sparingly by PSJH clinicians in this study since the study cut-off date was December 2019 and FDA approvals for the use of these treatments in 1L were only granted in 2019 and 2020.
Myelosuppressive AEs and treatments
Among evaluable patients with longitudinal laboratory data (n = 338), 206 (60.9%) had at least one grade ≥3 myelosuppressive AE during the follow-up. Grade ≥3 neutropenia (44.9 events per 100 patients) and anemia (41.1 events per 100 patients) were the two most frequently observed AEs, followed by grade ≥3 thrombocytopenia (25.4 events per 100 patients; ). Similar results were observed in the analysis by the line of therapy. Rates of grade ≥3 myelosuppressive AEs (events per 100 patients) were 33.4 during 1L treatment (24.5 during 2L treatment) for neutropenia, 29.9 (21.6) for anemia, and 18.3 (15.7) for thrombocytopenia. Baseline demography and clinical presentation did not appear to predispose patients to have one or more types of grade ≥3 myelosuppressive AE (neutropenia, anemia, and/or thrombocytopenia; ).
Table 2. Grade ≥3 myelosuppressive AEs and treatments.
Table 3. Baseline characteristics among patients with no grade ≥3 myelosuppressive AEs and in patients with grade ≥3 myelosuppressive AEs in one, two, or three lineages.
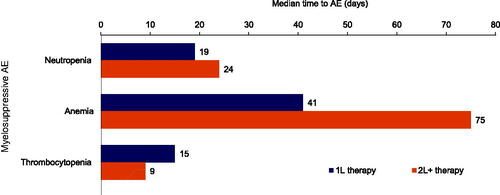
The median number of days from 1L therapy to myelosuppressive AE was shortest for thrombocytopenia (15 days) and neutropenia (19 days), and longest for anemia (41 days) (). A similar pattern was observed for the median number of days from 2L + therapy to myelosuppressive AEs, with time to thrombocytopenia being the shortest (9 days) and time to anemia the longest (75 days).
Figure 2. Median time from chemotherapy/immunotherapy to myelosuppressive AEs (N = 338). 1L: first-line; 2L+: second-line and beyond; AE: adverse event.
The most frequently used treatment for myelosuppressive AEs across all patients was G-CSF, with an incidence of 47.0 per 100 patients (1.7 prophylactic and 45.6 therapeutic; ). The rate of G-CSF use during 1L (40.5 per 100 patients; 1.7 prophylactic and 40.2 therapeutic) was higher than during 2L (17.6 per 100 patients; 0 prophylactic and 17.6 therapeutic). RBC transfusion was the second-most frequently used treatment, with an incidence of 41.7 per 100 patients (1L: 31.4; 2L: 41.2). The incidence of platelet transfusion was 13.3 per 100 patients (1L: 9.2; 2L: 11.8). The use of ESAs was low, at 2.0 per 100 patients (1L: 2.0; 2L: 1.9). There was a trend toward increased use of supportive care interventions among patients with AEs in more than one lineage (). For example, 25% of patients with no myelosuppressive AEs received G-CSF, vs. 54, 66, and 67% of patients with AEs across one, two, and three lineages, respectively.
Table 4. Supportive care treatments among patients with no grade ≥3 myelosuppressive AEs and in patients with grade ≥3 myelosuppressive AEs in one, two, or three lineages.
HCRU
Percentages of patients with inpatient, outpatient, ED, and intensive-care-unit visits and numbers of each type of visit in the 12 months following chemotherapy initiation in patients with grade ≥3 myelosuppressive AEs in none, one, two, or three lineages are shown in . Among patients with SCLC who had received chemotherapy and were included in the analysis (n = 338), there was a trend toward increasing resource use with multilineage myelosuppression. The hospitalization rate among patients with no myelosuppressive AEs, for example, was 60.6%, vs. 63.3, 85.2, and 85.5% for patients with myelosuppressive AEs in one, two, and three lineages, respectively ().
Table 5. Healthcare resource utilization among SCLC patients within 12 months of chemotherapy initiation.
Subgroup analysis of myelosuppressive AEs
The number of patients with grade ≥3 myelosuppressive AEs was stratified by G-CSF use (prophylactic and therapeutic), disease stage, prior radiation, and index chemotherapy (). Among patients receiving G-CSF, 59.6% had neutropenia, 52.5% had anemia, and 36.9% had thrombocytopenia. Among the most common index chemotherapy regimens, etoposide plus cisplatin was associated with the highest rates of neutropenia and anemia.
Table 6. Incidence of grade ≥3 myelosuppressive AEs, stratified by G-CSF use, disease stage, prior radiation, and index chemotherapy.
Discussion
This real-world retrospective EMR study evaluated the burden of myelosuppression among patients diagnosed with SCLC in US clinical practice. To our knowledge, this was the first study to evaluate multilineage myelosuppression in SCLC in the real-world community cancer care setting using EMR data, with prior studies focusing on patients with cancers across different tumor sites (e.g. breast, lung, or colon) and using other data sources (e.g. insurance claims or national inpatient databases). Our findings revealed that almost two-thirds of patients experienced grade ≥3 myelosuppressive AEs, most commonly neutropenia and anemia, during the study period. Rates of events were similar between patients receiving 2L + therapy and those receiving 1L, with the most common event being neutropenia in both patient groups. Baseline characteristics were consistent across patient subgroups with no grade ≥3 myelosuppressive AEs and grade ≥3 myelosuppressive AEs in one, two, or three lineages, and there was no clear pattern in AE rates according to disease stage, suggesting that multilineage myelosuppression should be of equal concern for patients with LS- and ES-SCLC being treated with chemotherapy.
Although published incidence rates of myelosuppression in patients with SCLC vary owing to factors, such as patient population characteristics, study design, treatment regimens, AE definitions, and methods of reporting (e.g. event rate vs. percentage of patients), the findings in this study are generally in line with those of the refereed literature. Neutropenia was the most common grade ≥3 chemotherapy-induced cytopenia observed in this study, experienced at a rate of 44.9 events per 100 patients across all lines of therapy. In clinical trials of treatments for ES-SCLC, the percentage of patients with grade 3/4 neutropenia has ranged from 22.7% for atezolizumab plus etoposide and carboplatinCitation5, and 24.5%Citation5, and 33.0%Citation30 for etoposide plus carboplatin, to higher rates of 44.0–86.5%Citation31–34 for etoposide plus cisplatin. An observational study by Igawa et al. reported a rate of chemotherapy-induced neutropenia of 40.0% with etoposide plus carboplatin in their patient populationCitation35, which is broadly comparable with the current study.
As the second-most common grade ≥3 myelosuppressive AE, chemotherapy-induced anemia was observed at a rate of 41.1 events per 100 patients in this study. Several clinical trials of therapies for ES-SCLC (e.g. atezolizumab plus etoposide and carboplatin, etoposide plus carboplatin, and etoposide plus cisplatin) have reported rates of grade ≥3 anemia of <20%Citation5,Citation31,Citation32,Citation34,Citation36. This apparent disparity may be due to differences in study methodologies and differences in patient populations between clinical trials and real-world practice; for example, the inclusion of patients who would typically be excluded from Phase 3 drug trials, methodological differences due to heterogeneity in AE definitions and severity gradingCitation10, or the utilization of laboratory data to evaluate grade ≥3 myelosuppressive AEs rather than investigator assessment. Observational studies have generally observed higher prevalence rates of anemia than in clinical trials. For example, in the 2001 European Cancer Anemia SurveyCitation37—a large, prospective, observational, epidemiologic survey that assessed the prevalence, incidence, and treatment of anemia in 24 countries—the prevalence of anemia (defined as hemoglobin <12 g/dL) was reported as 83% among patients with lung cancer who were receiving chemotherapy; however, that study included anemia that is less severe than grade ≥3, and the prevalence of severe anemia is likely to be lower.
It is notable that 42.1% of patients in the current study had received radiation therapy since previous studies have shown that radiotherapy is associated with bone marrow suppression and contributes to severe myelosuppression in patients with SCLC receiving chemotherapyCitation38,Citation39. Subgroup analysis indicated that patients who received radiation had higher rates of grade ≥3 myelosuppressive AEs (particularly neutropenia and anemia) compared with those without/with unknown radiation, suggesting that prior receipt of radiation therapy may have contributed to some of the myelosuppressive AEs reported in this study.
High usage of supportive therapies to manage myelosuppression was reported in this study, including 41.7 RBC transfusions and 47.0 G-CSF regimens per 100 patients. Notably, patients who received G-CSF also had high rates of grade ≥3 anemia and thrombocytopenia, highlighting the large multilineage burden of myelosuppression among these patients. The trend toward increasing use of supportive care interventions, as well as increasing numbers and frequencies of visits to healthcare facilities as the number of myelosuppressive AEs in each lineage increased underscores the substantial real-world burden that multilineage myelosuppression places on the healthcare system.
Although not directly comparable due to differences in methodology and reporting, the rate of RBC transfusion appears high in relation to the proportion of patients with RBC transfusions reported in some other studies. For example, in a recent clinical trial among patients with ES-SCLC, 24% received RBC transfusionsCitation40. Similarly, in the European Cancer Anemia Survey observational studyCitation37, only 18% of anemic patients with lung cancer undergoing chemotherapy received RBC transfusions, and 53% received no treatment for anemia. Importantly, the high rate of transfusions observed in the current real-world study would have a significant impact on the patient burden, given the time and multiple visits required to complete blood testing and the RBC transfusion procedureCitation11.
United States guidelines recommend primary G-CSF prophylaxis for chemotherapy regimens that carry a >20% risk of FN, while patient-specific risk factors (e.g. age >65 years, comorbidities) should be considered for those receiving intermediate (10–20%) risk regimensCitation41. Prophylactic use of G-CSF was observed in 1.7% of patients in this study, which is lower than what might be expected based on the guidelines for primary G-CSF prophylaxis, given the patient population. In this study, most patients received either carboplatin or cisplatin in combination with etoposide as first-line treatment. Almost a third (29.7%) of patients received second-line treatment, with topotecan being the most common (given to ∼20% of patients who received a second-line treatment). Among these treatments, only topotecan is considered to present a high (>20%) risk for FNCitation41. The median age in this study was 65 years, indicating that only half of the patients might be considered to have a supervening risk factor for the development of FN with intermediate-risk chemotherapies based on age. It is also possible that what would be considered as secondary prophylaxis according to guidelines (administration of G-CSF before second and subsequent chemotherapy cycles in patients with a history of febrile or dose-limiting neutropenia)Citation41 was classed as therapeutic G-SCF in this analysis, contributing to the lower-than-expected use of prophylactic G-CSF. Of note, some cost-effectiveness models do not support G-CSF use for primary or secondary prophylaxis for patients with SCLC, which may be prohibitive to prescribing G-CSF in some settingsCitation42–44. Indeed, G-CSF utilization has been shown to vary in clinical practice, with trends suggesting potential underutilization in high-risk patients and overutilization in lower-risk patientsCitation45. This divergence in the use of G-CSF could be problematic, with underutilization resulting in adverse outcomes, and overuse of ineffective/unnecessary treatment having a substantial impact on costs and resource useCitation46. On the other hand, the MONITOR-GCSF study in patients receiving biosimilar filgrastim identified patterns of G-CSF prophylaxis above guideline recommendations and noted that this “over-prophylaxis” was associated with better outcomes among patients with chemotherapy-induced neutropenia and FNCitation18,Citation47,Citation48.
The risk of myelosuppression in patients receiving chemotherapy must be considered alongside the potential benefits of cytotoxic treatment for SCLC. If left untreated, ES-SCLC is usually fatal within 2–5 monthsCitation49. By comparison, median overall survival with 1L platinum plus etoposide is ∼8–10 months, and this further increases to ∼12–13 months with the addition of an immune checkpoint inhibitor (atezolizumab or durvalumab)Citation5,Citation30–34,Citation50,Citation51. Survival times with 2L topotecan treatment in patients with relapsed SCLC range from ∼6 to 8 months, with one study reporting a survival improvement of ∼3 months with the addition of oral topotecan to best supportive careCitation52–54. In addition to prolonging survival, chemotherapy treatment may also provide symptom control, thereby improving quality of lifeCitation52,Citation55. As such, the clinical benefit of cytotoxic chemotherapy may outweigh the risk of toxicity for many patients with SCLC. Nonetheless, the potential impact of myelosuppression on treatment outcomes must also be deliberated, since hematologic AEs commonly lead to dose delays, dose reductions, and reductions in relative dose intensity, the latter of which is significantly associated with decreased survival outcomes and quality of life in certain cancer typesCitation12–14,Citation56. Furthermore, data suggest that patients with lung cancer and febrile neutropenia have higher mortality than those without febrile neutropenia (incidence per 100 person-months: 44.3 vs. 29.6, respectively)Citation57.
Results from this study highlight that myelosuppression places a significant real-world burden on patients with SCLC and the healthcare system. The burden of myelosuppression shown in this study supports the need for innovation in a discipline where current mitigation approaches (G-CSF and ESA) were first approved over 30 years ago. Fortuitously, several newer agents are under clinical investigation for the treatment of single- or multilineage myelosuppression in various cancer types, including plinabulin (Phase 3), ALRN-6924 (Phase 1/2), roxadustat (Phase 2), romiplostim (Phase 3), and avatrombopag (Phase 3)Citation58. Of course, further assessment of the benefit: risk ratio of these investigational agents in controlled clinical evaluations is needed before any conclusions can be drawn regarding their value in the prevention/mitigation of chemotherapy-induced myelosuppression. In March 2021, trilaciclib, an intravenous cyclin-dependent kinase 4/6 inhibitor, was approved by the US FDA to decrease the incidence of chemotherapy-induced myelosuppression in patients with ES-SCLC when administered before a platinum/etoposide- or topotecan-containing regimenCitation59. In three randomized, double-blind, placebo-controlled Phase 2 trials, the addition of trilaciclib before chemotherapy resulted in clinically meaningful reductions in multilineage myelosuppression, a reduced need for supportive care interventions, and dose reductions, and an improved safety profileCitation40,Citation60,Citation61. Ultimately, it is hoped that these newer approaches may help to overcome some of the limitations of G-CSF and ESAs.
A key strength of this study is that grade ≥3 myelosuppressive AEs were reported based on laboratory values rather than relying on physician reports in medical charts or claims data, therefore increasing the reliability of the dataset compared with other commonly utilized real-world data sources. The dataset also reflects a broad oncology setting from multiple hospital-based oncology clinics across seven states and thus is likely to provide a good representation of the US SCLC patient population. Although the current analysis was of a static dataset from a defined period (January 2016 to December 2019), the dynamic nature of real-world data sources, such as EMRs, which can be used to collect data almost continuously, is advantageous given that disease populations, clinical practice patterns, and healthcare systems are continually evolving. The use of EMRs for dynamic evaluation of a large variety and volume of clinical data may also facilitate the prediction of future healthcare trends, including in the burden of myelosuppression and its management.
This study possesses some limitations, yet it also spotlights important areas of future research. Our analysis focused on grade ≥3 myelosuppressive AEs only; however, patients reported as having no (grade ≥3) myelosuppressive AEs or myelosuppressive AEs in a particular lineage (e.g. neutropenia only) may have also experienced grade 1 or 2 AEs in other blood cell lineages. This is notable as lower-grade AEs may also require supportive care interventions, such as transfusions in some cases. Some outpatient infusions and transfusions may not have been captured if they were conducted in clinics not included in this analysis or not recorded in the EMR. Therefore, possibly, HCRU associated with myelosuppression may have been underestimated slightly in this study. Of interest for further study would be the rates of myelosuppressive events associated with the constellation of chemotherapy regimens that are preferred or acknowledged in published clinical guidelines. Lastly, a follow-on study that examines myelosuppressive AEs in ES-SCLC specifically would be especially useful. The NCCN Guidelines® now recommend that the preferred regimen for primary treatment of ES-SCLC includes a checkpoint inhibitor (atezolizumab or durvalumab) in combination with etoposide and platinum chemotherapy for 4–6 treatment cyclesCitation3. Considering nearly two-thirds of patients with SCLC have the extensive-stage disease at diagnosisCitation2, the findings of the present study in SCLC should provide clinicians with important insights regarding preventing and managing myelosuppressive AEs in patients with ES-SCLC.
Conclusions
In this real-world study, a large and meaningful proportion of patients with SCLC experienced grade ≥3 hematologic toxicity, which was associated with a substantial increase in HCRU. Multilineage myelosuppression places an ever greater real-world burden on patients and the healthcare system in a community cancer care setting. Innovative or improved management strategies, which may include trilaciclib and other novel agents for the treatment of one or more cytopenias, have the potential to address this burden.
Transparency
Declaration of funding
This study was funded by G1 Therapeutics, Inc. The study sponsor provided support in the form of salary for TS at the time of study and consultancy fees to RSE, RKW, ASP, and JK, and was involved in the study design, collection, analysis, and interpretation of data, writing of the report, and the decision to submit the report for publication. Epstein Health LLC. and Providence Health & Services provided support in the form of salaries for RSE and JK, and RKW and ASP, respectively, but did not have any additional role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Declaration of financial/other relationships
RE is an employee of Epstein Health, LLC and has received research funding and consultancy fees from G1 Therapeutics, Inc. Outside of the submitted work, RE is a board member for Fate Therapeutics, Illumina, and Veracyte, and has received consultancy fees from Halozyme, Intracellular Therapies, Merck, Otsuka, Radius Health, and Taiho Oncology. RKW is an employee of Providence Health & Services, and has received consultancy fees from G1 Therapeutics, Inc. ASP is an employee of Providence Health & Services, and has received consultancy fees from G1 Therapeutics, Inc. Outside of the submitted work, ASP owns stock in IQVIA. JK is an employee of Epstein Health, LLC and has received consultancy fees from G1 Therapeutics, Inc. Outside of the submitted work, JK owns stock in Cigna, Express Scripts, and Medco Health Solutions. Outside of the submitted work, RS has received honoraria from Amgen and AstraZeneca, consultancy fees from Amgen, AstraZeneca, AbbVie, Ariad, Celldex, EMD Serono, Genentech-Roche, Peregrine Pharmaceuticals, Seattle Genetics, and Takeda, expenses from Five Prime Therapeutics and Janssen Oncology, and institutional research grants from Bristol-Myers Squibb, MedImmune, and Merck. TS was an employee of G1 Therapeutics, Inc., at the time of study completion, and is currently a paid employee of Taiho Oncology, Inc.
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Authors contributions
All authors were involved in the study conception and design and/or analysis and interpretation of the data, drafting the paper and/or revising it critically for intellectual content, approved the final version to be published, and agree to be accountable for all aspects of the work.
This work was presented at the American Society of Clinical Oncology 2020 congress and published in the abstract supplement (DOI: 10.1200/JCO.2020.38.15_suppl.e19300, Journal of Clinical Oncology 38, no. 15_suppl).
Acknowledgements
The medical writing support was provided by Curo and Alligent Europe (Divisions of the Envision Pharma Group), funded by G1 Therapeutics, Inc. The authors would like to thank Huan Huang (G1 Therapeutics, Inc.) for her invaluable support in the development and revision of this manuscript.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- American Society of Clinical Oncology. Lung cancer – small cell: statistics [cited 2021 Jul 1]. Available from: https://www.cancer.net/cancer-types/lung-cancer-small-cell/statistics
- American Society of Clinical Oncology. Lung cancer – small cell: stages [cited 2021 Jul 1]. Available from: https://www.cancer.net/cancer-types/lung-cancer-small-cell/stages
- National Comprehensive Cancer Network. Small cell lung cancer (version 3.2021); 2021 [cited 2021 Jul 1]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS Meta-analysis of individual patient data. J Clin Oncol. 2012;30(14):1692–1698.
- Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229.
- Tecentriq. Prescribing information; 2021 [cited 2021 Jul 1]. Available from: https://www.gene.com/download/pdf/tecentriq_prescribing.pdf
- Imfinzi. Prescribing information; 2021 [cited 2021 Jul 1]. Available from: https://www.azpicentral.com/imfinzi/imfinzi.pdf#page=1
- US Food and Drug Administration. FDA grants accelerated approval to lurbinectedin for metastatic small cell lung cancer; 2020 [cited 2021 Jul 1]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-lurbinectedin-metastatic-small-cell-lung-cancer
- Carey PJ. Drug-induced myelosuppression : diagnosis and management. Drug Saf. 2003;26(10):691–706.
- Bryer E, Henry D. Chemotherapy-induced anemia: etiology, pathophysiology, and implications for contemporary practice. IJCTM. 2018;6:21–31.
- Corey-Lisle PK, Desrosiers MP, Collins H, et al. Transfusions and patient burden in chemotherapy-induced anaemia in France. Ther Adv Med Oncol. 2014;6(4):146–153.
- Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology. 2015;29(4):282–294.
- Weycker D, Hatfield M, Grossman A, et al. Risk and consequences of chemotherapy-induced thrombocytopenia in US clinical practice. BMC Cancer. 2019;19(1):151.
- Lalami Y, Klastersky J. Impact of chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) on cancer treatment outcomes: an overview about well-established and recently emerging clinical data. Crit Rev Oncol Hematol. 2017;120:163–179.
- Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010;28(26):4086–4093.
- Extermann M. Interaction between comorbidity and cancer. Cancer Control. 2007;14(1):13–22.
- Bohlius J, Bohlke K, Lazo-Langner A. Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH clinical practice guideline update. JOP. 2019;15(7):399–402.
- Aapro M, Ludwig H, Bokemeyer C, et al. Predictive modeling of the outcomes of chemotherapy-induced (febrile) neutropenia prophylaxis with biosimilar filgrastim (MONITOR-GCSF study). Ann Oncol. 2016;27(11):2039–2045.
- Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162(3):205–213.
- Lyman GH. Issues on the use of white blood cell growth factors in oncology practice. Am Soc Clin Oncol Educ Book. 2016; 35:e528–e532.
- Liou SY, Stephens JM, Carpiuc KT, et al. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig. 2007;27(6):381–396.
- Tai E, Guy GP, Dunbar A, et al. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. JOP. 2017;13(6):e552–e561.
- Wong W, Yim YM, Kim A, et al. Assessment of costs associated with adverse events in patients with cancer. PLoS One. 2018;13(4):e0196007.
- Association of Community Cancer Centers; 2021 [cited 2021 Jun 8]. Available from: https://www.accc-cancer.org/home/about
- Community Oncology Alliance. FACT Sheet: What is community oncology? 2017 [cited 2021 Jun 8]. Available from: https://communityoncology.org/wp-content/uploads/2017/08/What-is-Comm-Onc.pdf
- Kirkwood MK, Hanley A, Bruinooge SS, et al. The state of oncology practice in America, 2018: results of the ASCO practice census Survey. J Oncol Pract. 2018;14(7):e412–e420.
- Providence. 2021 [cited 2021 Jun 8]. Available from: https://www.providence.org/locations/st-joseph-hospital-orange/about-us
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139.
- US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0; 2017 [cited 2021 Jul 1]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- Jalal SI, Lavin P, Lo G, et al. Carboplatin and etoposide with or without palifosfamide in untreated extensive-stage small-cell lung cancer: a multicenter, adaptive, randomized phase III study (MATISSE). J Clin Oncol. 2017;35(23):2619–2623.
- Sun Y, Cheng Y, Hao X, et al. Randomized phase III trial of amrubicin/cisplatin versus etoposide/cisplatin as first-line treatment for extensive small-cell lung cancer. BMC Cancer. 2016;16:265.
- Tiseo M, Boni L, Ambrosio F, et al. Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: the GOIRC-AIFA FARM6PMFJM trial. J Clin Oncol. 2017;35(12):1281–1287.
- Zatloukal P, Cardenal F, Szczesna A, et al. A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol. 2010;21(9):1810–1816.
- Hanna N, Bunn PA, Jr., Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24(13):2038–2043.
- Igawa S, Shirasawa M, Ozawa T, et al. Comparison of carboplatin plus etoposide with amrubicin monotherapy for extensive-disease small cell lung cancer in the elderly and patients with poor performance status. Thorac Cancer. 2018;9(8):967–973.
- Yilmaz U, Polat G, Anar C, et al. Carboplatin plus etoposide for extensive stage small-cell lung cancer: an experience with AUC 6 doses of carboplatin. Indian J Cancer. 2011;48(4):454–459.
- Crawford J, Kosmidis PA, Hirsch FR, et al. Targeting anemia in patients with lung cancer. J Thorac Oncol. 2006;1(7):716–725.
- Barney CL, Scoville N, Allan E, et al. Radiation dose to the thoracic vertebral bodies is associated with acute hematologic toxicities in patients receiving concurrent chemoradiation for lung cancer: results of a single-center retrospective analysis. Int J Radiat Oncol Biol Phys. 2018;100(3):748–755.
- Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18(8):1116–1125.
- Weiss JM, Csoszi T, Maglakelidze M, et al. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized phase II trial. Ann Oncol. 2019;30(10):1613–1621.
- National Comprehensive Cancer Network. Hematopoietic growth factors (version 1.2021); 2021 [cited 2021 Mar 2]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf
- Chouaid C, Bassinet L, Fuhrman C, et al. Routine use of granulocyte colony-stimulating factor is not cost-effective and does not increase patient comfort in the treatment of small-cell lung cancer: an analysis using a Markov model. J Clin Oncol. 1998;16(8):2700–2707.
- Adams JR, Lyman GH, Djubegovic B, et al. G-CSF as prophylaxis of febrile neutropenia in SCLC. Expert Opin Pharmacother. 2002;3(9):1273–1281.
- Timmer-Bonte JN, Adang EM, Smit HJ, et al. Cost-effectiveness of adding granulocyte colony-stimulating factor to primary prophylaxis with antibiotics in small-cell lung cancer. J Clin Oncol. 2006;24(19):2991–2997.
- Ramsey SD, McCune JS, Blough DK, et al. Colony-stimulating factor prescribing patterns in patients receiving chemotherapy for cancer. Am J Manag Care. 2010;16(9):678–686.
- Wright JD, Neugut AI, Ananth CV, et al. Deviations from guideline-based therapy for febrile neutropenia in cancer patients and their effect on outcomes. JAMA Intern Med. 2013;173(7):559–568.
- Gascón P, Aapro M, Ludwig H, et al. Treatment patterns and outcomes in the prophylaxis of chemotherapy-induced (febrile) neutropenia with biosimilar filgrastim (the MONITOR-GCSF study). Support Care Cancer. 2016;24(2):911–825.
- Bokemeyer C, Gascón P, Aapro M, et al. Over- and under-prophylaxis for chemotherapy-induced (febrile) neutropenia relative to evidence-based guidelines is associated with differences in outcomes: findings from the MONITOR-GCSF study. Support Care Cancer. 2017;25(6):1819–1828.
- Pelayo Alvarez M, Westeel V, Cortés-Jofré M, et al. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev. 2013;(11):CD001990.
- Chan BA, Coward JI. Chemotherapy advances in small-cell lung cancer. J Thorac Dis. 2013;5:S565–S578.
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939.
- O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24(34):5441–5447.
- Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25(15):2086–2092.
- von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17(2):658–667.
- Hermes A, Bergman B, Bremnes R, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase III trial. J Clin Oncol. 2008;26(26):4261–4267.
- Crawford J, Denduluri N, Patt D, et al. Relative dose intensity of first-line chemotherapy and overall survival in patients with advanced non-small-cell lung cancer. Support Care Cancer. 2020;28(2):925–932.
- Lyman GH, Michels SL, Reynolds MW, et al. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer. 2010;116(23):5555–5563.
- Lyman GH, Kuderer NM, Aapro M. Improving outcomes of chemotherapy: established and novel options for myeloprotection in the COVID-19 era. Front Oncol. 2021;11:697908.
- US Food and Drug Administration. COSELA™ Prescribing Information; 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214200s000lbl.pdf
- Daniel D, Kuchava V, Bondarenko I, et al. Trilaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer: a multicentre, randomised, double-blind, placebo-controlled phase II trial. Int J Cancer. 2021;148(10):2557–2570.
- Hart LL, Ferrarotto R, Andric ZG, et al. Myelopreservation with trilaciclib in patients receiving topotecan for small cell lung cancer: Results from a randomized, double-blind, placebo-controlled phase II study. Adv Ther. 2021;38(1):350–365.
