Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is associated with substantial morbidity, mortality, and economic burden. With currently no approved treatment, an effective pharmaceutical intervention for this disease must be both clinically- and cost-effective.
Methods
A Markov model was constructed to estimate the clinical outcomes, costs, and quality of life impact of a hypothetical pharmaceutical intervention. Lifetime clinical outcomes, life-years, quality-adjusted life-years (QALYs), costs (2020 $US), incremental cost-effectiveness ratios (ICERs), and economically justifiable prices (EJPs) were quantified. Only patients with fibrosis stage F2–F4 were assumed eligible to initiate pharmaceutical treatment.
Results
Over a mean life expectancy of approximately 21 years in the simulated cohort, drug treatment reduced liver-related mortality by 6.0% (2.7% absolute reduction). Assuming an annual drug cost of $36,000, total discounted medical costs were $574,238 and $120,312 for drug and usual care, respectively, with discounted QALYs estimated to be 9.452 and 9.272 for the two comparators. This yielded an ICER of $2,517,676/QALY gained. The EJP of the drug at an ICER threshold of $150,000/QALY gained was $2,633, a 93% reduction from a base case. Sensitivity analyses suggest that, without a substantial decrease in the drug price, ICERs would exceed $500,000/QALY gained even with the most favorable efficacy assumptions.
Conclusions
For a pharmaceutical intervention to be considered cost-effective in the NAFLD fibrosis population, the substantial clinical benefit will need to be coupled with a modest annual price. Annual drug costs exceeding $12,000 likely will not provide reasonable value, even with favorable efficacy. More work is needed to estimate the cost-effectiveness of lifestyle modifications.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the United States (US), affecting 30% of adultsCitation1. NAFLD encompasses a spectrum of histological states ranging in severity from simple intrahepatic fat accumulation or nonalcoholic fatty liver (NAFL) to necrotic inflammation in the presence of ballooned hepatocytes and possibly fibrosis or nonalcoholic steatohepatitis (NASH)Citation2. While patients with NAFL do not typically require medical therapy, NASH increases the risk of advanced hepatic complications, such as fibrosis, cirrhosis, and hepatocellular carcinoma (HCC).
NAFLD patients have an increased mortality risk compared to the general population. Among adults with NAFLD, liver-related mortality is the third-leading cause of death after cardiovascular disease and malignancyCitation3. The presence and severity of fibrosis are the most significant predictors of adverse outcomes in NAFLD. In a meta-analysis of 1,495 biopsy-confirmed NAFLD patients, the risk of both all-cause mortality and liver-related deaths increased substantially with increases in the fibrosis stagesCitation4. In this meta-analysis, NAFLD patients with stage four fibrosis had 6.40 higher risks of all-cause mortality and 42.30 times the risk of liver-related deaths compared to fibrosis-free NAFLD individuals.
The economic healthcare burden of NAFLD/NASH is substantial. In the US, the total annual cost associated with NAFLD is estimated to be $292.2 billion, of which $103.3 billion are indirect costsCitation5. The lifetime per-patient cost of NASH in the US was found to be $32,249, with a total cost of $222.6 billion for all patientsCitation5. Between 2007 and 2014, the total inpatient hospitalization charges for NAFLD patients increased from $7.7 billion to $19.9 billion, respectivelyCitation6. In a health economics analysis using claims data, the annual per-person cost of a new NAFLD diagnosis was $7,804 and $3,789 annual per-person cost for long-term managementCitation7.
Currently, there are no approved pharmacologic treatment modalities for NAFLD. Lifestyle modifications suggested as a treatment for NAFLD follow those recommended for metabolic syndrome, including increased physical activity and weight loss. Such lifestyle changes have been shown to reduce the risk of NAFLD progression and significantly improve histological NASH biomarkers. In a meta-analysis of randomized trials, weight loss, meeting or exceeding 7%, was associated with improved hepatic histological markersCitation8. However, fewer than 50% of subjects across several trials achieved this level of weight loss.
Given the lack of standard of care treatment for NAFLD, numerous pharmacologic agents are currently under evaluation. In turn, there is an increasing interest in quantifying the potential lifetime clinical outcomes, cost, and cost-effectiveness benchmarks for an optimal NAFLD-fibrosis treatment. Such benchmarks will provide the comparative data needed to evaluate the cost-effectiveness of multiple treatment modalities over the natural history of the disease. To address this research gap, we conducted a comprehensive analysis to assess the lifetime clinical implications and cost-effectiveness of a hypothetical NAFLD treatment compared to current standard care for adults with NAFLD-fibrosis. Our primary objective was to generate comparative data that aid researchers in ascertaining the optimal cost-effectiveness of future NAFLD pharmacologic treatment modalities.
Methods
Model structure
We developed a Markov model in Microsoft Excel (version 2019; Microsoft Corporation) to assess the potential value of a pharmaceutical treatment of NAFLD-fibrosis. In this model, we quantified the cost-effectiveness of a hypothetical pharmaceutical product and compared its performance to usual care, which was defined as the disease natural history. The model was developed following good modeling practicesCitation9 with methodology and results reporting adhering to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guideline and checklistCitation10. Human participants were not included in the study, and institutional review board approval was not required.
The model health states included fibrosis stages zero to three (F0-F3), stage 4 compensated cirrhosis (F4-CC), decompensated cirrhosis (DCC), HCC, liver transplant (LT), and longer-term post-LT states, which described post-liver transplant (PLT) and the first year after liver transplant (1YPLT) ( and ). In addition, modeled patients could die and enter one of three absorbing states associated with cardiovascular mortality (CVM), liver-related mortality (LRM), or other/all-cause mortality. We also estimated the probability of other clinical events such as liver transplants. We quantified costs, life-years (LYs), and quality-adjusted life-years (QALYs) over a lifetime horizon (i.e. up to 50 years). We limited our model cycle length to one year to provide comparative results consistent with previous NAFLD/NASH economic modelsCitation12–14. All estimated costs reflected a payer perspective, and all outcomes were considered both undiscounted and discounted at a 3% annual rateCitation15
Figure 1. is a simplified model schematic that lists the modules, and health states within each module, with key health state transitions, denoted with arrows. A full listing of all allowable health state transitions is adjacent to the schematic. Abbreviations: CC, compensated cirrhosis; CV, cardiovascular; CVM, cardiovascular-related mortality; DCC, decompensated cirrhosis; F0-F4, fibrosis stages; HCC, hepatocellular carcinoma; LRM, liver-related mortality; LT, liver transplant; Yr 1 PLT, first year after liver transplant. Hagström et al. Citation11, Pearson et al. Citation12.
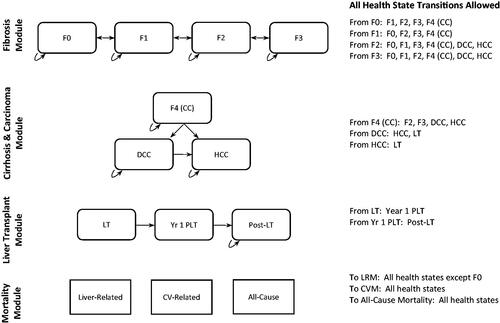
Table 1. Example transition matrix representing model health states and annual transitions (natural history, years 1–5) a.
Treatment strategies and transition probabilities
The model included two treatment strategies (1) usual care and (2) usual care with the concomitant use of a hypothetical pharmaceutical product. Patients transitioned through health states characterized largely by disease progression with possible fibrosis regression for usual care. Transition probabilities for the natural history of liver disease were informed by published literature and several NAFLD/NASH economic modelsCitation6,Citation16–29. presents the annual transition probability matrix for Years 1–5 in the natural history arm.
We calculated the incidence rates (IR) of fibrosis/cirrhosis transitions using the transition status and the total person-years of follow-up. The quantified IRs were subsequently transformed to cumulative incidence estimates and health state-specific transition probabilities using standard methods (additional text in the Supplement). We used newly published literature for DCCCitation5,Citation18 or HCC to ascertain the probability of liver transplant (additional text in the Supplement)Citation19,Citation28.
Mortalities from cardiovascular disease or liver-related causes were assumed to vary by health state. Similar to previous NAFLD/NASH economic analysesCitation6,Citation14, LRM estimates were derived from the clinical literatureCitation19–21,Citation23–25,Citation27,Citation29. We combined mortality data from the Third National Health and Nutrition Examination Survey (NHANES III) to calculate CVM (additional text in the Supplement)Citation17,Citation30. Mortality from other causes was assumed to be age-dependent and was adjusted in the 5-year windows using the US lifetable dataCitation31. We proportionally adjusted for the transitions between fibrosis and cirrhosis stages to account for the effect of aging on all-cause mortality.
Adding pharmaceutical treatment to usual care was hypothesized to exert a clinical effect in three potential domains: (a) increased disease regression in the F0-F3 fibrosis stages; (b) increased disease regression from F4-CC to F3; and (c) reduced disease progression among the fibrosis stages and F4-CC. Two separate parameters for modification of disease regression were included in the model as it is expected that earlier fibrosis stages would be more amenable to improvement than F4-CC, which would be less likely to regress. Furthermore, the drug effect on disease progression was assumed to be modest relative to its effects on regression due to other unaltered factors (e.g. co-morbidities) that play a role in fibrosis progression.
Only patients in the F2, F3, and F4-CC health states were deemed to be candidates for starting the pharmaceutical intervention, although, once initiated, treatment was assumed to be ongoing in all fibrosis health states. Upon disease progression to DCC, HCC, or LT, pharmaceutical treatment ceased, and patients were subject to natural history progression.
Costs
Unit costs were derived primarily from published sourcesCitation12,Citation14,Citation32–34. In the few cases where robust evidence was unavailable, conservative assumptions based on clinical expert opinion were used to supplement published estimates (). For fibrosis stages, costs cited in a recent economic analysis of NASH patients with type 2 diabetesCitation34 were inflated from the 2017 US dollars (USD) to the 2020 USD using the Medical Care component of the US Consumer Price Index (CPI)Citation37. Cirrhosis costs and HCC health states (in 2017 USD) were similarly obtained from a recently published healthcare claims analysisCitation33 and were also inflated using the Medical Care CPI.
Table 2. Base-case model parameter estimates.
Liver and other organ transplants are extensively studied by Milliman (an actuarial and consulting firm) and summarized in a triennial research report. The costs of a liver transplant for the year in which it occurs were informed by liver transplant charges reported in the 2020 Milliman report and using a cost-to-charge ratio derived from Younossi and colleaguesCitation34. Less robust cost estimates were available for the time periods after the year of transplant (health states 1YPLT and PLT). Thus, to develop cost estimates for these two health states we used published resource utilization and immunosuppression costsCitation32, costs for similar health states from other NASH economic analysesCitation12,Citation14, and from clinical expert opinion (VKR, MIE). With no approved drug to treat NASH fibrosis in the US, we set the annual base case cost for the hypothetical drug to $36,000 (), assuming a substantial reduction from some lead compounds already on the marketCitation43,Citation44.
Utilities
Health-related quality of life (QOL) utility weights were used to calculate QALYs (). We identified relevant values in utility research studies and in economic analysesCitation14,Citation38–41. Due to the lack of NASH-specific utilities, the utility weights for fibrosis stages were based on transformed SF-36 data collected from NAFLD/NASH patientsCitation38,Citation39. Standard gamble utilities were used for cirrhosis, HCC, and liver transplant stagesCitation14,Citation40. For the longer-term post-transplant health states, we used utilities cited in an earlier NASH modelCitation14, which were based on data from AbergCitation41. All utilities’ estimates were age- and gender-adjusted using nationally representative utilities derived from the US adult populationCitation42.
Validation and analyses
Consistent with best practices guidanceCitation45, the model was subjected to extensive validity testing. The face validity of the model was evaluated by comparison to other NAFLD/NASH model structures and through a clinical applicability review (VKR; MIE). Internal validity was assessed by a review of the model programming by a second modeler (MIE) and through a series of 25 quality assurance tests and analyses to confirm proper model functionality. Finally, the external validity of the natural history model foundation was appraised by comparisons of clinical outcomes to previously published studiesCitation11,Citation12.
Upon the conclusion of the validation process, base-case analyses using the default parameter estimates were conducted, which included quantifying clinical events, cost-effectiveness analyses, and estimation of an economically justifiable price (EJP) for the pharmaceutical at three incremental cost-effectiveness ratio (ICER) thresholds. Several sensitivity analyses were performed to assess the robustness of the model results and conclusions.
Results
Validation results
The model was deemed valid based on the evaluations conducted. The external validity assessment included comparisons of survival by fibrosis stage, liver-related mortality, and the cumulative incidence of important clinical outcomes (eTable 1 in the Supplement). An exact comparison of median survival by Fibrosis stage was limited by the insufficient demographic details in the paper by HagstromCitation11, which may account for some differences in our comparative results. There was good alignment between our model results and relevant published estimates in instances where parameters could be more accurately matched.
Base case results
Using the default parameter estimates, the model showed that eligible patients (mix of F2, F3, and F4-CC) initiating drug therapy would be treated for an average of 19.3 years. The clinical outcomes associated with drug therapy and natural history are detailed in eTable 2 in the Supplement. Drug treatment is predicted to modestly reduce liver-related mortality, although the incidence of other clinical outcomes is only nominally decreased. Notably, liver transplants' lifetime occurrence probability is estimated to decline by only 0.2% (from 3.8% to 3.6%).
The base case economic analysis results are presented in . Drug treatment increased mean survival by approximately 6.3 months (21.1 years vs. 20.6 years; undiscounted). Lifetime discounted treatment costs were $120,312 and $574,238 for natural history and drug treatment, respectively, yielding an incremental cost of $453,926. Offsets to the lifetime drug acquisition costs ($460,572) were nominal ($6,646), primarily due to reductions in cirrhosis rather than liver transplants. Finally, drug treatment increased the lifetime discounted QALYs by 0.18 (9.272 and 9.452 for natural history and drug treatment, respectively). The ICER exceeded $2.5 million/QALY gained, which substantially exceeds commonly cited thresholds in the US of acceptable value for money—typically cited in the range of $100,000-$150,000/QALY gainedCitation46.
Table 3. Results of base-case cost-effectiveness analyses and economically justifiable pricea.
Because the default drug cost yielded a relatively high ICER, we conducted EJP analyses at three ICER thresholds—$50,000, $100,000, and $150,000/QALY gained (). These analyses demonstrated that the annual drug cost would need to decrease by 93%–97% (to <$2,640) to meet these thresholds.
Numerous additional scenario analyses were performed to further explore the impact of alternative parameter values and assumptions related to drug efficacy and price. Targeting treatment only to certain disease stages (F2, F3, or F4-CC) was evaluated (). Although mean treatment duration decreased with increasing fibrosis severity (23.2, 21.2, and 15.0 years, respectively); thus, decreasing both total and incremental costs, the QALY benefit gained also decreased. The resulting ICERs were similar to the base case result ().
Table 4. Results of base case-cost-effectiveness analyses and economically justifiable prices by starting fibrosis stage a.
Sensitivity analysis results
We conducted sensitivity analyses using the ranges specified in (eFigure 1 in the Supplement). As expected, the ICER is sensitive to the three-drug efficacy factors, especially the factor representing increased disease regression in the F0–F3 fibrosis stages. Patient age at treatment initiation also has a substantial impact on the cost-effectiveness results. Drug treatment in younger patients with a longer life expectancy is relatively more cost-effective (although still unfavorable) than treatment of older patients with fewer years to accrue the clinical benefits of treatment. Finally, because drug treatment costs are ongoing with most clinical benefits not received until further in the future, the results are sensitive to the time horizon and the discount rate. Analysis of a time horizon shorter than 50 years only increases the ICER while the discount rates of 0% and 5% decrease and increase the ICER by 28% and 45%, respectively.
We also varied all drug efficacy factors simultaneously across a wide range while adjusting the annual drug cost (eFigure 2 in the Supplement). In a Best-Case scenario, critical clinical outcomes such as liver transplant and liver-related mortality were predicted to decrease by up to 30% relative to natural history (eTable 2 in the Supplement). However, even in this scenario, the annual drug cost would still need to be <$11,000 to achieve an ICER below $150,000/QALY gained.
Discussion
NAFLD and NASH are associated with substantial morbidity and increased mortality risk compared to the general populationCitation2. The economic burden of disease, mainly driven by hepatic complications, is measured in the hundreds of billions of dollars in the US alone. Similarly, the medical and societal costs associated with NAFLD in just three major European countries add nearly €200 billion annuallyCitation5.
While lifestyle modifications, such as weight loss, are the current standard of care and potentially effective for obesity and NAFLDCitation47, long-term maintenance is notoriously difficultCitation48. Certainly, in other chronic illnesses, such as diabetes, lifestyle modification has been noted to be worthwhile though more data are neededCitation49. Bariatric surgery results in long-term improvements in NAFLD markersCitation50. However, surgery was shown to be cost-effective only for obese patients with NASH, independent of fibrosis stage, and for overweight NASH patients with F3Citation14.
The addition of an effective pharmaceutical intervention to lifestyle changes is an attractive treatment strategy, although a safe and effective agent is not yet available. It is anticipated that the first such medication will be premium-priced. However, the budgetary implications associated with millions of eligible patients will be of concern to payers. The cost-effectiveness (or value) of treatment is sure to be questioned if clinical benefit is perceived as negligible, drug costs exorbitant, or both.
Both fibrosis presence and severity are the most significant predictors of adverse outcomes in NAFLD. In a study of NAFLD patients with 33 years of follow-up, fibrosis stage and not the NAFLD activity score (NAS) was a significant predictor of both overall and cause-specific mortalitiesCitation51. Therefore, our model focused on examining the effectiveness of fibrosis treatment in NAFLD.
Our analysis shows that in patients most likely eligible to initiate treatment, and with our best estimates of potential clinical efficacy and cost, the addition of drug treatment would not be cost-effective compared to usual care alone. Even under the most favorable clinical efficacy assumptions, achieving a favorable ICER would require more modest drug pricing. The latter notion was demonstrated in our scenario analyses that simultaneously varied the drug efficacy and cost and in which only a small fraction of simulations at high efficacy/low cost yielded ICERs that would be considered favorable. These results are consistent with the findings of two economic analyses by the Institute for Clinical and Economic Review, which concluded that obeticholic acid was not cost-effective as a treatment for NASHCitation12,Citation43.
As with any modeled analysis, some limitations warrant further discussion. First, intensive lifestyle intervention was not explicitly modeled as a comparator to the hypothetical drug. However, because this strategy has a nominal cost relative to drug treatmentCitation52 and some efficacy (albeit transient in many patients), we concluded that this comparison would yield even less favorable results for a pharmaceutical intervention than when compared to natural history. Second, our analysis did not attempt to model drug adherence, nor did it account for any safety-related issues, either of which would have likely increased the ICER. Third, we did not examine the effects of diabetes on disease progression in NAFLD. Because disease severity and progression would be expected to be worse in a population with this co-morbidity, drug treatment may be more cost-effective in these patients. Finally, some parameter estimates are not available for NASH patients specifically (e.g. some utility values), an issue identified in other economic analyses in this populationCitation12,Citation14. However, we structured our natural history modules based on validated natural history models for NAFLD and NASH. We also assessed the validity of our natural history model against peer-reviewed models (eTable 1).
The limitations are balanced by the unique strengths of our economic analysis. Our model builds on the widely accepted approach to simulating liver disease progression but improves upon past efforts by adopting new methods and updated data sources for key elements such as liver transplant risk, cardiovascular-related mortality, and health state costs. Furthermore, while our conclusions are consistent with another comprehensive analysis of the potential value of NASH's drug treatmentCitation12, our analysis offers new insights as to the combinations of drug efficacy and cost that may yield acceptable ICERs.
While the potential downstream clinical consequences of NAFLD/NASH are severe, progression to cirrhosis, HCC, and liver transplant (the most deleterious health states) is slow, occurring for most patients many years in the future. Besides, only a relatively small percentage of patients suffer the costliest of these outcomes (i.e. lifetime cumulative incidence of a liver transplant is <4% in our analysis), yielding only a modest opportunity for clinical, cost, and QOL benefit after many years of chronic, costly treatment. While lifestyle modification will remain a mainstay of care for NAFLD and NASH, a pharmaceutical intervention for the disease is inevitable. Researchers' challenge will be to develop a medication that addresses both NASH and fibrosis, ameliorating or preventing the most harmful sequelae at a cost that provides reasonable value. Failing that, a drug will not achieve optimal adoption and limit public health benefits in this critical disease population.
Conclusion
In this cost-effectiveness study of pharmaceutical intervention for NAFLD-fibrosis, in order for an intervention to be considered cost-effective, the substantial clinical benefit will need to be coupled with a modest annual price. Annual drug costs higher than $12,000 will likely not provide reasonable value, even with favorable efficacy. More work is needed to estimate the cost-effectiveness of lifestyle modifications.
Transparency
Declaration of funding
No funding was received to produce this article
Declaration of financial/other relationships
All authors declare that they have no relevant financial or other relationships to disclose.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in data analysis, writing the manuscript, and provided input on data queries.
Supplemental Material
Download MS Word (151.1 KB)Acknowledgements
None stated
Data availability statement
All relevant data were included in the manuscript. Further information about any additional data could be requested by contacting the corresponding author.
References
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313(22):2263–2273.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357.
- Younossi ZM, Tampi R, Priyadarshini M, et al. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology. 2019;69(2):564–572.
- Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133.
- Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586.
- Hirode G, Vittinghoff E, Wong RJ. Increasing clinical and economic burden of nonalcoholic fatty liver disease among hospitalized adults in the United States. J Clin Gastroenterol. 2019;53(10):765–771.
- Allen AM, Van Houten HK, Sangaralingham LR, et al. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large U.S. Claims database. Hepatology. 2018;68(6):2230–2238.
- Musso G, Cassader M, Rosina F, et al. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in nonalcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55(4):885–904.
- Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices-overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012;15(6):796–803.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health. 2013;16(2):e1–e5.
- Hagstrom H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–1273.
- Pearson S, Banken R, Chapman R, et al. Obeticholic Acid for the Treatment of Nonalcoholic Steatohepatitis: Comparative Clinical Effectiveness and Value: final report: The Institute for Clinical and Economic Review (ICER). 2016.
- Chongmelaxme B, Phisalprapa P, Sawangjit R, et al. Weight reduction and pioglitazone are cost-effective for the treatment of non-alcoholic fatty liver disease in Thailand. Pharmacoeconomics. 2019;37(2):267–278.
- Klebanoff MJ, Corey KE, Chhatwal J, et al. Bariatric surgery for nonalcoholic steatohepatitis: a clinical and cost-effectiveness analysis. Hepatology. 2017;65(4):1156–1164.
- Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276(15):1253–1258.
- Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–1978.
- US Centers for Disease Control and Prevention National Center for Health Statistics. The Linkage of National Center for Health Statistics Survey Data to the National Death Index – 2015 Linked Mortality File (LMF): Methodology Overview and Analytic Considerations 2018. [cited January 2, 2020]. Available from: https://www.cdc.gov/nchs/data-linkage/mortality-methods.htm.
- Davis KL, Mitra D, Medjedovic J, et al. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol. 2011;45(2):e17-24–e24.
- Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565.
- Hui JM, Kench JG, Chitturi S, et al. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38(2):420–427.
- Jain A, Reyes J, Kashyap R, et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232(4):490–500.
- National Institutes of Health Surveillance, Epidemiology, and End Results Program. Liver and Intrahepatic Bile Duct Cancer Recent Trends in SEER Age-Adjusted Incidence Rates, 2000–2016. 2020. [cited April 6, 2020]. Available from: https://seer.cancer.gov/explorer/application.html?site=35&data_type=1&graph_type=2&compareBy=sex&chk_sex_1=1&race=1&age_range=1&stage=101&rate_type=2&advopt_precision=1&advopt_display=2.
- Ratziu V, Bonyhay L, Di Martino V, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35(6):1485–1493.
- Saab S, Hunt DR, Stone MA, et al. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: a decision analysis model. Liver Transpl. 2010;16(6):748–759.
- Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43(4):682–689.
- Thuluvath PJ, Guidinger MK, Fung JJ, et al. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4 Pt 2):1003–1019.
- Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4 Pt 2):961–972.
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555.
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
- Unalp-Arida A, Ruhl CE. Noninvasive fatty liver markers predict liver disease mortality in the US population. Hepatology. 2016;63(4):1170–1183.
- Arias E. United States life tables, 2017. Natl Vital Stat Rep. 2019;68(7):1–66.
- Habka D, Mann D, Landes R, et al. Future economics of liver transplantation: a 20-year cost modeling forecast and the prospect of bioengineering autologous liver grafts. PLOS One. 2015;10(7):e0131764.
- Rustgi VL, John T, Catalano C, et al. Health care resource use and cost burden of chronic kidney disease in patients with chronic liver disease: a real-world claims analysis. Hepatol Commun. 2020;4(10):1404–1418.
- Younossi ZM, Tampi RP, Racila A, et al. Economic and clinical burden of nonalcoholic steatohepatitis in patients with type 2 diabetes in the US. Diabetes Care. 2020;43(2):283–289.
- Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53(3):810–820.
- Poynard T, Imbert-Bismut F, Munteanu M, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3(1):8.
- US Bureau of Labor Statistics. Consumer price index for medical care in the US city average, all urban consumers, seasonally adjusted 2020. [cited April 6, 2020]. Available from: https://www.bls.gov/cpi/data.htm.).
- David K, Kowdley KV, Unalp A, et al. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(6):1904–1912.
- Nichol MB, Sengupta N, Globe DR. Evaluating quality-adjusted life years: estimation of the health utility index (HUI2) from the SF-36. Med Decis Making. 2001;21(2):105–112.
- Chong CA, Gulamhussein A, Heathcote EJ, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterology. 2003;98(3):630–638.
- Aberg F, Maklin S, Rasanen P, et al. Cost of a quality-adjusted life year in liver transplantation: the influence of the indication and the model for end-stage liver disease score. Liver Transpl. 2011;17(11):1333–1343.
- Hanmer J, Lawrence WF, Anderson JP, et al. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26(4):391–400.
- Rind DM, Hansen R, Guzauskas G, et al. Obeticholic Acid for the Treatment of Nonalcoholic Steatohepatitis with Fibrosis: Effectiveness and Value: The Institute for Clinical and Economic Review (ICER); 2020.
- Thompson Reuters. Red book 2020: pharmacy's fundamental reference. Montvale (NJ): PDR Network LLC; 2020.
- Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task Force-7. Value Health. 2012;15(6):843–850.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness-the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797.
- Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–846.
- Moroshko I, Brennan L, O'Brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obes Rev. 2011;12(11):912–934.
- Jacobs-van der Bruggen MA, van Baal PH, Hoogenveen RT, et al. Cost-effectiveness of lifestyle modification in diabetic patients. Diabetes Care. 2009;32(8):1453–1458.
- Fakhry TK, Mhaskar R, Schwitalla T, et al. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15(3):502–511.
- Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554.
- Rushing J, Wing R, Wadden TA, et al. Cost of intervention delivery in a lifestyle weight loss trial in type 2 diabetes: results from the look AHEAD clinical trial. Obes Sci Pract. 2017;3(1):15–24.
