Abstract
Aim
National health care expenditures have been increasing each year, although the Japanese government has annually revised official drug prices. Managing the health care system to pay for expensive drugs is a major concern. The reimbursement restriction, which is the only way that a drug can be implemented before market entry in Japan, is crucial for managing expenditures. Therefore, this study identifies the impact of the reimbursement restriction on drug market sales in Japan, particularly in the situation where health technology assessment or other market access regulations are not applicable before market entry.
Method
All new drugs listed in fiscal years 2011–2019, along with their market size forecast, were identified using the materials from the Central Social Insurance Medical Council. We then calculated the percentage rate of reimbursement amounts based on the National Database of Health Insurance Claims relative to the predicted market size as a dependent variable. Using the reimbursement restriction for each drug as an independent variable, we performed descriptive and univariate analyses on each variable, followed by generalized linear mixed-effects model regression analysis.
Results
We identified 211 drugs. The mean rates of drugs that required physicians, facilities, and patients to meet criteria for use were 30.85% (n = 2), 31.42% (n = 2), and 72.11% (n = 6), respectively. The mean rate of drugs that required diagnostic testing was 22.99% (n = 7), which was 3.7 times lower than the rate of drugs that did not require such testing (p < .05).
Conclusion
Our results indicate that the reimbursement restriction requiring diagnostic testing has a substantial impact on decreasing market sales. As the number of cases for each requirement is small, further study is needed to measure the impact of the other reimbursement restrictions.
1. Introduction
Health care expenditure is a major concern in high-income countries, and brand-name pharmaceuticals have been largely responsible for high overall spending, especially in the USCitation1. Although proposals to regulate drug pricing have been made by US scholarsCitation2,Citation3, pricing regulation in European countries has greater government involvement in terms of financing and organizationCitation4. These policies vary by country, but the governmental policies include the following regulations: health technology assessment (HTA), a limit on the growth of total pharmaceutical spending, objective standards to set prices, and/or guidelines or programs on the rational use of medicinesCitation4–9.
In Japan, access to innovative drugs has been beneficial to patients in terms of cost and speed. Most of the newly approved drugs, except preventive drugs, are listed on the National Health Insurance (NHI) list within 60 daysCitation10, and patient co-payments at medical institutions are typically inexpensive. However, the national health care expenditures have been increasing each year, now exceeding 40 trillion yenCitation11, of which drug costs account for about 10 trillion yen. The Japanese government even revises official drug prices annuallyCitation12,Citation13. In addition, although HTA has been introduced to recalculate official prices, which involves using incremental cost-effectiveness ratios (ICERs) as price adjustment according to the degree of ICER estimates, the impact of the drug expenditure has been lower than that of the recalculation policy based on market expansionCitation13,Citation14. These recalculating policies are adopted after a drug becomes commercially available by setting the official price on the NHI list. Conversely, a reimbursement policy not to reduce the price of the drug but to optimize the volume of the drug is considered before market entry, which marks the beginning of drug distribution and insurance reimbursement, by regulating usage for doctors, hospitals, and patients. This reimbursement restriction plays an important role because it can be implemented when the drug is added to the NHI list before market entry.
Recently, because of the increasing number of expensive drugs being approvedCitation15,Citation16, managing the health care system to pay for these drugs has become a major concernCitation17,Citation18. In Japan, the reimbursement restriction, which is the only way that a drug can be implemented before market entry, is crucial for managing expenditures for expensive drugs. However, no previous research explores the effect of this reimbursement restriction. Therefore, this study identifies the impact of the reimbursement restriction on drug market sales in Japan, particularly in the situation where HTA or other market access regulations are not applicable before market entry.
2. Method
2.1. Selection of drugs
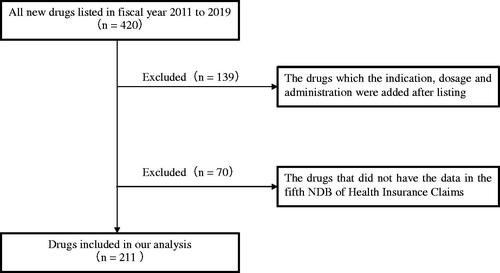
All new drugs listed in fiscal years 2011–2019, along with their market size forecast, were identified using the materials from the Central Social Insurance Medical CouncilCitation19. The market size forecast at the time of listing is expected to change if the indication, dosage, and administration of the drug are added after listing. We excluded these drugs using the list of newly approved drugs from the Pharmaceutical and Medical Devices AgencyCitation20. To investigate the actual market sales of drugs, we excluded drugs that were not included in the fifth National Database (NDB) of Health Insurance ClaimsCitation21.
2.2. Dependent variable
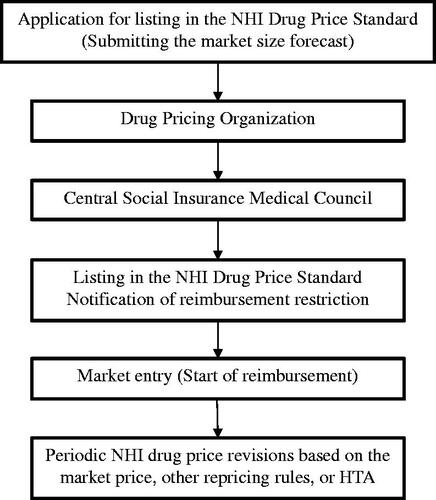
We used the percentage rate of the amount of NDB reimbursement relative to predicted market size as a dependent variable derived from the materials from the Central Social Insurance Medical Council and the fifth NDB of Health Insurance Claims and Specific Health Checkups of JapanCitation19,Citation21. NDB reimbursement and predicted market size are based on NHI price; they include taxes but exclude margins, refunds, and discounts. The reason why this variable is an appropriate indicator for estimating the impact of reimbursement restriction is that a company needs to submit its market size forecast with the NHI price listing request form to the Ministry of Health, Labour, and Welfare (MHLW) by the company before the notification of reimbursement restriction can be issued (see ). The company does not know whether the notification of reimbursement restriction will be issued at the time of submission of its market size forecast; thus, by comparing the difference between the amount of NDB reimbursement and the market size forecast, the effect of the restriction can be measured.
2.3. Independent variables
The year of the newly listed drug and the name of the drug’s manufacturer were extracted from the materials from the Central Social Insurance Medical Council and the fifth NDB of Health Insurance ClaimsCitation19. The reimbursement restriction for each drug in the notification (Supplemental Material 1)—issued by the Director of Medical Economics Division, Health Insurance Bureau, MHLW—was categorized into 10 regulations: (1) physician requirements, (2) facility requirements, (3) patient requirements, (4) dosing period limitations, (5) removal of dosing period limitations, (6) additional entries in the receipt, (7) compliance with the package insert, (8) diagnostic testing, (9) prioritizing other medicines, and (10) explanation of technical fees that can be charged together. presents the details of these regulations.
Table 1. Characteristics of each reimbursement restriction.
2.4. Statistical methods
We performed descriptive analyses upon each variable, including our dependent variable, which is the percentage rate of the amount of NDB reimbursement relative to market size forecast—and applied univariate analysis to the explanatory variables. We performed Mann–Whitney U tests on the categorical variables. We constructed a generalized linear mixed-effects model (GLMM) regressing each response against the explanatory variable, allowing the intercept to vary by the year of the newly listed drug and the manufacturer. In the GLMM analysis, the gamma distribution with log link was assumed as the explanatory variable because the value was from zero to positive infinite. We did not include the independent variables that showed high correlation to avoid multicollinearity in the analysis, because some policies are applied simultaneously as a set. To confirm the fitting of the GLMM, we performed a log-likelihood test and compared the results with those of a generalized linear model (GLM), which removed the random effect variables. We estimated beta coefficients and 95% confidence intervals using STATA version 14.2.
3. Results
We identified 420 drugs and their market size forecast from the Central Social Insurance Medical Council’s materials. We excluded 139 drugs for which the indication, dosage, and administration were added after listing, and 70 drugs that did not have data in the fifth NDB of Health Insurance Claims. The remaining 211 drugs were identified as objects of analysis ().
3.1. Characteristics of selected drugs and univariate analysis
summarizes the drugs’ characteristics. The mean rate of the amount of NDB reimbursement relative to predicted market size is 96.42% for the 211 examined drugs. The mean rates of the drugs with physician, facility, and patient requirements are 30.85% (n = 2), 31.42% (n = 2), and 72.11% (n = 6), respectively. Six drugs have dosing period limitations with a mean rate of 55.20%, while four drugs have no dosing period limitations, with a mean rate of 557.50%. Drugs requiring additional entries on receipts have a mean rate of 31.26% (n = 8). Twenty-five drugs have reminders to comply with the package insert, and the mean rate is 136.73%. The mean rate of drugs that require diagnostic testing is 22.99% (n = 7). The eight drugs recommended to prioritize other medicines have a mean rate of 112.63%. The drugs that entail an explanation of technical fees that can be charged together have a mean rate of 68.77% (n = 21). In the univariate analysis, the drugs that required additional entries in receipts correlate negatively and significantly with the percentage rate (p = .082). In addition, the requirement of diagnostic testing (p = .022) correlates negatively and significantly with the percentage rate.
Table 2. Results of the univariate analysis: association between percentage rate and covariates.
3.2. GLMM regression analysis
In our model, the independent variables, such as drugs that required additional entries in the receipt and prioritizing other medicines were excluded because of their high correlation coefficient with diagnostic testing (0.66) and patient requirements (0.71), respectively. displays the regression results. The percentage rates for drugs with a requirement to remove dosing period limitation are 6.3 times higher than those for drugs without such requirement, and the result is significant (p < .05). Conversely, the percentage rates for drugs that require diagnostic tests are 3.7 times lower than those for drugs not requiring diagnostic tests, and the result is significant (p < .05). This result is consistent even when the variable of “additional entries in the receipt” is used instead of “diagnostic testing” (Supplemental Material 2). The result of the likelihood ratio tests shows that the GLMM is a better model than the GLM, which removes random effect variables (p < .001).
Table 3. GLMM regression analysis (with family = gamma, link = log).
4. Discussion
Reimbursement policies that impose requirements on healthcare providers reduce the market sales of drugs while the predictions submitted by pharmaceutical companies are almost the same as actual market sales when drugs have no reimbursement restrictions (). A restriction that requires diagnostic testing significantly reduces market sales (). The same trend is observed for a restriction requiring additional entries on receipts, which is often imposed alongside diagnostic testing. The imposition of physician or facility requirements also reduces market sales to a large extent (), although the reductions are not significant because the sample contains only a small number of drugs with such restriction requirements. Conversely, drugs without dosing period limitations have increased market sales () because drugs with no prescription restrictions are perceived to have sufficient experience and fewer safety issues. The rate of compliance with package inserts and prioritizing other medicines are almost the same as that for the case with no restriction because these are easily anticipated by the company upon the approval of the drug.
Limited research has explored the effect of reimbursement restrictions, but in terms of the accuracy of sales forecasts submitted by pharmaceutical companies, researchers have reported that companies are, on average, too optimistic in their forecastsCitation22,Citation23. Considering that the total mean percentage rate in our study is lower than 100%, we consider our work to be in line with existing research, although the medical environment varies across countries.
The results indicate that a reimbursement restriction is a key to managing drug expenditure. People usually tend to focus on the price, and the high unit price has been the target of criticism. However, by utilizing reimbursement policies, it is possible to achieve a decrease in market sales, while maintaining the unit price. Although Japan has a recalculating system of up to a 50% reduction in price when drug sales exceed market expectationsCitation13, the advantage of the reimbursement restriction is that it can be implemented before market entry. Our study is significant in that it is the first to show the impact of the restriction in objective figures by comparing the expected drug sales amount derived without knowing whether the restriction would be issued.
It is important to note that when an expensive long-term use drug is listed, it will be addressed through reimbursement policies that require diagnostic testing or impose requirements on physicians and medical institutions because in Japan, HTA is not used for insurance benefit availability and most new drugs are automatically listed. Conversely, we must avoid making these policies so strict that the new drug does not reach patients who need the treatment. Although some specialists in Japan argue that certain drugs should not be covered by insurance, further discussion is needed to explore policies, such as changing the insurance benefit rate while ensuring continued excellent patient access to new drugs, an advantage of the Japanese system.
This study has several limitations. First, the NDB only considers the top-selling items and does not extract items with low sales. We excluded drugs with no data in the NDB. If these drugs with low sales were included, then the actual difference between the amount of NDB reimbursement and market size forecast might indicate a more pronounced impact of the reimbursement restriction. Second, our dependent variable is the percentage rate. Precautions should be taken when the expected sales amount is small because the percentage rate tends to be more susceptible. Third, we explore the effect of restriction on market sales directly, but there might be indirect costs and other factors, such as restriction administration cost, entry of competitors, and more. Finally, this study is based on a limited number of cases, that is, the number of drugs subject to each of the 10 restriction requirements is small. Specifically, the number of cases for physician and facility requirements is very limited and insufficient to perform statistical testing. Therefore, even if the differences of mean percentage rate with and without requirements are large, there may be no real effect of the requirements. Future studies should be conducted when the number of policies is enough to explore the robust impact of the reimbursement restriction.
5. Conclusion
Our results indicate that the reimbursement restriction requiring diagnostic testing has a substantial impact on decreasing market sales. As the number of cases for each requirement is small, further study is needed to measure the impact of the other reimbursement restrictions.
Transparency
Declaration of funding
No specific funding was received for conducting this study.
Declaration of financial/other relationships
The authors declare that they have no competing interests.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Both authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by HM. The first draft of the manuscript was written by HM, and both authors read and approved the final manuscript.
Acknowledgements
None stated.
Supplemental Material
Download MS Word (109 KB)Data availability statement
All necessary data used for this study are included in the manuscript.
References
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024–1039.
- Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA. 2016;316(8):858–871.
- Kaltenboeck A, Bach PB. Value-based pricing for drugs: theme and variations. JAMA. 2018;319(21):2165–2166.
- Emanuel EJ, Zhang C, Glickman A, et al. Drug reimbursement regulation in 6 peer countries. JAMA Intern Med. 2020;180(11):1510–1517.
- Vogler S, Paris V, Ferrario A, et al. How can pricing and reimbursement policies improve affordable access to medicines? Lessons learned from European countries. Appl Health Econ Health Policy. 2017;15(3):307–321.
- Vogler S, Zimmermann N, Leopold C, et al. Pharmaceutical policies in European countries in response to the global financial crisis. South Med Rev. 2011;4(2):69–79.
- Thornhill MH, Dayer MJ, Forde JM, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ. 2011;342(1):d2392.
- Rogers Van Katwyk S, Grimshaw JM, Nkangu M, et al. Government policy interventions to reduce human antimicrobial use: a systematic review and evidence map. PLOS Med. 2019;16(6):e1002819.
- Gong Y, Yang C, Yin X, et al. The effect of essential medicines programme on rational use of medicines in China. Health Policy Plan. 2016;31(1):21–27.
- Association JPM. Health insurance programs and drug pricing in Japan. Pharmaceutical Regulations in Japan; 2020 [cited 2022 Jan 7]. Available from: https://www.jpma.or.jp/english/about/parj/eki4g6000000784o-att/2020e_ch06.pdf
- Japanese Ministry of Health, Labour, and Welfare. Annual trends in national health care expenditures; 2021 [cited 2021 Jul 14]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/k-iryohi/18/dl/toukei.pdf
- Japanese Ministry of Health, Labour, and Welfare. Annual trends in drug costs and estimated deviation rates; 2019 [cited 2021 Jul 23]. Available from: https://www.mhlw.go.jp/content/12404000/000376813.pdf
- Japanese Ministry of Health, Labour, and Welfare. About rule of NHI price calculation method in FY 2020; 2021 [cited 2021 Jul 27]. Available from: https://www.mhlw.go.jp/web/t_doc?dataId=00tc5663&dataType=1&pageNo=1
- Kamae I, Thwaites R, Hamada A, et al. Health technology assessment in Japan: a work in progress. J Med Econ. 2020;23(4):317–322.
- Lin PJ, Cohen JT, Neumann PJ. Preparing the health-care system to pay for new Alzheimer's drugs. Alzheimers Dement. 2020;16(11):1568–1570.
- Braendstrup P, Levine BL, Ruella M. The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19. Cytotherapy. 2020;22(2):57–69.
- Crosson FJ, Covinsky K, Redberg RF. Medicare and the shocking US food and drug administration approval of aducanumab: crisis or opportunity? JAMA Intern Med. 2021;181(10):1278–1280.
- Senior M. Rollout of high-priced cell and gene therapies forces payer rethink. Nat Biotechnol. 2018;36(4):291–293.
- Japanese Ministry of Health, Labour, and Welfare. Central Social Insurance Medical Council. [cited 2020 Jul 3]. Available from: https://www.mhlw.go.jp/stf/shingi/shingi-chuo_128154.html
- Pharmaceuticals and Medical Devices Agency (PMDA). The list of newly approval drugs [cited 2021 Apr 20]. Available from: https://www.pmda.go.jp/review-services/drug-reviews/review-information/p-drugs/0010.html
- Japanese Ministry of Health, Labour, and Welfare. The fifth NDB open data [cited 2021 May 19]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000177221_00008.html
- Kossmeier M, Themanns M, Hatapoglu L, et al. Assessing the accuracy of sales forecasts submitted by pharmaceutical companies applying for reimbursement in Austria. Front Pharmacol. 2021;12:2072.
- Keeping S, Deslandes PN, Haines KE, et al. Estimated versus observed expenditure associated with medicines recommended by the all Wales medicines strategy group. Pharmacoecon Open. 2019;3(3):343–350.