?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Various methods exist for the induction of labor (IOL), and there is limited consensus as to optimal methods. Off-label misoprostol is recommended by the World Health Organization (WHO) for IOL but preparing it into doses suitable for IOL lacks precision, with potential adverse outcomes if dosing is inaccurate. This study explores potential outcomes and costs associated with increased uptake of a low-dose (25 µg) oral misoprostol formulation (Angusta; Norgine BV, Amsterdam) approved for IOL, in France, Belgium, and the Netherlands.
Methods
A literature review was undertaken to derive probabilities of delivery outcomes (vaginal, instrumental, and cesarean sections) for IOL methods, from published meta-analyses. Outcomes for oral misoprostol tablets (25 µg) were unavailable in the meta-analyses, so were estimated using data from two published retrospective cohort studies. A model was developed to predict the frequency of IOL outcomes and associated costs at the national level, across multiple scenarios. Scenarios were tested using a moderate, medium, and high increase in oral misoprostol tablet (25 µg) uptake. Market shares, costs, and induction rates were defined for each country using multiple data sources.
Results
Increased uptake of oral misoprostol tablets (25 µg) was estimated to be associated with a slightly increased rate of routine vaginal deliveries, and concurrent decreases in instrumental vaginal deliveries and cesarean sections. Since routine vaginal deliveries are less costly than other delivery outcomes, increased uptake of oral misoprostol tablets (25 µg) within the IOL market has the potential to be cost-saving. These trends were predicted using 25 µg oral misoprostol tablet outcomes informed by both retrospective studies.
Conclusion
Preliminary outcomes suggest that oral misoprostol tablets at 25 µg per dose may improve outcomes in IOL and be cost-saving. Further study is required to validate these findings and assess the comparative efficacy of IOL methods, including oral misoprostol tablets (25 µg).
Introduction
Induction of labor (IOL) is recommended where there is a clear medical indication and the expected benefits outweigh its potential harmsCitation1. The benefits of IOL (where clinically indicated) in pregnancies at or beyond term include improvement of neonatal and maternal outcomes and reducing the risk of cesarean sectionsCitation2. Globally, rates of IOL have increased over time. Across European countries, induction rates differ. In France, 22.0% of all births involve IOL, whereas in Belgium the proportion is 28.5%, and in the Netherlands, 21.3%Citation3–10.
Guidelines on IOL exist internationally, nationally, and at the local hospital levelCitation1,Citation11–15. A recent systematic review of guidelines found consistency in guidance for inducing labor for prolonged pregnancy and premature rupture of membranes, but inconsistencies in the timing of IOL for other indicationsCitation16. There are also large disparities in IOL rates between hospitals, based on local guidelines and practices.
Multiple methods exist for inducing labor, including pharmacological, mechanical, and alternative interventions. Options recommended by the World Health Organization (WHO) are low dose oral or vaginal misoprostol, low dose vaginal prostaglandins, and balloon catheter; oxytocin is an alternative when prostaglandins (including misoprostol) are not availableCitation17. There is considerable variation between countries in choice of method, due to differing opinions nationally and locallyCitation18–21.
Misoprostol is a synthetic prostaglandin E1 analogueCitation22. It is sold under the brand name Cytotec (Pfizer Inc, Groton, CT) and other brand names, and is indicated for the treatment of gastric and duodenal ulcersCitation23. Misoprostol is among the options recommended by the WHO and has commonly been used off-label for IOL; however, off-label use is associated with various concerns. Cytotec is formulated as 200 µg tablets, but the dosing required for IOL is either 25 or 50 µg per dose. There is no single protocol for the preparation of the correct dose, and practitioners use differing preparation methods and dosing regimensCitation24. Cutting up the tablet is difficult to do precisely and may lead to dosing inaccuracyCitation25,Citation26. Further, compounded Cytotec deteriorates rapidly and therefore has a short shelf lifeCitation27. Correct misoprostol dosing is vital to ensure efficacy and safety and minimize adverse eventsCitation28. Too high a dose can increase the risk of uterine hyperstimulationCitation29, too low a dose can lead to ineffective inductionCitation30. Because of the risk of negative outcomes associated with off-label use, Cytotec has been withdrawn for all indications in France due to safety concernsCitation23,Citation31, and is subject to warnings and restrictions in other countriesCitation32,Citation33.
Oral misoprostol tablets (25 µg) (Angusta, Norgine BV, Amsterdam) are designed specifically for IOL, providing a fixed ready-to-use dose of misoprostol requiring no additional preparation time in the pharmacyCitation28,Citation34. They are the only oral formulation of misoprostol approved for IOL and were developed following requests from the Danish and French authorities for a controlled low dose form of misoprostol. Evidence from real-world studies shows favorable obstetric outcomes for oral misoprostol tablets (25 µg) in various IOL protocolsCitation30,Citation34–36.
Comparing different IOL methods in terms of both clinical outcomes and costs is important for clinical and payer decision-making. However, there are significant gaps in clinical evidenceCitation37, and considerable uncertainty has been found when ranking methods in terms of clinical outcomesCitation38. Moreover, limited trial sizes have rendered analyses of adverse outcomes, such as uterine rupture impreciseCitation37. The aim of IOL is to achieve vaginal delivery, without the use of instruments or cesarean section. Instrumental vaginal delivery and cesarean section are suboptimal outcomes for IOL and are also associated with greater costs than routine vaginal deliveryCitation39,Citation40. Therefore, interventions that improve these outcomes also have the potential to save costs.
The objective of this study is to analyze the outcomes and associated costs for the different IOL methods available in three countries (the Netherlands, Belgium, France), and to explore the effects of increased use of oral misoprostol tablets (25 µg) at a population level, assuming displacement of various other interventions (based on country-specific market shares). This is performed by a cost-calculator model using a national healthcare insurance perspective, with clinical outcomes informed by a targeted literature review (TLR). These three countries were selected based on the broad similarity and comparability of their healthcare systems and associated costs.
Methods
Targeted literature review
A TLR was conducted to assess the efficacy of IOL interventions and to inform the cost calculator model. Searches of databases (PubMed, Embase) were conducted to identify systematic literature reviews (SLRs) comparing mechanical or pharmacological interventions for IOL. The Cochrane Pregnancy and Childbirth Group website was also searchedCitation41. Findings were grouped by study type (network meta-analysis [NMA] or meta-analysis [MA]) and by the number of comparisons in MA studies (>2 or ≤2).
Eligibility criteria were applied following the PICOS framework (population, intervention, comparison, outcome, and study design), in line with Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) guidanceCitation42. In brief, only SLR studies written in English and published from 2015 to June 2020 (the date of the search) were included; the date limit was applied to obtain recent information relevant to current practice. Full details of the PICOS criteria, search strategy, and PRISMA diagram are given in the Supplementary Material (Tables S1–S4 and Figure S1). The population of interest was women with a viable fetus undergoing third trimester IOL, and interventions of interest were pharmacological or mechanical interventions for IOL (i.e. not alternative interventions, such as acupuncture), and not methods for augmentation of labor, such as oxytocin. Ideally, data would have been limited to women with an unripe cervix, to ensure that only IOL, rather than labor augmentation, was evaluated. However, the evidence available specifically for this subpopulation is limited and therefore the population was not restricted based on cervical ripeness. An overall population of women undergoing IOL was evaluated; no specific subpopulations were assessed and studies assessing a specific subpopulation (e.g. nulliparous women only, or women with a previous cesarean section) were excluded due to the differences in risk and outcomes in these specific subpopulations.
Review outputs and calculation of probabilities
A total of 369 studies were identified of which 33 met the inclusion criteria. Three were NMA and the remainder were MA. Of the MA, five evaluated two or more interventions (Supplementary Material, Figure S1). None of the identified studies evaluated oral misoprostol tablets (25 µg). Three studies have been published which describe delivery outcomes with oral misoprostol tablets (25 µg) (), however, only two are of interestCitation35,Citation36. The third evaluated outcomes in nulliparous women only so did not meet the inclusion criteriaCitation30. One of the studies evaluated two treatment regimens, one of which did not reflect the recommended dosing in the Summary of Product Characteristics (SmPC),Citation34 and was therefore not incorporatedCitation35. The sensitivity of the model to outcome probabilities reported within the two included studies for oral misoprostol tablets (25 µg) (Bendix et al.Citation35 and Helmig et al.Citation36) was assessed in scenario analyses.
Table 1. Population and dosing in oral misoprostol tablets (25 µg) studies.
Absolute probability values for each outcome and intervention of interest were calculated (). These data were assessed for clinical plausibility by expert clinicians (RH, FP, and RD) and were deemed plausible.
Table 2. Absolute probability values for each intervention and outcomes.
Comparators were selectively drawn from the TLR outputs based on the interventions used within each of the scope countries. Outcomes of interest were cesarean section rates (from which total vaginal delivery rates could be calculated), and instrumental vaginal delivery rates, as these were the only outcomes routinely published across all oral misoprostol tablets (25 µg) and comparator studies.
Ideally, a single study that included all interventions and associated probabilities for the outcomes of interest would have been used to inform the cost calculator inputs, but none of the identified studies fulfilled these criteria. To minimize variability in values due to different study methods, the number of studies used was kept to a minimum. In total, four were selected based on the following order of priorities (Supplementary Material, Table S5):
Absolute probabilities directly reported in NMA studies
Odds ratios reported in NMA studies, where absolute probability could be calculated
Absolute probabilities directly sourced from the minimum number of MA studies to fulfill all the interventions
To capture the absolute probability of outcomes, probabilities had to be calculated from odds ratios vs. placebo. Given that:
and:
The probability of an outcome with the intervention can be calculated as:
Where P( ) is the probability with either intervention or comparator. Therefore, knowing the probability of an outcome with the comparator, the probability of an outcome with the intervention of interest may be calculated using:
To capture the absolute probability of outcomes, from risk ratios, probabilities were calculated using the following equation:
Where P() is the probability with either intervention or comparator
Cost calculator model
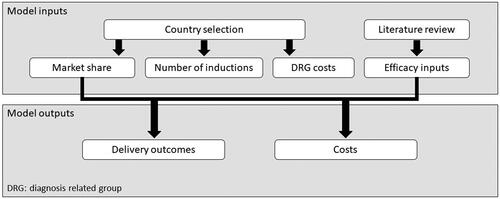
A cost calculator () was developed in Microsoft Excel to calculate the costs and number of events associated with IOL for each country. This used a one-year time horizon without discounting rates (due to 1-year horizon). The cost calculator evaluated core obstetric outcomes (vaginal delivery and cesarean section rates) for various interventions, and market shares for these interventions. The model was developed from a national healthcare insurance perspective, focusing on diagnosis-related group (DRG) costs for the outcomes of interest. DRG costs are fixed fees that encompass all the costs in the relevant episode, regardless of the length of stay and other individual variations in a patient’s care. The cost calculator was quality checked before analysis of results (calculations, and face validity of outputs).
Model inputs
Inputs into the model comprised absolute probabilities of outcomes for each IOL intervention, and country-specific data on the number of interventions, unit costs for outcomes, and market shares for each intervention () Drug costs are inherently included within these unit costs for France and the Netherlands (based on their payment system). In Belgium these are not included as they are paid for by the individual undergoing IOL (and therefore are not explicitly included given the perspective used), except for a small proportion of the cost of Prostin E2 (Pfizer Ltd.); this cost was considered negligible and was not included. Assumptions were made on country-specific details, for example on the route of Prostin E2 and Cytotec delivery, and exclusion of oxytocin (based on the assumption that studies evaluated induction rather than augmentation of labor). Model inputs and assumptions were discussed with expert clinicians (RH, FP, and RD) and were deemed clinically plausible.
Table 3. Summary of country-specific inputs.
Scenario testing in cost calculator
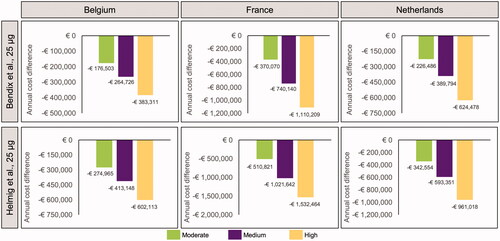
Efficacy, market share, and cost data reflecting current practice at the time of writing were incorporated into the cost calculator. For each country, scenarios were evaluated using hypothetical moderate, medium, and high increases in oral misoprostol tablet (25 µg) market share compared to current values. For each country and set of market share scenarios, two different datasets from retrospective observational cohort studies were used to inform outcome probabilities with oral misoprostol tablets (25 µg) (). The model was run twice, once for the outcomes reported in each retrospective study.
Results
Market share scenarios
The market share of oral misoprostol tablets (25 µg) differs between countries. In Belgium and the Netherlands, oral misoprostol tablets (25 µg) had not been introduced at the time that market share data was obtained, so began from a base of zero. Under the moderate, medium, and high increases, its estimated market share reached 27.0, 40.7, and 60.0%, respectively in Belgium (Supplementary Material, Table S6) and 19.9, 34.9, and 57.7%, respectively in the Netherlands (Supplementary Material, Table S7). In France, from a baseline market share of 29.0%, the share rose to 36.1, 43.2, and 50.3% under the respective scenarios (Supplementary Material, Table S8). Other interventions were displaced accordingly.
Outcomes assessment
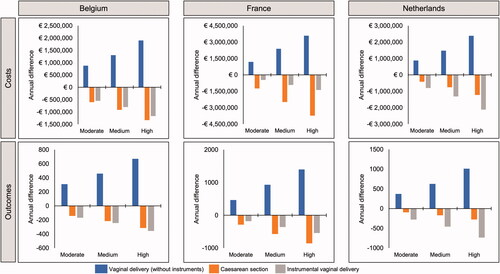
Based on data for oral misoprostol tablets (25 µg) informed by Bendix et al.Citation35, increased use of this formulation was predicted to result in a reduction in instrumental vaginal deliveries and cesarean sections in each country, with a corresponding increase in routine vaginal deliveries (). The same trends were predicted based on the data from Helmig et al.Citation36, again driven by predicted reduced cesarean section and instrumental vaginal delivery rates with oral misoprostol tablets (25 µg) (). This result was driven by the low cesarean section and instrumental vaginal delivery rate reported for oral misoprostol tablets (25 µg) in the original datasets. For Belgium and the Netherlands, there was a greater predicted reduction in the number of instrumental deliveries than cesarean sections, whereas in France the reverse was true (i.e. a greater reduction in cesarean sections).
Figure 2. Differences in annual costs and outcomes of induction of labor under scenarios of moderate, medium, and high uptake of oral misoprostol tablets (25 µg), based on data from Bendix et al.Citation35, 25 µg per dose (N = Belgium 21,100; France 110,773; Netherlands 23,349).
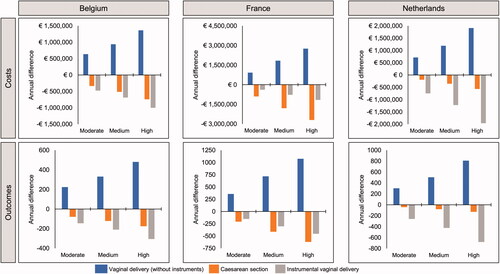
Figure 3. Differences in annual costs and outcomes of induction of labor under scenarios of moderate, medium and high uptake of oral misoprostol tablets (25 µg), based on data from Helmig et al.Citation36, 25 µg per dose (N = Belgium 21,100; France 110,773; Netherlands 23,349).
Impact on costs
Since both instrumental vaginal deliveries and cesarean sections have a greater unit cost than routine vaginal deliveries, this translated into predicted cost savings. In all three countries, increasing the use of oral misoprostol tablets (25 µg) was predicted to be associated with a net annual cost saving with both of the relevant datasets (). As oral misoprostol tablets (25 µg) use increased, so did cost savings.
Discussion
Our study describes potential clinical and cost advantages from increased use of oral misoprostol tablets (25 µg) for IOL in France, Belgium, and the Netherlands. The study focuses on core delivery outcomes for IOL, namely routine vaginal delivery, instrumental delivery, and cesarean section. Comparing outcomes from the Bendix et al.Citation35 and the Helmig et al.Citation36 observational studies of oral misoprostol tablets (25 µg) in real-world practice with evidence from published meta-analyses, our study suggests that an increased population-level uptake of oral misoprostol tablets at 25 µg per dose (dosing every 2 h) may be associated with improved outcomes compared with other IOL methods, namely slightly lower rates of instrumental vaginal delivery and cesarean section. This trend was predicted in all three countries. However, the differences estimated are small and their significance is uncertain, as the analysis was descriptive and generation of confidence intervals and significance testing were not undertaken. Clinical experience as the formulation is used more widely could be evaluated to confirm whether improvement in outcomes and the associated cost savings are realized in real-world practice.
Since instrumental vaginal delivery and cesarean section are more expensive than routine vaginal deliveryCitation39,Citation40, increased use of oral misoprostol tablets (25 µg) has the potential to be cost-saving if the estimated differences in outcomes were confirmed in practice. This was predicted in all three of the scope countries, based on the data available. In France, savings were predicted even though instrumental and standard vaginal delivery are assigned the same cost by the French healthcare system. The cost savings predicted may be conservative, as the study did not take into account additional cost savings associated with reducing primary cesarean section rates, such as the consequent reduction in repeat caesareans for subsequent pregnanciesCitation43 and the cost of neonatal complications. Relatively small improvements in primary and repeat cesarean rates have been found to be associated with substantial reductions in hospital costsCitation44.
There is a paucity of data explicitly comparing outcomes between different IOL methods. Few head-to-head comparisons between interventions are available in the literature, and therefore alternative methods had to be used to estimate absolute probability values for outcomes with each intervention. To make the estimates as robust as possible given the available data, NMA studies were used in preference to MA studies, and the number of studies used was kept to the minimum necessary to obtain all the data. However, since oral misoprostol tablets (25 µg) have only been approved for use since 2017, it is not explicitly included in any of the identified NMA and MA studies that were used to evaluate efficacy and outcomes for the other methods. Therefore, a naïve comparison had to be undertaken between outcomes in the oral misoprostol tablet (25 µg) studies and calculated absolute probabilities from the literature. This is a limitation of the study since there is no adjustment to control for heterogeneity between populations across studies. A further limitation is that the analysis was descriptive only, meaning that the degree of uncertainty around the point estimates, or the statistical significance of the differences calculated, are not known. Additionally, this paucity of data meant that outcomes, such as neonatal intensive care admissions could not be evaluated, as this was not sufficiently captured in the NMA and MA studies.
An additional limitation of the data is the lack of information on whether women in the studies have an unripe cervix. Our study focuses on cervical ripening and IOL, and as such would ideally only incorporate data from women with an unripe cervix in whom methods, such as oxytocin, are used for the augmentation of labor as opposed to cervical ripening, would be excluded. One SLR attempted to evaluate a low Bishop score population, aligning to an unripe cervix, but inconsistencies within the data meant this was not possibleCitation29. Other authors have commented that no or very few relevant clinical trials specifically recruited women with an unripe cervixCitation38,Citation45. Therefore, although the data included in our study does not specifically represent the subpopulation of women with an unripe cervix, it would not be feasible to limit the analysis to this group, and the majority of women in studies of IOL receiving the interventions of interest are likely to have an unripe cervix at baseline.
There are various avenues for future research building on the analyses presented herein. For example, incorporating neonatal and post-natal maternal outcomes, and assessment of individual subpopulations (such as nulliparous women). Full cost-effectiveness analyses would generate additional information; these may be undertaken in the future depending on the reimbursement requirements for each country. Using a full cost-effectiveness analysis, further impacts on costs, labor outcomes, and quality of life may be evaluated at a patient level. However, the paucity of high-quality comparative effectiveness evidence noted above is likely to result in uncertainty. A comprehensive cost-effectiveness evaluation comparing 19 different IOL interventions (not including oral misoprostol tablets [25 µg]) in the UK reported “considerable uncertainty around effect estimates” and “a high degree of uncertainty in [our] estimates of cost-effectiveness”Citation29.
There is a need for a low-dose oral misoprostol formulation, such as oral misoprostol tablets (25 µg), that is specifically designed and approved for IOL, to extend the options available to women and their physicians and obviate the risks associated with off-label use of Cytotec. A prospective qualitative study in Germany reported that the most common reasons for not using misoprostol were concern over the lack of a license (69%) and uncertainty of the legal situation (27%)Citation46. Furthermore, gaps in providing information on the choice of method for inducing labor, the associated risks, and reduced ability to make informed choices, are reported as reducing maternal satisfaction with IOLCitation47. The introduction of an approved oral formulation provides additional choices and may improve women’s satisfaction with their treatment.
The preliminary outcomes reported here suggest that increased use of oral misoprostol tablets (25 µg) within IOL could potentially increase the proportion of routine vaginal deliveries, with concurrent decreases in instrumental vaginal deliveries and cesarean sections, both of which may be associated with negative implications for both maternal and fetal health. If confirmed in clinical practice, this predicted improvement in obstetric outcomes has the potential to be cost-saving vs. current IOL practice, based on the increased cost associated with instrumental vaginal deliveries and cesarean sections, compared with routine vaginal deliveries. Further study is required to validate these findings and assess the comparative efficacy of IOL methods, including oral misoprostol tablets (25 µg).
Transparency
Declaration of funding
This study was supported by Norgine Ltd.
Declaration of financial/other relationships
A.C.P. is an employee of Norgine Ltd. K.P. is an employee of Health Economics and Outcomes Research Ltd., which received fees from Norgine in relation to this study and development of the manuscript. R.H. received consulting fees from Norgine Ltd. for this article and reports no other potential conflict of interest relevant to this work. F.P. received consulting fees from Norgine Ltd. for this article, has received consulting fees from Roche Diagnostics France, and reports no other potential conflict of interest relevant to this work. R.D. received consulting fees from Norgine Ltd. for this article, has received research grants from Ferring and consulting fees from Metagenics, and reports no other potential conflict of interest relevant to this work.
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
All authors: study conception and design. KP: analysis of data. All authors: interpretation of data. K.P. and A.C.P.: drafting of the manuscript. All authors: critical revision of the manuscript and final approval of the manuscript. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (47.7 KB)Acknowledgements
Editorial assistance was provided by Jo Whelan of Health Economics and Outcomes Research Ltd., funded by Norgine.
References
- World Health Organization. WHO recommendations: Induction of labour at or beyond term; 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/277233/9789241550413-eng.pdf
- Middleton P, Shepherd E, Crowther CA. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev. 2018;5(5):Cd004945.
- Norgine. Confidential market research.
- Institut national de la statistique et des études économiques. INSEE and official statistics; 2021. Available from: https://www.insee.fr/fr/statistiques/5012724
- Perined. Perined database 2018. Available from: perined.nl/
- Belgian Federal Public Service. STATBEL. Available from: https://statbel.fgov.be/fr
- Leroy C, Van Leeuw VS. Santé périnatale en Wallonie – Année 2019; 2020.
- Devlieger R, Goemaes R, Laubach M. Perinatale activiteiten in Vlaanderen 2019; 2019.
- Van Leeuw V, Daelemans C, Debauche C, et al. Santé périnatale en Région bruxelloise – Année 2017; 2019.
- Coulm B, Bonnet C, Blondel B, et al. Enquête nationale périnatale Rapport 2016: Les naissances et les établissements. Situation et évolution depuis 2010; 2017.
- Iannuzzi L, Branchini L, Clausen JA, et al. Optimal outcomes and women's positive pregnancy experience: a comparison between the world health organization guideline and recommendations in European National Antenatal Care guidelines. Minerva Ginecol. 2018;70(6):650–662.
- Vayssiere C, Haumonte J-B, Chantry A, et al. Prolonged and post-term pregnancies: guidelines for clinical practice from the French College of Gynecologists and Obstetricians (CNGOF). Eur J Obstet Gynecol Reprod Biol. 2013;169(1):10–16.
- Mandruzzato G, Alfirevic Z, Chervenak F, et al. Guidelines for the management of postterm pregnancy. J Perinat Med. 2010;38(2):111–119.
- Nederlandse Vereniging voor Obstetrie & Gynaecologie. Guideline Prolonged Pregnancy; 2007.
- Belgian Health Care Knowledge Centre. Guideline relative to low risk birth; 2010.
- Coates D, Homer C, Wilson A, et al. Induction of labour indications and timing: a systematic analysis of clinical guidelines. Women Birth. 2020;33(3):219–230.
- World Health Organization. WHO recommendations for Induction of labour; 2011. Available from: https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241501156/en/
- Mambourg F, Gailly J, Wei-Hong Z. Guideline relative to low risk birth. Good clinical practice (GCP). Brussels: Belgian Health Care Knowledge Centre (KCE); 2010.
- Blanc-Petitjean P, Salomé M, Dupont C, et al. Labour induction practices in France: a population-based declarative survey in 94 maternity units. J Gynecol Obstet Hum Reprod. 2018;47(2):57–62.
- Haute Autorité de Santé (HAS). Déclenchement artificiel du travail à partir de 37 semaines d'aménorrhée; 2008.
- Federatie Medisch Specialisten. Elective inductie; 2020.
- World Health Organisation. WHO recommendations: Induction of labour at or beyond term; 2018.
- Pfizer Ltd. Cytotec 200 mcg tablets – summary of product characteristics (SmPC)-(eMC); 2013 [cited 2021 Feb 10]. Available from: https://www.medicines.org.uk/emc/product/1642/smpc
- Weeks A, Navaratnam K, Alfirevic Z. Simplifying oral misoprostol protocols for the induction of labour. BJOG. 2017;124(11):1642–1645.
- Amini M, Reis M, Wide-Swensson D. A relative bioavailability study of two misoprostol formulations following a single oral or sublingual administration. Front Pharmacol. 2020;11:50.
- Pfizer Ltd. New Zealand Data Sheet: Cytotec 200 microgram tablets. New Zealand Medicines and Medical Devices Safety Authority (MEDSAFE); 2019. Available from: https://www.medsafe.govt.nz/profs/Datasheet/c/Cytotectab.pdf
- Berard V, Fiala C, Cameron S, et al. Instability of misoprostol tablets stored outside the blister: a potential serious concern for clinical outcome in medical abortion. PLOS One. 2014;9(12):e112401.
- Amini M, Reis M, Wide-Swensson D. A relative bioavailability study of two misoprostol formulations following a single oral or sublingual administration. Front Pharmacol. 2020;11:50.
- Alfirevic Z, Keeney E, Dowswell T, et al. Methods to induce labour: a systematic review, network meta-analysis and cost-effectiveness analysis. BJOG. 2016;123(9):1462–1470.
- Eriksson A, Jeppesen S, Krebs L. Induction of labour in nulliparous women-quick or slow: a cohort study comparing slow-release vaginal insert with low-dose misoprostol oral tablets. BMC Pregnancy Childbirth. 2020;20(1):79.
- Agence nationale de sécurité du medicament et des produits de santé. Cytotec (misoprostol): arrêt de commercialisation à compter du 1er mars 2018 – Communiqué; 2017 [cited 2021 Feb 10]. Available from: https://ansm.sante.fr/S-informer/Communiques-Communiques-Points-presse/Cytotec-misoprostol-arret-de-commercialisation-a-compter-du-1er-mars-2018-Communique
- National Collaborating Centre for Women's and Children's Health. Clinical guideline: induction of labour (CG70); 2016.
- Federal Institute for Drugs and Medical Devices (BfArM). Dear Doctor Letter (Rote-Hand-Brief) on Cytotec® (misoprostol): risks associated with an off-label use for birth initiation; 2020. Available from: https://www.bfarm.de/SharedDocs/Risikoinformationen/Pharmakovigilanz/EN/RHB/2020/rhb-cytotec.pdf;jsessionid=2D391EF527C0DFCC253B8912F668A983.2_cid354?__blob=publicationFile&v=2
- Norgine BV. ANGUSTA summary of product characteristics (SmPC); 2020 [cited 2020 Oct 30]. Available from: https://mri.cts-mrp.eu/Human/Downloads/DK_H_2584_001_FinalSPC.pdf
- Bendix JM, Friis Petersen J, Andersen BR, et al. Induction of labor with high- or low-dosage oral misoprostol–A Danish descriptive retrospective cohort study 2015–16. Acta Obstet Gynecol Scand. 2020;99(2):222–230.
- Helmig RB, Hvidman LE. An audit of oral administration of Angusta® (misoprostol) 25 µg for induction of labor in 976 consecutive women with a singleton pregnancy in a University Hospital in Denmark. Acta Obstet Gynecol Scand. 2020;99(10):1396–1402.
- Mozurkewich EL, Chilimigras JL, Berman DR, et al. Methods of induction of labour: a systematic review. BMC Pregnancy Childbirth. 2011;11(1):84.
- Alfirevic Z, Keeney E, Dowswell T, et al. Which method is best for the induction of labour? A systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess. 2016;20(65):1–584.
- Technische Cel. Nationale Databank Medische Diagnose 2021. Available from: tct.fgov.be/
- Nederlandse Zorgautoriteit. Open data from the Dutch Healthcare Authority 2021. Available from: https://www.opendisdata.nl/
- Cochrane Pregnancy and Childbirth Group. Our reviews 2020 [cited 2020]. Available from: https://pregnancy.cochrane.org/our-reviews
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
- DeJoy SA, Bohl MG, Mahoney K, et al. Estimating the financial impact of reducing primary cesareans. J Midwifery Womens Health. 2020;65(1):56–63.
- Moran PS, Normand C, Gillen P, et al. Economic implications of reducing caesarean section rates – analysis of two health systems. PLOS One. 2020;15(7):e0228309.
- Vogel JP, Osoti AO, Kelly AJ, et al. Pharmacological and mechanical interventions for labour induction in outpatient settings. Cochrane Database Syst Rev. 2017; 9(9):CD007701.
- Voigt F, Goecke TW, Najjari L, et al. Off-label use of misoprostol for labor induction in Germany: a national survey. Eur J Obstet Gynecol Reprod Biol. 2015;187:85–89.
- Coates R, Cupples G, Scamell A, et al. Women's experiences of induction of labour: qualitative systematic review and thematic synthesis. Midwifery. 2019;69:17–28.
- Alfirevic Z, Keeney E, Dowswell T, et al. Labour induction with prostaglandins: a systematic review and network meta-analysis. BMJ. 2015;350(10):h217.
- de Vaan MDT, ten Eikelder MLG, Jozwiak M, et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;10(10):CD001233.
- ScanSante. Stats ATIH 2021. Available from: https://www.scansante.fr/
- Norgine. Confidential Market research 2020.
- Blanc-Petitjean P, Salomé M, Dupont C, et al. [Overview of induction of labor practices in France]. Gynecol Obstet Fertil Senol. 2019;47(7–8):555–561.