Abstract
Aims
In Egypt, cardiovascular (CV) diseases are not only the cause of 33% of disability-adjusted life years but are also a leading cause of death. This study aimed to evaluate dapagliflozin’s cost-effectiveness as an add-on to the standard of care (SOC) for the treatment of heart failure with reduced ejection fraction (HF-rEF) from the Egyptian healthcare system perspective.
Materials and methods
A state transition model was utilized to assess the cost-effectiveness of dapagliflozin as an add-on to the SOC and a cost-minimization analysis was performed to compare dapagliflozin to sacubitril/valsartan, as they have had similar efficacy. Patients were stratified into four health states using the KCCQ-TSS, in addition to a CV and non-CV mortality health states. Urgent heart failure (HF) visits and hospitalizations were captured as transient states. Clinical parameters and baseline characteristics were based on the DAPA-HF trial, utility scores were extracted from published articles, and costs were derived from the Universal Health Insurance Authority national database. Deterministic and probabilistic sensitivity analyses were performed.
Results
The treatment costs of HF-rEF patients receiving dapagliflozin compared to SOC are 47,901EGP ($10,550) and 34,377EGP ($7,572), respectively. The quality-adjusted life-years (QALYs) of dapagliflozin compared to SOC are 4.57 and 4.20, respectively. This resulted in an incremental cost per effectiveness ratio (ICER) of 36,449EGP ($8,028) per QALY gained over the lifetime horizon, suggesting this is cost-effective. Results of the cost-minimization analysis showed cost savings where the annual costs of dapagliflozin vs. sacubitril/valsartan are 10,914EGP ($2,404) and 32,242EGP ($7,101), respectively.
Conclusion
Dapagliflozin was found to be a highly cost-effective and cost-saving medication when compared to SOC and sacubitril/valsartan, respectively, in the treatment of HF-rEF from Egyptian healthcare system perspective. The ICER was below the willingness-to-pay threshold because dapagliflozin improved outcomes (less frequent hospitalization and mortality).
Introduction
Heart failure (HF) is a condition caused by the decreased function of either both heart ventricles or the left ventricle, leading to signs of congestion and symptoms, such as excessive fatigue and shortness of breathCitation1. The complexity of HF arises mainly from the ventricles’ inability to adequately circulate blood to the rest of the body, which consequently leads to decreased physical activity and increased fluid retentionCitation2. If left untreated, this manifestation could eventually lead to edema in the periphery and pulmonary congestion, thus significantly decreasing the patient’s quality of life and increasing the risk of mortalityCitation2.
As HF is a progressive disease, the New York Heart Association (NYHA) developed a system to classify the state of the patient, ranging from I to IV, where “Class” I refers to a patient at risk for heart failure, who does not have any symptoms, limitations, or structural abnormalityCitation3. “Class” II refers to patients with mild symptoms (mild shortness of breath and/or angina) and a slight limitation to physical activity. “Class” III includes patients with considerable limitations in physical activity due to symptoms, even during minimal activity, such as walking short distances (20—100 m). “Class” IV refers to patients with severe limitations who experience symptoms even while at rest or who are mostly bedbound patientsCitation3.
HF is also commonly described using the left ventricle ejection fraction (LVEF), which reflects the risks, progression, and need for intervention. The LVEF is used to classify HF patients into three classes. The first, those with LVEF >50%, are said to have HF with a preserved ejection volume (HF-pEF); the second, those with LVEF between 40 and 50%, are said to have HF with a mid-range ejection fraction (HF-mrEF); and patients with an LVEF <40% are said to have HF with a reduced ejection fraction (HF-rEF)Citation4.
More than 30 million people worldwide suffer from HF; however, the lifetime risk of developing HF is estimated to be 1 in 5Citation5. Moreover, HF has morbidity and mortality rates similar to some types of cancerCitation5. Globally, patients hospitalized for HF die within 5 years and 17–45% die within the first year after hospitalizationCitation6.
HF has resulted in 9.91 million years lost due to disability (YLD) and estimated costs of 346.17 billion USDCitation7. Risk factors for HF and HF-caused disability include male sex, age > 60 years, and low- and middle-income countriesCitation7.
In Egypt, cardiovascular diseases (CVDs) not only account for 33% of disability-adjusted life years (DALYs), but are also the leading cause of death since the 1990sCitation8,Citation9. It was estimated by the World Health Organization (WHO) that CVD causes 46.25% of all deaths in Egypt; thus, there is an urgent need to assess the burden of HF in Egypt, especially due to the lack of data on HF prevalenceCitation10. In 2020, the European Society of Cardiology Heart Failure Long-Term (ESC-HF-LT) Registry, of which Egypt is a member country, published a study on the burden and demographics of HF in Egypt, which included patients recruited from 2011 to 2014 in various regions of EgyptCitation9. Of the 1,661 HF patients recruited, the mortality among hospitalized patients ranged from 2.9–7.7%, with the most common HF condition being ischemic diseases. Other common characteristics observed in the participants included mitral regurgitation and complications due to infection, while the most common type of HF was HF-rEF.
The standard of care (SOC) for HF-rEF according to the 2016 guidelines of the European Society of Cardiology (ESC) includes angiotensin-converting enzyme (ACE) inhibitors, mineralocorticoid receptor antagonists (MRAs), and beta-blockers, as they have been proven to have beneficial outcomes in the treatment of HF-rEFCitation4. ACE inhibitors could be replaced by the combination of sacubitril and valsartan for patients who remain symptomatic despite optimized regimensCitation4. In addition, if patients on ACE inhibitors cannot take MRAs for tolerance-related reasons, angiotensin II receptor blockers (ARBs) could be considered, but the use of ARBs should be limited because there is limited evidence that their use improves the likelihood of patient survivalCitation4.
Dapagliflozin (Forxiga®, AstraZeneca) is a novel drug with a unique mechanism of actionCitation11. Dapagliflozin is a sodium-glucose co-transporter-2 inhibitor (SGLT2i) that has proven efficacy and safety in the treatment of type 2 diabetesCitation12.
However, in the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial, an investigation of dapagliflozin as a beneficial treatment for HF-rEF was conducted because, in a previous study, it reduced the risk of hospitalization due to HF in diabetic patientsCitation13. The DAPA-HF trial was a controlled, randomized, phase 3 clinical trial that enrolled 4,744 patients with HF-rEF (<40% EF), who were “class” II or more HF according to the NYHA classification. Participants received either 10 mg dapagliflozin or a placebo, in addition to standard care. The trial concluded that the risk of complications among HF-rEF patients was lower with 10 mg dapagliflozin compared to the placebo, regardless of whether the patient had type 2 diabetes.
In the current study, we aimed to evaluate the long-term economic consequences and cost-effectiveness of including dapagliflozin as an addition to the SOC for the treatment of HF-rEF from the Egyptian health insurance perspective. All direct medical costs were calculated, including the cost of medication and the cost of complication management.
Methods
Model design
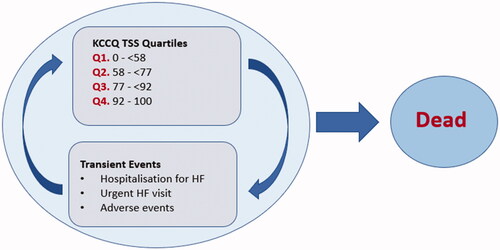
The current study utilized a state-transition Markov model to assess the cost-effectiveness of dapagliflozin, as shown in . The model consists of five health states according to the Kansas City Cardiomyopathy Questionnaire-Total Symptom Score (KCCQ-TSS), which quantifies the overall health of an individual, including physical function, social function, and quality of life, where the highest score represents the best overall health. The KCCQ-TSS ranges are Q1: 0–<58, Q2: 58–<77, Q3: 77–<92, Q4: 92–100. In addition to the four health states represented by the KCCQ-TSS ranges, urgent HF visits, hospitalization for HF, and adverse events were captured as transient states. Lastly, mortality was captured in the model as the absorbing state of death stratified by CV mortality and non-CV mortality. Considering HF is a chronic and progressive disease, the model time horizon is lifelong, assuming that most patients (>97%) died at 85. The cycle length was 1-month with a half-cycle correction.
Two base cases were considered for the evaluation of dapagliflozin. The first base case analysis was dapagliflozin compared to placebo as an add-on to the SOC, which was defined as ACE inhibitors and ARB, plus beta-blockers, and diuretics with or without MRAs. In the second base case analysis, a cost-minimization analysis was conducted comparing dapagliflozin to sacubitril/valsartan assuming similar efficacy based on the results of a matching adjusted indirect comparison (MAIC) performed by the National Institute for Health and Care Excellence (NICE)Citation14. The MAIC showed that there was no statistically significant difference between dapagliflozin and sacubitril/valsartan in terms of the time to CV death, hospitalization for HF, or all-cause mortality.
Target population
The target population for our analysis was adult HF-rEF patients aged 18 years or older with an ejection fraction of 40% or less, and NYHA “class” II, III, or IV symptoms. These criteria were based on the DAPA-HF trial, which was a randomized controlled phase 3 trial, conducted on 4,744 HF-rEF patients at 410 centers in 20 countries. Participants received either dapagliflozin or a placebo for the management of HF, in addition to the SOCCitation13.
Clinical parameters
All model inputs are listed in . The baseline characteristics of the two cohorts were extracted from the DAPA-HF trialCitation13. The DAPA-HF trial findings were used to build the model, including the incidence of urgent HF visits and hospitalizations, mortality rates, rates of adverse events, and therapy discontinuation. Furthermore, the change in KCCQ-TSS was also extracted from the trial and utilized in the model as the transition probability between the KCCQ-TSS quartile. The transition probabilities extracted from the DAPA-HF trial were used for the first four months, after which extrapolation was used to determine the remaining transition probabilities, as reported by NICECitation14.
Table 1. Model input values.
Extrapolation for overall survival (OS) Kaplan-Meier probability curves were performed to estimate lifetime extrapolation based on parametric models fitted to patient-level data. For OS, we used patients who died from any cause in the Kaplan-Meier probability curveCitation13. The Weibull parametric function provided a plausible fit for the OS curves, as reported in the NICE technology appraisalCitation14.
The five health states in our model were defined as follows: KCCQ-TSS quartile 1 (1–<58) represented the most complicated health state (i.e. patients were hospitalized, susceptible for monitoring, urgent HF visits, and high CV mortality); KCCQ-TSS quartile 2 (58–<77) included monitoring, urgent HF visits and hospitalizations, and the same CV mortality risk in KCCQ-TSS 1; starting with KCCQ-TSS quartile 3 (77–<92), the CV mortality risk decreased and monitoring, urgent HF visits and hospitalizations were applied to the patient; finally, in KCCQ-TSS quartile 4 (92–100), patients are not at risk for HF hospitalizations and urgent visits due to a high KCCQ-TSS, which corresponds to better health, accounting for subsequent monitoring costs, decreased CV mortality risk, and death by any cause.
Costs
Total healthcare costs were calculated as the quantity of each resource used multiplied by the corresponding unit cost in Egyptian Pounds (EGP) derived in 2021. In the first base case analyses, the annual cost of each class of medication (ACE inhibitors, ARBs, MRAs, beta-blockers, and diuretics), in addition to dapagliflozin and sacubitril/valsartan were derived from unit costs on the Universal Health Insurance Authority (UHIA) national database (secondary data). Dapagliflozin was calculated per the recommended dose (10 mg/day), ACE inhibitors (most commonly used: enalapril) at a dose of 10–20 mg per day, ARBs at 16 mg given twice daily, and MRAs (eplerenone) calculated at 25 mg administered twice daily. All costs were calculated based on the percentage of HF patients estimated to be on each medication. All medication doses were based on the official recommended doses and were validated by an expert panel.
Monitoring costs were also considered and included kidney function tests, complete blood count, potassium level tests, and echocardiograms (ECHOs). Tests were performed every 3 months with the exception of ECHOs that were performed every 6 months. The unit cost for the tests was extracted from UHIA national database. The cost of hospitalizations for HF, CV events, and urgent HF visits was captured from UHIA national database, and transient health states were applied to represent each complication. Background therapy costs do not differ according to the KCCQ-TSS quartiles as the progression of the disease is captured through increased HF hospitalization over the time horizon. The model considered direct medical costs and health effects from the healthcare payer’s perspective. A macro-costing approach was used to determine the costs.
A cost-minimization analysis was conducted to compare dapagliflozin and sacubitril/valsartan, assuming similar efficacy to that of a MAIC performed by NICECitation14.
Utility
The utility of each health state and the disutility values due to HF hospitalizations and urgent HF visits were extracted from the NICE technology appraisal for dapagliflozinCitation14. These utility and disutility values were derived from the DAPA-HF trial using a mixed-effect model. In the DAPA-HF trial, EQ-5D-5L questionnaires were used to evaluate the patient’s health-related quality of life. The questionnaires were administered on days 0, 120, 240, and 360 during every study year.
The utility value corresponding to each health state was multiplied by the number of patients in the mentioned health states based on transition probability to calculate the cumulative quality-adjusted life years (QALYs). HF hospitalizations and urgent HF visits were calculated as one-off decrements for the patients in these transient health states.
Sensitivity analyses
Deterministic sensitivity analyses (DSAs) were conducted to assess the robustness of the model and the effect of the most important parameters on the cost-effectiveness of the intervention. The inputs included in the sensitivity analyses were the cost of dapagliflozin, SOC, sacubitril/valsartan, HF hospitalizations, urgent HF visits, and all utilities, in addition to the CV and non-CV mortality rates.
To assess how a simultaneous change in several parameters affects the ICER, a probabilistic sensitivity analysis was performed. This analysis runs a large number of repeated simulations (e.g. 1,000) by drawing samples from the probability distributions of the input parameters and thus provides a probability distribution of the incremental costs, incremental effectiveness, and ICERs.
Results
The results of our model are presented in . In the first base case analysis, the treatment costs for HF-rEF patients using dapagliflozin plus SOC compared to SOC alone are 47,901EGP ($10,550) and 34,377EGP ($7,572), respectively. The QALYs of dapagliflozin plus SOC compared to SOC alone were 4.57 and 4.20, respectively. This resulted in an incremental cost per QALY gained of 36,449EGP ($8,028) over the lifetime horizon. Dapagliflozin use resulted in improved health outcomes compared to SOC but at a slightly higher cost. However, it is still considered cost-effective because it is less than the Egyptian Cost-effectiveness Threshold (3XGross Domestic Product per capita; 150,000EGP) recommended by the WHO. The improved outcomes were consistent throughout the patient’s disease journey.
Table 2. Decision analytic model results.
In the second base case analysis, a cost-minimization analysis was conducted to compare dapagliflozin and sacubitril/valsartan, assuming similar efficacy. The annual costs, including the cost of medications and monitoring of dapagliflozin and sacubitril/valsartan were 10,914EGP ($2,404) and 32,242EGP ($7,101), respectively, at a difference of −21,327EGP (−$4,697) per patient per year. Thus, dapagliflozin is cost-saving vs. sacubitril/valsartan as it is associated with lower costs than the sacubitril/valsartan group.
Sensitivity analyses
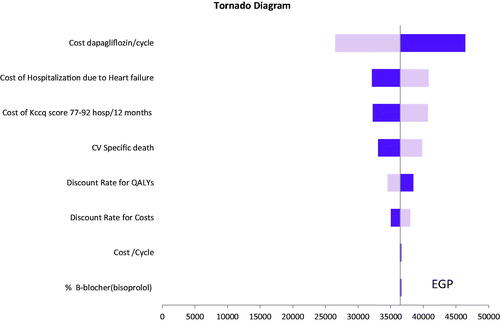
The robustness of the model and the results were tested using DSA. The tornado diagram () suggests that the results are robust in one-way sensitivity analyses. The most sensitive parameters of our model results were the cost of dapagliflozin per cycle and the cost of hospitalization due to HF.
Figure 2. Deterministic sensitivity analysis of dapagliflozin vs. standard of care. Abbreviations. QALY, quality adjusted life year; HF, heart failure; KCCQ-TSS, Kansas City Cardiomyopathy Questionnaire-Total Symptom Score; CV, cardiovascular. Base case = EGP 36,449, The light blue bar corresponds with the upper range, and the dark blue bar with the lower range of an input.
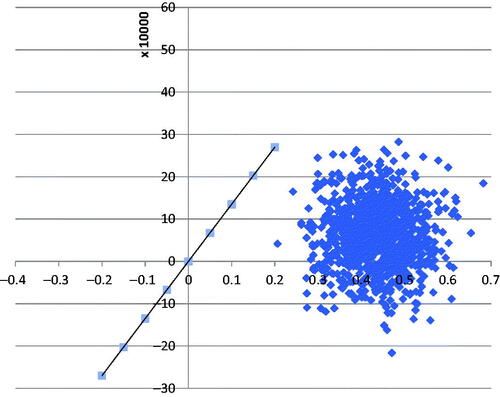
To consider the uncertainty around the parameters, an incremental cost-effectiveness plane was constructed. All inputs were simultaneously varied within several distributions. The plane in demonstrates the 1,000-iteration cost outcome difference pairs. As shown, most difference pairs are found in the northeast and southeast quadrants of the cost-effectiveness plane, which indicates that dapagliflozin use in HF patients is more effective (i.e. positive incremental QALY scores) and that there is a large proportion in the southeast quadrant, suggesting that the intervention is cost-effective.
Discussion
Dapagliflozin is an innovative SGLT2i used for the treatment of type 2 diabetes and was recently approved by the Food and Drug Administration for the treatment of HF-rEF in adultsCitation16. It had better efficacy compared to the placebo as an add-on to the SOC. In the DAPA-HF trial, dapagliflozin was associated with better outcomes in HF-rEF, which reduced the risk of HF-related mortality and HF hospitalizationsCitation13. Our results demonstrated that dapagliflozin is highly cost-effective and is a good value compared to the SOC due to fewer hospitalizations and lower mortality, in addition to cost-savings compared to sacubitril/valsartan. Furthermore, dapagliflozin improved tolerance in patients from different age groupsCitation17. Besides clinical benefits, dapagliflozin also had significant benefits for patients’ physical function and quality of lifeCitation18. Importantly, dapagliflozin increased the number of patients with improved health statusCitation18.
Dapagliflozin was evaluated by NICE and was found to be cost-effective and recommended for the treatment of HF-rEF in addition to the optimum SOC; ACE inhibitors, ARBs, or sacubitril/valsartanCitation19. The aforementioned NICE technology appraisal report also noted the unmet need that dapagliflozin fulfills. Specifically, hope for patients with a condition that has a poor prognosis, especially for those with low ejection fractions. In addition, a new medication was needed for healthcare providers to treat this condition, thus adding to the value of dapagliflozin. Dapagliflozin was previously approved for the treatment of diabetes and therefore has added benefit for a large portion of the population with both diabetes and HF. The approval of dapagliflozin for HF-rEF treatment is a potential step toward a whole new class of treatment for HF. It also has preventive benefits, such as the prevention of diabetic eye disease due to its glycemic lowering effect.
A literature review was performed to compare our results with those of other published studies. Three studies investigated the cost-effectiveness of dapagliflozin for the treatment of HFCitation20–22. The first is a multinational study investigating the cost-effectiveness of dapagliflozin as an add-on to optimized SOC compared to placebo from the German, British, and Spanish payer’s perspectiveCitation20. The study found that the ICER was £5,822/QALY from the UK perspective, €5,379/QALY from the German perspective, and €9,406/QALY from the Spanish perspective, thus deeming them all cost-effective in comparison to the SOC. Furthermore, more than 90% of all simulations during the probabilistic sensitivity analysis were cost-effective. The second study was conducted from the Australian healthcare perspective to assess the cost-effectiveness of dapagliflozin as an add-on to the SOC vs. the SOC alone for the treatment of chronic HFCitation21. The study calculated an ICER of $8,875 per year life saved and $12,482 per QALY gained, both of which were less than the Australian willingness-to-pay threshold of $50,000. Accordingly, dapagliflozin was found to be a cost-effective add-on therapy to the SOC for the treatment of HF with reduced lifetime ejection fraction. The third study from the Chinese healthcare perspective also evaluated dapagliflozin as an add-on therapy to the SOC for HF-rEF vs. the SOC aloneCitation22. The study estimated an ICER of $4,412.50 per QALY gained with a willingness-to-pay threshold of $8,573. The most influential factors on the results were medication cost, hospitalization cost, and CV deaths in the dapagliflozin and placebo groups. The analysis concluded that dapagliflozin was very cost-effective for the treatment of HF-rEF from the Chinese public healthcare perspective.
To the best of our knowledge, our model is the first in Egypt and the Middle East/North Africa (MENA) region to evaluate the cost-effectiveness of adopting dapagliflozin but we conducted other cost-effectiveness studies on different products using the Egyptian cost-effectiveness thresholdCitation23. Our findings lend further support to results from previously published trials and to the NICE recommendations to use dapagliflozin as an add-on for HF-rEF patients. The robustness of our model was investigated using DSA to increase the internal validity of the model. In addition, the clinical parameters for the model were extracted from the DAPA-HF clinical trial, which had a large number of participants, thus increasing the generalizability of the model.
In our model, different scenarios and cases were investigated. The first base case analysis was dapagliflozin compared to placebo as an add-on to the SOC. The second base case analysis was a cost-minimization analysis comparing dapagliflozin to sacubitril/valsartan, assuming similar efficacy. The different base cases ensured that the model captured the expected results and outcomes in the different subgroups, who were expected to benefit from the addition of dapagliflozin to their treatment regimen.
However, there are a few limitations to our model. Although the DAPA-HF is a high evidence source, due to strict inclusion and exclusion criteria, there is a difference between a clinical outcome in clinical trials and outcomes observed in real-world practice. The results of our cost-effectiveness analysis cannot be generalized to other countries because it considered the local inputs costs in Egypt. Another limitation was the novel values for dapagliflozin, such as the value of hope for HF-rEF patients and the value of scientific spillover. This was the first SGLT2i to be approved for the treatment of HF, so these values were not able to be quantified. However, it is likely that such values would have further validated our findings and increased the cost-effectiveness of dapagliflozin.
Conclusion
Dapagliflozin was found to be a highly cost-effective and cost-saving medication for the treatment of HF-rEF from the Egyptian healthcare system perspective when compared to the SOC and sacubitril/valsartan, respectively. The ICER was below the Egyptian willingness-to-pay threshold due to less frequent hospitalizations and lower mortality. A budget impact analysis is needed to identify its affordability.
Transparency
Declaration of funding
This work was supported by AstraZeneca, Egypt under Grant [number 4470067354].
Declaration of financial/other relationships
No conflicts of interest.
A reviewer on this manuscript has disclosed that they have recently received a grant from Mundipharma for a health economic evaluation of canagliflozin in renal disease. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
The Deputy Editor in Chief helped with adjudicating the final decision on this paper.
Author contributions
MA, MS, SM, and AE analyzed and interpreted the collected data. GE wrote the manuscript. SE and AS collected needed data about cost and outcomes. MR and MA reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
None reported.
References
- Rossignol P, Hernandez AF, Solomon SD, et al. Heart failure drug treatment. Lancet. 2019;393(10175):1034–1044.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure). J Am Coll Cardiol. 2005;46(6):e1–e82.
- New York Heart Association. New York Heart Association (NYHA) classification. NYHA; 1995.
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 42(36):3599–3726.
- Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41.
- Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1(1):4–25.
- Lippi G, Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Med J. 2020;5:15.
- Elrabbat MS, Sedrak AS, Elsebaie EH, et al. Cost-Analysis of heart failure cases: a pilot single center study in Egypt. Egypt J Commun Med. 2019;37(1):48–53.
- Hassanin A, Hassanein M, Bendary A, et al. Demographics, clinical characteristics, and outcomes among hospitalized heart failure patients across different regions of Egypt. Egypt Heart J. 2020;72(1):1–9.
- World Health Organization. Global health estimates: Life expectancy and leading causes of death and disability; 2020.
- Plosker GL. Dapagliflozin: a review of its use in type 2 diabetes mellitus. Drugs. 2012;72(17):2289–2312.
- List JF, Woo V, Morales E, et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32(4):650–657.
- McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
- NICE. Single technology appraisal dapagliflozin for treating heart failure with reduced ejection fraction [ID1656]; 2020. Available from: https://www.nice.org.uk/guidance/ta679/evidence/committee-papers-pdf-9016515613
- World bank website: PPP conversion factor, GDP (LCU per international $) – Egypt, Arab Re. Available from: https://data.worldbank.org/indicator/PA.NUS.PPP?locations=EG
- US Food and Drug Administration. FDA approves new treatment for a type of heart failure; 2020.
- Martinez FA, Serenelli M, Nicolau JC, et al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA-HF. Circulation. 2020;141(2):100–111.
- Kosiborod MN, Jhund PS, Docherty KF, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141(2):90–99.
- NICE. Final appraisal document–Dapagliflozin for treating chronic heart failure with reduced ejection fraction; 2020. Available from: https://www.nice.org.uk/guidance/gid-ta10560/documents/final-appraisal-determination-document
- McEwan P, Darlington O, McMurray JJ, et al. Cost‐effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health‐economic analysis of DAPA‐HF. Eur J Heart Fail. 2020;22(11):2147–2156.
- Savira F, Wang BH, Kompa AR, et al. Cost-effectiveness of dapagliflozin in chronic heart failure: an analysis from the Australian healthcare perspective. Eur Heart J. 2020;41(Supplement_2):ehaa946.1051.
- Yao Y, Zhang R, An T, et al. Cost‐effectiveness of adding dapagliflozin to standard treatment for heart failure with reduced ejection fraction patients in China. ESC Heart Failure. 2020;7(6):3582–3592.
- Hamdy Elsisi G, Nada Y, Rashad N, et al. Cost-effectiveness of sorafenib versus best supportive care in advanced hepatocellular carcinoma in Egypt. J Med Econ. 2019;22(2):163–168.