Abstract
Aim
This study evaluates the economic impact to US commercial payers of MMDx-Kidney used in conjunction with histologic evaluation of for-cause kidney transplant biopsies.
Materials and methods
An Excel-based model was developed to assess the cost impact of histology plus MMDx-Kidney versus histology alone for the evaluation of potential rejection in kidney transplant patients who receive a for-cause biopsy. Different model time periods were assessed, ranging from 1 to 5 years post-biopsy. A targeted literature review was used to identify parameter estimates, validated by two external clinicians with expertise in managing kidney transplant rejection. A sensitivity analysis was conducted to evaluate the relative impact of key clinical and cost parameters. In particular, the model identified the magnitude of MMDx-Kidney’s impact on graft failure from rejection that would be required for MMDx-Kidney to be cost-neutral.
Results
By more accurately characterizing rejection, MMDx-Kidney is estimated to increase antirejection treatment costs by $1,126 per test. Nevertheless, a break-even analysis shows that the costs of MMDx-Kidney and anti-rejection medication, as well as the costs associated with an increase in the number of patients with functioning transplants, may be offset by reductions in costs associated with graft failure (i.e. costs of hospitalizations, dialysis, and repeat transplants) over 5 years, assuming MMDx-Kidney reduces annual graft failure from rejection by at least 5%. For the base case, with a 25% relative reduction in annual rate of graft failures from rejection, MMDx-Kidney increases overall costs incurred in the first year of the model but starts generating savings by the second year of the model.
Conclusions
Compared with histologic evaluation of for-cause kidney transplant biopsies alone, the use of MMDx-Kidney in conjunction with histologic evaluation improves the diagnoses of graft dysfunction and may have the potential to generate overall savings from reductions in rejection-related graft failure.
Introduction
According to the Organ Procurement and Transplantation Network (OPTN), 22,817 kidney transplants were performed in the United States in 2020Citation1. Kidney transplantation has grown steadily by 20% in the last 5 years aloneCitation1. This increase is driven by the rise in eligible deceased-donor kidney transplants to over 77% of all kidney transplants from 40% in 2015Citation1. While greater opportunities for transplant benefit patients, deceased-donor transplants present an increased risk of rejection; approximately 60% of kidney graft failures in the US today are due to chronic or acute rejectionCitation2. Currently, 21% of deceased-donor kidney transplant recipients and 14% of living-donor kidney transplant recipients experience graft failure within 5 years of transplant.
Potential treatment options following kidney graft failure are dialysis or another kidney transplant. The median wait-time for a kidney transplant recorded in 2013 was 49.2 months, due to the limited availability of donor kidneysCitation3. Patient survival rates with dialysis are also poor when compared to survival rates for patients with a functioning kidney transplantCitation4. The median overall survival after kidney graft failure is estimated to be 3 yearsCitation5.
The economic burden of graft failure in kidney transplant recipients is substantial. Based on a 2017 US transplant cohort, graft failure from rejection imposes a cumulative economic burden to Medicare of $698 M, amounting to roughly half of the total economic burden of graft failureCitation2. According to the United States Renal Data System (USRDS), at the individual patient level, a functioning transplant cost Medicare $28,106 per year in 2018, compared to $126,771 for dialysis in the year of graft failure and $136,696 for a repeat transplant in the year of the initial graft failureCitation3.
Optimal therapy directed at preserving transplant viability for as long as possible requires accurate, timely assessment of the cause of a failing graft. Histologic biopsy interpretation and measurements of serum creatinine, glomerular filtration rate (GFR), and proteinuria are the current gold standards for assessing renal allograft function after kidney transplant, but these tools often do not accurately detect whether allograft dysfunction derives from injury, antibody-mediated rejection (ABMR), T-cell-mediated rejection (TCMR), or ABMR and TCMR combined (mixed rejection).
Grades of rejection are defined by the Banff Classification of Allograft Pathology – established in 1991 and updated regularly as additional research informs consensus, with the most recent criteria set at the 2019 meetingCitation6. Guided by a histology assessment based on the Banff criteria, pathologists exhibit a 30% error rate when evaluating kidney allograft dysfunctionCitation7. In addition to inadequate detection of rejection type, clinical interpretation of histology is subjective and, thus, remains prone to inter- and intra-observer variabilityCitation8–15. Further, as the tissue becomes more abnormal, the likelihood that the pathologist’s assessment departs from Banff criteria increasesCitation16. The Banff guidelines themselves can be a limitation, too, as classifications in the guidelines are often ambiguousCitation6,Citation17,Citation18. Lastly, the sample type required by histology may also be a limitation. For example, histology requires 10 glomeruli and two arteries, but the medulla contains none of the required glomeruli; thus, histology alone cannot assess rejection in the medulla of biopsiesCitation19.
The Banff 2019 meeting report advocates for the implementation of molecular diagnostics in conjunction with histology to improve the evaluation of renal allograft rejection and prognosis of graft failure, particularly when Banff classifications are ambiguous (i.e. ABMR in the absence of donor-specific antibodies). More specifically, the report names Molecular Microscope MMDx-Kidney (MMDx-Kidney) as a “more pathogenesis-driven way” to improve diagnostic capabilitiesCitation6.
MMDx-Kidney uses microarrays to measure messenger ribonucleic acid (mRNA) levels and profiles 1,494 genes in fresh tissue transplant biopsies. With predefined algorithms, MMDx-Kidney assesses the probability of rejection or injury based on observed patterns in gene expression in the biopsy. In particular, MMDx-Kidney is intended to complement all available diagnostic information for more precise evaluation and management of TCMR, ABMR, acute injury, chronic injury, atrophy fibrosis (AF), arteriolar hyalinosis (AH), acute kidney injury (AKI), and disturbance (inflammation). MMDx-Kidney diagnoses abnormalities in histologically problematic kidney transplant biopsies in all donor types, unrelated and related, living and deceasedCitation8. In addition, unlike histology, which generates binary or semi-quantitative grades (0, 1, 2, or 3), MMDx-Kidney provides continuous results, which are especially critical when a biopsy has values near boundaries.
In particular, MMDx-Kidney is intended to be used with for-cause biopsies (also known as indication biopsies), which are defined as biopsies conducted outside pre-determined intervals. Such biopsies are more likely to present with rejection than protocol or surveillance biopsies, and, thus, MMDx-Kidney is more likely to provide needed guidance when used with for-cause biopsiesCitation20.
Accurate diagnoses of dysfunctional kidney grafts may allow for optimized immunosuppressive treatment and improved outcomes, including graft survival. Based on our extensive review of published literature, this paper is the first to explore the economic impact associated with a reduction in rates of graft failure with MMDx-Kidney. The budget-impact model assesses the potential overall costs or savings associated with the use of MMDx-Kidney for a US commercial payer compared to the current standard of care: histopathology evaluation.
Methods
Study design
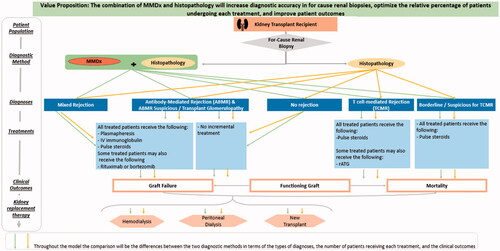
To estimate the economic impact to a US commercial payer of histology plus MMDx-Kidney versus histology alone following a for-cause biopsy in kidney transplant patients, a budget impact model was developed in Excel. The dynamic model was intended to allow easy examination of several scenarios, including varying assumptions about the impact of MMDx-Kidney on graft failure from rejection. The target population comprises kidney transplant recipients receiving a for-cause biopsy. The care pathway that the model follows is illustrated in .
In the base case of the model, patients presenting with symptoms of abnormal kidney graft activity receive a for-cause biopsy and are allocated to the following outcome categories:
Functioning initial transplant;
Graft failure and re-transplant in the same year;
Graft failure and dialysis in the same year;
Death with a functioning graft; and
Death after graft failure.
In the base case scenario, the model assumes MMDx-Kidney reduces the annual rate of graft failure from rejection by 25% in relative terms (e.g., from a 5.5% graft failure rate in the first year post-biopsy to 4.1%). While the magnitude of MMDx-Kidney’s impact is an assumption, a recent MMDx-Kidney study has shown that adding ABMR scores to the conventional assessment improves the prediction of 3-year graft failure by 23%Citation21. The model calculates undiscounted savings or losses in five separate model duration scenarios, ranging from 1 to 5 years after a patient receives a for-cause biopsy.
Model development
A focused literature review was used to develop a clinical care pathway following the initial for-cause biopsy, as well as treatment costs for rejection and outcomes post-biopsy. The estimates identified as the baseline parameters are from peer-reviewed publications, the United States Renal Data System (USRDS) database, Scientific Registry of Transplant Recipients (SRTS)/OPTN database, and Centers for Medicare and Medicaid Services Fee-for-Service (CMS FFS) data. The identified parameters were further verified through consultation with two practicing physicians experienced in the diagnosis and management of kidney transplant rejection (G.G. and C.L.) to ensure values reflected real-world experience.
Model assumptions
Given current limitations in longitudinal testing and outcomes data, the base-case model relies on several simplifying assumptions. First, patients receive a single diagnosis after exhibiting symptoms of graft dysfunction that results in a for-cause biopsy. Consequently, the model reflects one set of antirejection treatments per patient diagnosed with rejection and does not account for additional for-cause biopsies or multiple uses of MMDx-Kidney per patient in the time span of the model. Second, patients who start a certain type of dialysis after a graft failure do not switch to another type of dialysis in the time frame of the model. Third, while the model identifies patients who receive a second transplant in the year of graft failure, patients with graft failure who start dialysis do not receive a second transplant in the time frame of the model. Fourth, outcomes of death, functioning transplant, and graft failure occur after patients prescribed therapy for rejection have fully completed their treatment. Fifth, while the model identifies patients who do not receive a rejection diagnosis, the model does not specify the number of patients who are non-adherent to immunosuppression therapy or who have graft injury or scarring.
Outside the base case, the assumption regarding the number of MMDx-Kidney tests per patient is adjusted in a single scenario: an evaluation of the number of MMDx-Kidney tests required to eliminate savings over 5 years, given a 25% reduction in annual graft failure from rejection with the test.
Kidney rejection diagnosis and treatment
Based on the prospective INTERCOMEX study of kidney transplant biopsies in North America and Europe, presents the percentage distribution of patients by diagnosis status with the standard of care (histology) versus histology plus MMDx-KidneyCitation22. With MMDx-Kidney, 54% of indication biopsies in the study showed “no-rejection” and 46% showed “rejection”. MMDx-Kidney further classified rejection biopsies by rejection type.
Table 1. Percentage distribution of diagnoses after for-cause kidney biopsy.
While all patients diagnosed with TCMR, mixed rejection, and suspicious/borderline for acute TCMR receive treatment, 80% of the patients with an ABMR diagnosis and 20% of patients with an ABMR suspicious and transplant glomerulopathy diagnosis receive treatment, based on the experience of two clinical experts (G.G. and C.L.). Treated patients are assigned diagnosis-specific therapies derived from peer-reviewed sources and confirmed by clinical practice (G.G. and C.L.) ( and Supplementary Table S1). Based on an analysis of hospital claims for the ICD-10 T86.11 code for transplant rejection, approximately one third of treated patients are assumed to receive antirejection therapies in an inpatient setting, with the rest receiving outpatient treatmentCitation23. Patients without rejection do not receive any additional treatment in the model.
Table 2. Antirejection treatment percentage distribution and costs for outpatient visit.
Clinical outcomes
The proportion of patients with functioning transplants, mortality, graft failure from rejection, and graft failure from other causes over 5 years was estimated using the 30-year simulation model developed by Sussell et alCitation2. (). Further, the relative proportion of annual mortality in patients with graft failure versus patients with a functioning graft was estimated from an assessment of USRDS data reported by Kaplan and Meier-KriescheCitation24.
Table 3. Rates of outcomes 5 years after for-cause kidney biopsy.
Costs
Cost estimates following for-cause kidney biopsies include: (1) the cost of MMDx-Kidney, (2) the cost of antirejection treatment in all settings, and (3) the average annual costs associated with each of the clinical outcomes, including renal replacement therapy (dialysis or re-transplant).
Cost of MMDx-Kidney test
The cost per MMDx-Kidney test was assumed to be $3,159.42 based on Medicare reimbursement rates for CPT Code 0088 U: “transplantation medicine (kidney allograft rejection), microarray gene expression profiling of 1,494 genes, utilizing transplant biopsy tissue, algorithm reported as a probability score for rejection”, as published in the CMS CY 2022 Clinical Lab Fee Schedule Final Payment DeterminationCitation25,Citation26.
Cost of outpatient antirejection treatment
The outpatient costs for each antirejection treatment, including administration costs, were estimated using the CMS FFS schedule and based on the recommended dosage and length of treatment for each medication (). The total Medicare costs were then multiplied by an adjustment factor of 126% to reflect the approximate amounts incurred by commercial payers relative to MedicareCitation27.
Cost of inpatient antirejection treatment
For patients treated in the hospital inpatient setting, Medicare costs associated with three Diagnosis-Related Group (DRG) codes for “other kidney and urinary tract diagnoses” (698, 699, 700) were identified using CMS Fiscal Year (FY) 2021 Final Rule Tables and the CMS ICD-10-CM/PCS MS-DRG Definitions ManualCitation28,Citation29. All three DRG codes map to the International Classification of Disease (ICD-10) CM T.86-11 code for the diagnosis of kidney transplant rejection in general; the ICD-10 code is not specific to the type of rejection (ABMR versus TCMR versus mixed). Payment distribution across the three DRG codes was calculated using hospital claims data from the Definitive Healthcare database. The weighted average of the Medicare costs was multiplied by a factor of 189% to reflect the amounts incurred by commercial payersCitation30.
Cost of outcomes
reports the annual costs of each outcome following a for-cause biopsy. Data from USRDS models were used to estimate annual costs for surviving patients within each outcome group. These models calculate costs from ESRD Medicare inpatient, outpatient, skilled nursing facility, hospice, home health, physician/supplier, durable medical equipment, and Part D claims data from 2018. To estimate Medicare costs associated with death with graft function and death after graft failure, 2014 economic data from the OPTN/SRTR database were usedCitation31. To reflect commercial payer costs, the Medicare costs were multiplied by an adjustment factor of 146%, based on a weighted average of inpatient and outpatient adjustment factorsCitation27,Citation30. All costs were inflated to 2021 costsCitation32.
Table 4. Annual costs of outcomes following for-cause kidney biopsy.
Sensitivity analysis
Multiple one-way sensitivity analyses were conducted using a relative change of ±10% from the base-case values of model input parameters. Parameters that demonstrate the highest impact on base case values are presented.
Results
Base case budget impact analysis
The average antirejection treatment costs for biopsies diagnosed with rejection with the standard of care versus MMDx-Kidney plus the standard of care are provided in . In the base case scenario, MMDx-Kidney increases total rejection treatment costs by $1,126 per test as a result of identifying more patients with ABMR and mixed rejection in the first year of the model.
Table 5. Antirejection treatment costs per MMDx-Kidney test.
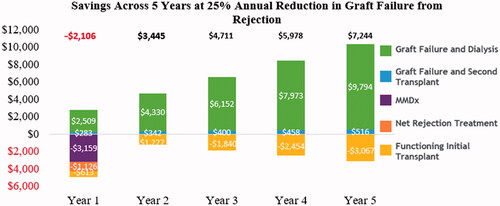
presents the savings with MMDx-Kidney by year in a 5-year model, given the assumption that MMDx-Kidney may reduce annual graft failure from rejection by 25% after a for-cause biopsy, in relative terms. In year one, the costs increase by $3,159 per patient for MMDx-Kidney, $1,126 per patient for anti-rejection treatment, and $613 per patient for the management of functioning transplants, while savings of $2,509 and $283 per patient occur because of fewer dialysis cases and fewer repeat transplants, respectively. From years two through five, overall savings are observed as a result of the reduced rates of graft failure; the decrease in the costs associated with patients receiving renal replacement therapy (dialysis and repeat transplants) outweighs the increase in costs from additional patients with functioning kidney transplants.
Overall, assuming one MMDx-Kidney test per patient, MMDx-Kidney results in an estimated undiscounted savings of $19,271 per test over a 5-year period, with positive savings starting with models of a 2-year duration ().
Table 6. Savings per MMDx-Kidney test with different model durations, assuming a 25% annual Impact of MMDx-Kidney on graft failure from rejection.
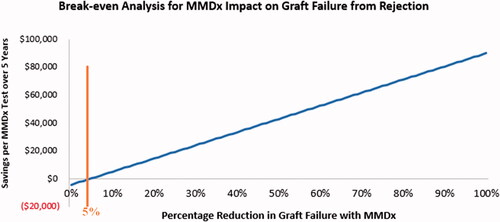
Over a 5-year timeframe, reductions in costs associated with graft failure (i.e. costs of hospitalizations, dialysis, and repeat transplants) completely offset the increased costs of antirejection treatment, functioning transplants, and a single-use of MMDx-Kidney per patient, assuming MMDx-Kidney reduces annual graft failure from rejection by at least 5%. This break-even point is illustrated in .
Figure 3. Break-even analysis: relationship between overall savings per MMDx-Kidney test over 5 years and MMDx-Kidney’s impact on graft failure from rejection. The orange line indicates the minimum percentage in graft failure reduction that will offset increased costs associated with MMDx-Kidney. The blue, line represents the savings per test at increasing percentages of reduction in graft failure from rejection with MMDx-Kidney.
Scenario analysis: number of MMDx-Kidney tests
If the model assumes MMDx-Kidney can be used multiple times per patient, one could potentially use MMDx-Kidney six times over 5 years before the cost of MMDx-Kidney ($3,159.42) eliminates the estimated savings from the test, assuming a 25% reduction in annual graft failure from rejection with MMDx-Kidney. This simplified assessment assumes MMDx-Kidney neither further improves the patient’s clinical trajectory beyond the 25% impact nor reduces the need for additional for-cause biopsies. This analysis also does not include the cost of the for-cause biopsy, as all costs are measured after the first for-cause biopsy. In this simplified scenario, the model continues to assume that each patient diagnosed with rejection receives one set of antirejection treatments over the 5 years of the model and that patients who start a certain type of dialysis after a graft failure do not switch to another type of dialysis in the time frame of the model. The model also continues to assume that patients with graft failure who start dialysis do not receive a second transplant in the time frame of the model.
Sensitivity analysis
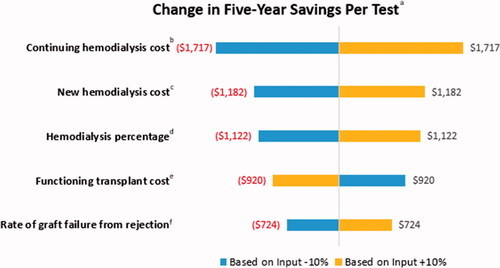
Results from the one-way sensitivity analysis indicate the parameters with the greatest impact on savings are the following: the annual cost of maintaining hemodialysis in the years after graft failure, the cost associated with hemodialysis in the year of graft failure, the percentage of patients receiving hemodialysis after graft failure, the annual cost of a functioning initial transplant, and the rate of graft failure from rejection in the year after for-cause biopsy. The absolute value of the changes in savings per MMDx-Kidney test over 5 years range from $724 to $1,717 with these inputs ().
Figure 4. Sensitivity analysis: top five input parameters with the most impact on the change in savings per test. aThe change in savings per MMDx-Kidney test is defined as the difference between (a) the savings per test over 5 years between the MMDx-Kidney plus the standard of care and the standard of care alone in the base case and (b) the savings per test over 5 years between MMDx-Kidney plus the standard of care and the standard of care alone with the adjusted input parameter defined in the sensitivity analysis. bContinuing hemodialysis cost: annual cost to payers for patients receiving hemodialysis after the year of kidney transplant failure. cNew hemodialysis cost: annual cost to payers for patients who continue to hemodialysis in the year of kidney graft failure. dHemodialysis percentage: percentage of patients with graft failure who receive hemodialysis. In this analysis, the percentage of patients receiving peritoneal dialysis remains constant; the change in hemodialysis percentage directly impacts the percentage receiving a second transplant after graft failure. eFunctioning transplant: annual cost to payers for patients who have a functioning kidney transplant. fRate of graft failure from rejection: standard of care rate of graft failure from rejection in year 1 after the for-cause biopsy. In this analysis, the model applies MMDx-Kidney’s relative percentage improvement in graft failure in the base case (25%) to this adjusted rate.
Discussion
MMDx-Kidney in conjunction with the standard of care (histopathologic evaluation of kidney biopsies) produces savings to commercial payers within 2 years because of its ability to clarify rejection classifications and reduce costs associated with rejection-related graft failure, including re-transplants and dialysis. Although treatment of active ABMR may not always result in complete and prolonged response, several recent studies have provided evidence that MMDx-Kidney improves clinical decisions and avoids over-treatment with immunosuppression therapyCitation9,Citation21,Citation33–36.
While MMDx-Kidney’s 25% impact on the annual rate of graft failure from rejection is an assumption in the base case model, a break-even analysis indicates that use of MMDx-Kidney will generate positive savings over 5 years, provided MMDx-Kidney delivers a minimum of only a 5% reduction in annual graft failure resulting from rejection. By reducing graft failure rates and the need for subsequent dialysis or second transplant, MMDx-Kidney lowers the overall costs commercial payers incur within 2 years of the initial for-cause biopsy. Overall, the savings per MMDx-Kidney test over 5 years is estimated to be $19,271 in the base case model.
This study is the first to estimate the economic impact associated with the use of MMDx-Kidney, and the study’s principal strength is the use of well-documented assumptions derived from peer-reviewed literature and validated by leading clinical experts.
There are nevertheless several limitations to our study. First, the model may not capture the complete range of events in the care pathway for kidney transplant patients undergoing for-cause biopsies. To address this limitation, clinicians reviewed input parameters that were derived from a literature review, and appropriate sensitivity analyses were performed to assess the impact of uncertainty on interpretation of results. Second, given the paucity of longitudinal testing and outcomes data, the model reflects one MMDx-Kidney test used in conjunction with a for-cause biopsy, followed by one set of antirejection treatments per patient diagnosed with rejection (i.e. one diagnosis per patient, with no additional for-cause biopsies). Although MMDx-Kidney can benefit patients who receive multiple for-cause biopsiesCitation9, the literature suggests the number of for-cause biopsies per kidney transplant patient is low in the 5 years following for-cause biopsy. For example, the total number of for-cause kidney biopsies at a transplant center in Belgium was 1,443 over a 20-year follow-up period, while 752 patients received at least one for-cause biopsy, suggesting each of these patients received 1.9 for-cause biopsies, on averageCitation37. Similar rates of for-cause biopsies per patient can be derived from French studiesCitation38,Citation39. Third, the model does not explicitly include the cost of adverse events related to antirejection medication. A literature search for possible adverse events associated with antirejection treatment identified heterogeneity in adverse event reporting, low adverse event rates in general, and a lack of clear control dataCitation40,Citation41. Consequently, MMDx-Kidney’s net impact on medication-related adverse events is unclear at this time; adverse events may rise with an increase in ABMR treatment following the use of MMDx-Kidney, while other adverse events may decrease as physicians are able to identify more accurately those patients who would not benefit from treatment. Fourth, the analysis does not account for indirect costs, quality-of-life, and impacts to patients diagnosed without rejection; consequently, the values presented likely reflect a conservative estimate of the impact of MMDx-KidneyCitation42,Citation43. For example, when MMD-Kidney discounts histologic rejection, expensive immunosuppressive drugs may be avoided and quality of life for patients may improve.
As more data become available, further research is needed to evaluate the economic impact of MMDx-Kidney on diagnosing injury and on immunosuppressive management. Additional research can be conducted to demonstrate the overall reduction in mortality and graft failure, including graft failure from causes other than rejection, associated with the use of MMDx-Kidney. It will also be helpful to evaluate the economic impact of MMDx-Kidney in the context of new treatments such as tocilizumab, which may become more widely used.
Conclusion
While a mainstay in the evaluation and management of kidney transplant dysfunction, histopathology based on for-cause biopsies provides insufficient information on the true state of kidney transplant grafts. The diagnostic uncertainty is further exacerbated by limitations in evaluation criteria and treatment guidelines, which increase the burden of management of suspected graft rejection on physicians and patients. Correct diagnosis of the underlying cause of observed graft dysfunction is critical for optimal immunosuppressive management, lowering the risk of graft failure and its corresponding costs.
By integrating machine learning with 1,494 unique gene profiles, MMDx-Kidney provides an opportunity to improve patient management, particularly for lesions classified by histology as ambiguous. Through more accurate diagnosis of rejection, MMDx-Kidney may facilitate better treatment options and reduce the total costs incurred by payers for post-transplant graft failure and the need for subsequent dialysis or replacement transplant. In sum, this budget impact model demonstrates MMDx-Kidney can be an effective complement to histology when performed in patients with suspected kidney dysfunction receiving a for-cause biopsy after transplant.
Transparency
Declaration of funding
This study was funded by a research grant from Thermo Fisher Scientific.
Declaration of financial/other relationships
The analysis was funded by Thermo Fisher Scientific Corporation; one co-author (CL) is currently employed by Thermo Fisher. Boston Healthcare Associates, Inc. (now a Veranex company) received consulting fees from Thermo Fisher for developing the model on which this manuscript is based. Co-authors LF; SM; SFM; and TFG were employed by Boston Healthcare Associates, Inc. (now a Veranex company) at the time of this research.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (21.9 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
Data availability statement
The datasets generated during and/or analyzed during the current study may be available from the corresponding author on reasonable request.
References
- Deceased donor transplants in the U.S by state [Internet]. 2021. [cited 2021 Nov 17]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/.
- Sussell J, Silverstein AR, Goutam P, et al. The economic burden of kidney graft failure in the United States. Am J Transplant. 2020;20(5):1323–1333.
- USRDS. 2021 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda (MD): United States Renal Data System; 2020.
- Kaballo MA, Canney M, O'Kelly P, et al. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin Kidney J. 2018;11(3):389–393.
- McCaughan JA, Patterson CC, Maxwell AP, et al. Factors influencing survival after kidney transplant failure. Transplant Res. 2014;3(1):18.
- Loupy A, Haas M, Roufosse C, et al. The Banff 2019 kidney meeting report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20(9):2318–2331.
- Halloran PF, Pereira AB, Chang J, et al. Potential impact of microarray diagnosis of T cell-mediated rejection in kidney transplants: the INTERCOM study. Am J Transplant. 2013;13(9):2352–2363.
- Reeve J, Böhmig GA, Eskandary F, et al. Generating automated kidney transplant biopsy reports combining molecular measurements with ensembles of machine learning classifiers. Am J Transplant. 2019;19(10):2719–2731.
- Halloran PF, Reeve J, Akalin E, et al. Real time central assessment of kidney transplant indication biopsies by microarrays: the INTERCOMEX study. Am J Transplant. 2017;17(11):2851–2862.
- Rush D. Can protocol biopsy better inform our choices in renal transplantation? Transplant Proc. 2009;41(6 Suppl):S6–S8.
- Furness PN, Taub N. International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project11A complete listing of the participants who all contributed equally to the work of the CERTPAP project includes peter N. Furness (Leicester, UK); NIC. Kidney Int. 2001;60(5):1998–2012.
- Reeve J, Sellarés J, Mengel M, et al. Molecular diagnosis of T cell-mediated rejection in human kidney transplant biopsies. Am J Transplant. 2013;13(3):645–655.
- Furness PN, Taub N, Assmann KJ. International variation in histologic grading is large, and persistent feedback does not improve reproducibility. Am J Surg Pathol. 2003;27(6):805–810.
- Sellarés J, Reeve J, Loupy A, et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant. 2013;13(4):971–983.
- Famulski KS, de Freitas DG, Kreepala C, et al. Molecular phenotypes of acute kidney injury in kidney transplants. J Am Soc Nephrol. 2012;23(5):948–958.
- Madill-Thomsen KS, Reeve J, Bohmig G, et al. editors. Automated histology lesion interpretation in kidney transplant biopsies shows that pathologists often deviate from Banff guidelines. American Transplant Congress; 2019. American Society of Transplantation and the American Society of Transplant Surgeons.
- Becker JU, Chang A, Nickeleit V, et al. Banff borderline changes suspicious for acute T cell-mediated rejection: where do we stand? Am J Transplant. 2016;16(9):2654–2660.
- Roufosse C, Simmonds N, Clahsen-van Groningen M, et al. A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102(11):1795–1814.
- Madill-Thomsen KS, Wiggins RC, Eskandary F, et al. The effect of cortex/medulla proportions on molecular diagnoses in kidney transplant biopsies: rejection and injury can be assessed in medulla. Am J Transplant. 2017;17(8):2117–2128.
- Einecke G, Reeve J, Gupta G, et al. Factors associated with kidney graft survival in pure antibody-mediated rejection at the time of indication biopsy: importance of parenchymal injury but not disease activity. Am J Transplant. 2021;21(4):1391–1401.
- Halloran PF, Chang J, Famulski K, et al. Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J Am Soc Nephrol. 2015;26(7):1711–1720.
- Madill-Thomsen K, Perkowska-Ptasinska A, Bohmig GA, et al. Discrepancy analysis comparing molecular and histology diagnoses in kidney transplant biopsies. Am J Transplant. 2020;20(5):1341–1350.
- Payments and Claims by DRG Code - Medicare [Internet]. Baltimore (MD): Definitive healthcare. 2021. [cited 2021 Sept 07]. Available from: https://www.definitivehc.com/.
- Kaplan B, Meier-Kriesche H-U. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant. 2002;2(10):970–974.
- CMS Manual System-Pub 100-04 Medicare Claims Processing [Internet]. Baltimore (MD): CMS Database; 2021. [cited 2021 Nov 09]. Available from: https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2019Downloads/R4326CP.pdf.
- CMS Clinical Laboratory Fee Schedule (CLFS) Annual Public Meeting [Internet]. Baltimore (MD): CMS Database; 2021. [cited 2021 Nov 09]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Laboratory_Public_Meetings.
- Johnson B, Kennedy K, Kurowski D. Comparing commercial and Medicare professional service prices: health care cost Institute; 2020. [cited 2021 Nov 21]. Available from: https://healthcostinstitute.org/hcci-research/comparing-commercial-and-medicare-professional-service-prices.
- ICD-10-CM/PCS MS-DRG v37.0 definitions manual [Internet]. Baltimore (MD): CMS Database; [cited 2021 Nov 09]. Available from: https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P1151.html.
- Table 5 (FY 2021 Final rule and correction notice MS-DRGs, relative weighting factors and geometric and arithmetic mean length of stay) [Internet]. Baltimore (MD): CMS Database; 2021. [cited 2021 Nov 09]. Available from: https://www.cms.gov/medicare/acute-inpatient-pps/fy-2021-ipps-final-rule-home-page#Tables.
- Lopez E, Neuman T, Jacobson G, et al. How much more than medicare do private insurers pay? A review of the literature: Kaiser Family Foundation. 2020. [cited 2021 Nov 09]. Available from: https://www.kff.org/medicare/issue-brief/how-much-more-than-medicare-do-private-insurers-pay-a-review-of-the-literature/.
- Schnitzler MA, Skeans MA, Axelrod DA, et al. OPTN/SRTR 2016 annual data report: Economics. Am J Transplant. 2018;18:464–503.
- CPI inflation calculator [Internet]. US Bureau of Labor Statistics. 2021. [cited 2021 Nov 09]. Available from: https://www.bls.gov/data/inflation_calculator.htm.
- Kumar D, Demehin M, Christensen J., et al. The role of the molecular microscope to guide therapy for T-cell mediated rejection in kidney transplants. 2020 American Transplant Congress; 2020.
- Lawrence C. Analysis of RNA transcripts by the molecular microscope diagnostic system (MMDx) can direct management after indication kidney transplant biopsy; 2020. 06/24.
- Lawrence C, Thiru S, Manoj A, et al. editors. First UK experience of the molecular microscope (MMDX™) in the kidney transplant clinic. 2019 American Transplant Congress; 2019.
- Myślak M, Deborska-Materkowska D, Ciszek M, et al., editors. The Polish troubled kidney transplant biopsy study: external validation of the molecular microscope system. 2019 American Transplant Congress; 2019.
- Naesens M, Kuypers DRJ, De Vusser K, et al. The histology of kidney transplant failure: a long-term follow-up study. Transplantation. 2014;98(4):427–435.
- Loupy A, Vernerey D, Tinel C, et al. Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts. JASN. 2015;26(7):1721–1731.
- Vargas GG, Caillard S, Parissiadis A, et al., editors. Identification and long-term consequences of antibodies which bound C1q in 118 kidney transplant recipients with donor specific antibodies. World Transplant Congress 2014; 2014.
- Lachmann N, Duerr M, Schönemann C, et al. Treatment of antibody-mediated renal allograft rejection: improving step by step. J Immunol Res. 2017;2017:6872046.
- Prescribing Information of THYMOGLOBULIN® FDA. [2021 Jul 29]. Available from: https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert-Thymoglobulin.pdf
- Tucker EL, Smith AR, Daskin MS, et al. Life and expectations post-kidney transplant: a qualitative analysis of patient responses. BMC Nephrol. 2019;20(1):175.
- Pinter J, Hanson CS, Chapman JR, et al. Perspectives of older kidney transplant recipients on kidney transplantation. Clin J Am Soc Nephrol. 2017;12(3):443–453.