Abstract
Aims
To compare long-term healthcare resource utilization (HCRU) and costs among patients who initiated ixekizumab (IXE) or adalimumab (ADA) for treatment of psoriasis in the United States.
Methods
Adult patients with psoriasis who had ≥1 claim for IXE or ADA were identified from IBM MarketScan claims databases prior to the COVID-19 pandemic (1 March 2016–31 October 2019). The index date was the date of first claim for the index drug of interest. Inverse probability of treatment weighting was employed to balance treatment cohorts. All-cause and psoriasis-related HCRU and costs were examined for 24 months of follow-up. Costs were reported as per patient per month. Costs of psoriasis-related biologics were adjusted using published Institute for Clinical and Economic Review (ICER) discount factors. Index drug costs were adjusted for adherence and ICER discount rates.
Results
The analyses included 407 IXE and 2,702 ADA users. IXE users had significantly higher inpatient admission rate (all-cause HCRU: 14.9% vs. 11.0%; p =0.012) and greater mean length of stay per admission (days, 6.6 vs. 4.1; p =0.004) than ADA users. ICER-adjusted costs were significantly higher in IXE than ADA users (all-cause costs: $4,132 vs. $3,610; p <0.001; psoriasis-related costs $3,077 vs. $2,700; p <0.001). After adjusting for ICER and adherence, IXE and ADA drug costs were comparable ($3,636 vs. $3,677; p =0.714).
Limitations
Study relied on administrative claims data, subjected to data coding limitations and data entry errors. Rebates, patient assistance programs, and commission to wholesalers are not always captured in claims. Adjustment made by ICER discount factors may lead to double-discounting if the discounts have been applied in claim payments.
Conclusions
All-cause HCRU was higher in IXE than ADA users. Healthcare costs were also higher in IXE than ADA users after ICER adjustment, over 24 months. Cost differences were largely driven by higher treatment adherence associated with IXE. Index drug costs were comparable after ICER and adherence adjustments.
Introduction
Psoriasis is a chronic systemic inflammatory disease leading to global burden of the healthcare systemCitation1,Citation2 and affects ∼7.4 million people in the United StatesCitation3,Citation4. It has a significant impact on patient quality-of-life and work productivity, and is associated with an increased risk for developing comorbidities, including psoriatic arthritis, depression, anxiety, cardiovascular disease, and diabetes mellitusCitation5. Moderate-to-severe psoriasis is often managed with biologic therapiesCitation6,Citation7 and most patients frequently inter-switch between themCitation8. The common biologic classes include tumor necrosis factor (TNF) inhibitors and interleukin (IL)-17A inhibitorsCitation7,Citation9. Adalimumab (ADA), a TNF inhibitor, has been the most commonly prescribed biologic therapy for psoriasisCitation10. Ixekizumab (IXE), a newer biologic for psoriasis, inhibits IL-17A, a key effector cytokine in psoriasis pathogenesisCitation11. Although both biologics are effective in treating psoriasis, no clinical trial studies have directly compared these two drugs.
Because of the cost of biologics and other factors, psoriasis has substantial economic burden, resulting in high healthcare resource utilization (HCRU) and expenditureCitation4,Citation12. The estimated direct economic burden of psoriasis ranged from $51.7 billion to $63.2 billion in the US; indirect costs ranged from $23.9–$35.4 billion, while medical comorbidities totaled $36.4 billion annually for the year 2013Citation13. In another study, the total economic burden (direct and indirect costs) was estimated to be $35.2 billion (2013 estimates), with indirect costs being the largest contributorCitation4. Currently, there are limited data available on HCRU and costs for newer therapies like IXE compared to established TNF inhibitors, such as ADACitation10,Citation14. While clinical efficacy parameters between IXE and ADA have been compared in the literature individuallyCitation15–18, the important measures of long-term costs have not previously been evaluated. Here, we assessed long-term HCRU and costs among patients in the US with psoriasis treated with IXE or ADA over 24 months of follow-up, accounting for payer discounts and treatment adherence.
Methods
Data sources
This observational study used administrative health insurance claims data from IBM Watson Health MarketScanDatabases (Commercial Claims and Encounter Database, Medicare Supplemental and Coordination of Benefits, and Early View Database). These databases contain the healthcare experience of privately insured individuals and those with Medicare supplemental insurance paid for by employers. Coverage is provided under a variety of fee-for-service and managed healthcare plans. The Early View Database includes the same components as Commercial Claims and Encounter Database, Medicare Supplemental and Coordination of Benefits. It captures healthcare services incurred as late as approximately 60 days before data release.
Data on healthcare costs and healthcare services in both inpatient and outpatient settings were included based on the International Classification of Diseases 9th/10th Revision Clinical Modification (ICD-9/ICD-10-CM), Current Procedural Technology 4th edition codes, and Health Common Procedure Coding System and National Drug Codes. All these datasets are statistically de-identified and certified to be fully compliant with United States patient confidentiality requirements, including the Health Insurance Portability and Accountability Act, 1996. Therefore, institutional review board approval was not required.
Patient cohorts
Patients had at least one inpatient or two non-diagnostic outpatient claims (at least 30 days apart), with a diagnosis of psoriasis (diagnosis code ICD-9-CM 696.1x or ICD-10-CM diagnosis codes L40.0–L40.4 or L40.8–L40.9) at any time from 1 March 2015 through 31 October 2019 (prior to the COVID-19 pandemic). Patients had at least one claim for IXE or ADA between 1 March 2016 and 31 October 2019, with a diagnosis of psoriasis before or coinciding with the first claim for that index drug. Index date was defined as the date of first claim for index drug of interest (IXE/ADA). Patients who were ≥18 years and had been continuously enrolled with medical and pharmacy benefits for at least 6 months pre-index date and 24 months post-index date were included. Patients with conditions other than psoriasis indicated for the index drug, such as psoriatic arthritis (IXE/ADA), ankylosing spondylitis, juvenile idiopathic arthritis, rheumatoid arthritis, Crohn’s disease, ulcerative colitis, hidradenitis suppurativa, and uveitis (ADA), were excluded. Patients with index medications within 90 days before the index date were also excluded.
Healthcare utilization and cost outcomes
Post-period all-cause and psoriasis-related HCRU and costs incurred over a 24-month follow-up period were reported. All-cause costs were based on all medical and pharmacy claims. Psoriasis-related costs were based on inpatient claims with a primary diagnosis for psoriasis, outpatient claims with a diagnosis for psoriasis, and pharmacy claims for the treatment of psoriasis. HCRU services included inpatient admissions, outpatient services (emergency room visits, office visits, other outpatient services [e.g. radiology services, lab services]), and outpatient pharmacy. Inpatient costs, outpatient costs, outpatient pharmacy costs, and total healthcare costs were assessed. Percentages of patients with services, number of services, average length of inpatient stay, and all-cause and psoriasis-related healthcare costs were reported. All-cause and psoriasis related costs captured all relevant costs during the follow-up period. If a patient switched treatment, the new treatment costs were included in these costs. In addition, index drug costs were reported, including all IXE costs for the IXE cohort and all ADA costs for the ADA cohort during the follow-up. If an ADA patient switched to IXE and switched back to ADA during the follow-up, all ADA costs were captured as index drug costs.
In addition, comorbidity-related costs in the follow-up period were examined. Comorbidities included anxiety, cerebrovascular disease, coronary heart disease (included peripheral vascular disease), depression, diabetes, hyperlipidemia, hypertension, obesity, osteoarthritis, other autoimmune disorders, and sleep apnea.
Costs for services provided under capitated arrangements were estimated using payment proxies that were computed based on paid claims at the procedure level using the MarketScan Commercial and Supplemental Databases. All HCRU and costs were reported as per patient per month and were inflated to 2019 US dollars by using the medical care component of the Consumer Price Index.
Cost adjustments
Payment rebates and discounts reported are not usually captured in claims (e.g. pharmacy rebates, patient assistance program, and commission to wholesalers). To account for discounts and rebates not captured in claims, published Institute for Clinical and Economic Review (ICER) discount factors based on net price divided by Wholesale Acquisition Cost were used to adjust the costs of biologics for psoriasis during 24 months of follow-up: Drug costs *[1-ICER discount factor]. The ICER discount factor applied for IXE was 0.44 and it was 0.31 for ADACitation19. In order to estimate ICER-adjusted all-cause and other psoriasis-related biologic costs, the ICER discount factors were also applied for other biologics accordingly: brodalumab (0.20), certolizumab (0.36), etanercept (0.31), guselkumab (0.33), infliximab (0.22), and ustekinumab (0.27). The ICER discounts were not available for tildrakizumab and risankizumab as they were approved for psoriasis in March 2018 and April 2019, respectively. Therefore, an average ICER factor (0.31) was used to estimate discounts for these drugs. Total healthcare costs adjusted by ICER discount factors were calculated as the sum of ICER-adjusted index drug costs, ICER-adjusted other psoriasis-indicated biologic costs, and other healthcare costs. Sensitivity analyses were performed to estimate mean ICER-adjusted all-cause and psoriasis-related costs by varying the discount factors of index drugs by ±5%.
Post-period index drug costs are impacted by treatment adherence because higher adherence leads to more consumption of medication. Index drug costs during the 24-month follow-up period were estimated, assuming the patients were fully adherent to treatment. This was calculated in two steps: (1) Applying the ICER discount factors to the index drug costs; and (2) Dividing the ICER-adjusted index drug costs by mean proportion of days covered (PDC) for each cohort to obtain the ICER-adherence adjusted index drug costs during the 24-month follow-up period. PDC was defined as the number of days the patients were covered by medication during the follow-up period divided by the total number of days during follow-up.
Covariates
Pre-period demographics and clinical characteristics included age, gender, payer, plan type, and region. Charlson comorbidity index, comorbidities (listed above), psoriasis-related costs, comorbidity-related costs, baseline psoriasis biologic use, systemic therapies/targeted oral therapies (apremilast, acitretin, systemic steroids, cyclosporine, methotrexate, azathioprine, hydroxyurea, isotretinoin, leflunomide, methoxsalen, mycophenolate mofetil, sulfasalazine, and thioguanine), topical agents (coal tar, ketoconazole, topical corticosteroids, anthralin, calcipotriene, calcitriol, pimecrolimus, tacrolimus, and tazarotene), or phototherapy were included as covariates.
Statistical analysis
Inverse probability of treatment weighting (IPTW) was applied to address cohort imbalances and minimize observed confounding. Propensity weights were estimated using logistic regression.Citation20,Citation21 Pre-period demographics, clinical characteristics, pre-period psoriasis-related costs, pre-period comorbidity-related costs, and interaction of pre-period biologic use and topical agents for psoriasis were included as covariates in the logistic regression model. The balance between IXE and ADA cohorts was evaluated for each variable using absolute standardized difference (Std. Diff), where a difference of lower than 0.10 was indicative of good balance.
A multivariate model was employed to estimate the adjusted comorbidity-related costs. First, the association between index treatment and any costs was estimated using a weighted multivariable logistic regression model. Then the association between index treatment and follow-up period comorbidity-related costs was estimated using a Gamma-family generalized linear model with a log link. Predicted costs were estimated for each index drug using the recycled predictions method. Recycled predictions are helpful for understanding/quantifying the marginal effect of a given independent variable (index drug) on a dependent variable (costs). Recycled predictions are obtained from regression models by averaging the predicted scores of the dependent variable after fixing to one value of the independent variable and using observed values on the remaining independent variables in the sampleCitation22,Citation23. In this study, every patient was taken into consideration and their predicted cost was calculated assuming that their index drug was IXE but using their actual values for all other model covariates; the mean across those predicted costs was reported in IXE. The same process was followed for ADA. This allowed comparisons of the average predicted costs for IXE and ADA after holding constant all other model covariates.
The following covariates were used in the cost model: demographic characteristics, pre-period CCI, pre-period use of psoriasis-related biologics, systemic agents/targeted oral therapies, and pre-period topical agents, pre-period phototherapy, log [pre-period psoriasis-related costs], and pre-period comorbidity-related costs.
Categorical variables were presented as the count and proportion of patients in each category. Continuous variables presented as mean and standard deviation (SD). Statistical test for significance was conducted using chi-square test for categorical variables and using pooled two sample t-test for continuous variables for weighted outcomes. A threshold p-value of 0.05 was considered statistically significant. Descriptive statistics were conducted using WPS Analytics version 4.02 (World Programming, UK), while IPTW and multivariable analyses were conducted using R (version 3.6.3).
Results
Baseline demographics and clinical characteristics
Out of 261,539 IXE/ADA users, the final analysis was conducted in 407 IXE users and 2,702 ADA users (Supplementary Figure S1). Before weighting, IXE users were approximately 2 years older than ADA users (). IXE users had higher rates of pre-period diabetes, sleep apnea, and prior biologic use, as well as higher mean pre-period all-cause healthcare costs (IXE: $3,525 vs. ADA: $1,763; ) and psoriasis-related healthcare costs than ADA users (IXE: $2,563 vs. ADA: $1,061; ). The baseline all-cause and psoriasis-related costs were higher for IXE compared to ADA, most likely due to higher baseline use of biologics among the IXE patients (56%) compared to ADA (29%). IXE being a relatively newer drug than ADACitation10,Citation11, most of the patients had used ADA (or another biologic) before switching to IXE, whereas ADA was more likely used as a first line biologic. In addition, psoriasis biologic costs account for the majority of healthcare costs for this disease population. The IPTW approach effectively balanced the cohort differences by including pre-period psoriasis-related costs, pre-period comorbidity-related costs, and interaction of pre-period biologic use and topical agents for psoriasis in the logistic regression model. All the baseline demographics and clinical characteristics were balanced after weighting.
Table 1. Baseline demographic and clinical characteristics for 24 months of follow-up (weighted and unweighted results).
Healthcare resource utilization
After weighting, IXE users had a significantly higher inpatient admission rate (14.9% vs. 11.0%; p =0.012; ) and a greater mean length of stay per admission (days; 6.6 vs. 4.1; p =0.004). Outpatient visits/services were similar for both groups (). Psoriasis-related HCRU were also similar for both groups.
Table 2. Healthcare resource utilization (all-cause and psoriasis-related healthcare utilization) weighted results during 24 months of follow-up.
Unadjusted healthcare costs (all-cause, psoriasis-related costs; index drug costs)
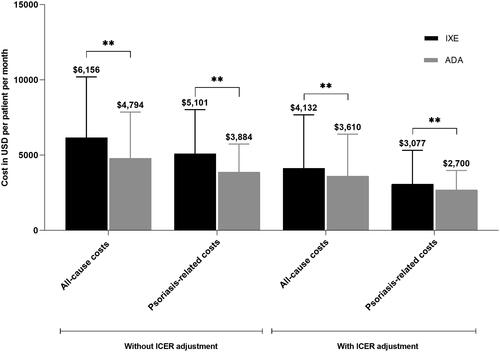
The unadjusted total all-cause healthcare costs were significantly higher in IXE users than ADA users ($6,156 vs. $4,794; p <0.001; ). IXE users had significantly higher unadjusted all-cause costs for outpatient costs ($916 vs. $552; p <0.001) and outpatient pharmacy costs ($4,929 vs. $4,009; p <0.001) than ADA users (). The unadjusted psoriasis-related healthcare costs were higher in IXE users compared to ADA users (total psoriasis-related healthcare costs: $5,101 vs. $3,884; p <0.001 (); outpatient costs: $382 vs. $71; p <0.001 (); outpatient pharmacy costs: $4,719 vs. $3,811; p <0.001 (). IXE users had higher unadjusted index drug cost than ADA users ($3,915 vs. $2,894, p <0.001) ().
Figure 1. Weighted all-cause and psoriasis-related healthcare costs (PPPM): Without ICER adjustment, 24-month follow-up (IXE vs. ADA). (a) All-cause healthcare costs (without ICER adjustment). (b) Psoriasis-related healthcare costs (without ICER adjustment).
Abbreviations. ADA, adalimumab; ICER, Institute for Clinical and Economic Review; IXE, ixekizumab; PPPM, per patient per month; SD, standard deviation.
Weighted two-tailed t-test, **p ≤ 0.001, *p < 0.01, ns: p > 0.1.
Note: Data are represented as mean with SD.
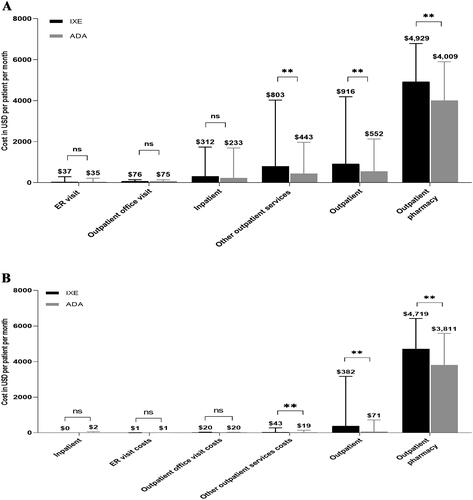
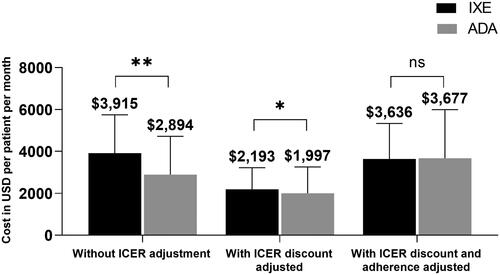
Figure 2. Weighted total healthcare costs (PPPM): Without ICER adjustment and with ICER adjustment, 24-month follow-up (IXE vs. ADA).
Abbreviations. ADA, adalimumab; ICER, Institute for Clinical and Economic Review; IXE, ixekizumab; PPPM, per patient per month; SD, standard deviation.
Weighted two-tailed t-test, **p < 0.001, *p < 0.01, ns: p > 0.1.
Note: Data are represented as mean with SD.
ICER-adjusted all-cause and psoriasis-related costs; ICER-adherence adjusted index drug costs
After applying ICER discounts, the adjusted total all-cause costs were significantly higher in IXE users than ADA users ($4,132 vs. $3,610; p <0.001; ). The ICER-adjusted psoriasis-related costs were $3,077 and $2,700 (p <0.001) for IXE and ADA, respectively (). Sensitivity analysis demonstrated significant cost differences of estimated mean ICER-adjusted all-cause and psoriasis-related costs between IXE and ADA (Supplementary Table S1).
The ICER-adjusted index drug costs associated with IXE ($2,193) remained higher than that of ADA ($1,997, p =0.001, ), but the cost difference was reduced from $1,021 to $196. The index drugs, IXE and ADA, accounted for 53.1% and 55.3% of the total adjusted all-cause costs, and 71.3% and 74.0% of the adjusted psoriasis-related costs, respectively. Mean adherence (PDC) was significantly higher during the 24-months follow-up for IXE users than ADA users after weighting (0.58 vs. 0.55, p =0.030). After adjusting for both ICER discounts and adherence, IXE and ADA drug costs were comparable ($3,636 vs. $3,677, p =0.714; ).
Comorbidity-related costs
The post-period unweighted co-morbidity-related costs for diabetes, hyperlipidemia, and sleep apnea were significantly higher for IXE users than ADA users (Supplementary Table S2). However, after weighting, there were no significant differences in the comorbidity-related costs between IXE users and ADA users (Supplementary Table S2). Post-period comorbidity-related costs (weighted) accounted for 5–7% of total all-cause healthcare costs for IXE users and ADA users. After multivariable adjustment, no significant differences were detected in post-period comorbidity-related costs between IXE users and ADA users ($358 vs. $402, p =0.881).
Discussion
This retrospective study compared long-term HCRU and costs between patients with psoriasis treated with IXE or ADA over a 24-month follow-up period. IXE users had higher inpatient admission rate and healthcare costs (mean unadjusted total and psoriasis-related healthcare costs) compared with ADA users. After adjustment for ICER discounts, the differences in all-cause and psoriasis-related costs were smaller, however, costs remained significantly higher for IXE users.
The plausible reasons for higher costs for IXE users could be attributed to higher adherence among IXE users than ADA users (0.58 vs. 0.55, p =0.030), and a higher number of induction doses for IXE (160-mg initial dose, followed by 80 mg at weeks 2, 4, 6, 8, and 12, then 40 mg every 4 weeks thereafter)Citation24 compared to ADA induction dosing (80-mg initial dose, followed by 40 mg every other week starting 1 week after initial dose)Citation25. The cost differences were predominantly driven by psoriasis-related pharmacy costs. After adjustment for ICER discounts and treatment adherence, index drug costs were comparable between IXE and ADA users over 24 months ($3,636 vs. $3,677, p =0.714).
Our findings of higher unadjusted costs for IXE users compared to ADA users are consistent with previous reports with shorter follow-up durationsCitation14,Citation26. In a 12-month follow-up study, the weighted mean all-cause health care costs were $6,535 and $5,557 (p =0.026), and mean psoriasis-related costs were $5,792 and $4,754 (p =0.017), for IXE and ADA users, respectivelyCitation26. The unadjusted costs for both IXE and ADA users reported in the current study were lower than that reported in a previous study of 12-month follow-up by Blauvelt et al.Citation26. Another study with variable length of follow-up of 7.5 months also reported higher total all-cause healthcare costs ($8,371) and pharmacy costs ($7,792) for IXE users compared to the current studyCitation14. The lower costs in this analysis could be attributed to a longer follow-up period of 24 months, since a longer maintenance therapy period costs less than the induction period.
Similar to healthcare costs, IXE users had higher inpatient admission rates, and average length of stay per admission compared to ADA users. The plausible reason for higher inpatient admission rates could be that patients who initiated IXE had worse baseline characteristics and had higher comorbidities such as diabetes (14.9% vs. 11.4%; p =0.024) and obesity (17.1% vs. 13.6%; p =0.046). Moreover, psoriasis-related HCRU was similar in both the groups, indicating that higher inpatient admissions and length of stay could be attributed to the presence of other comorbid conditions among IXE users. The percentage of inpatients admission rate in the current study was higher than that of a previous study (14.9% vs. 2.9%Citation14), likely due to the longer follow-up in the current study.
Psoriasis is a chronic condition that requires long-term pharmacologic treatment. Biologics have shown to be efficacious and safe over long-term useCitation27–29, but also impose high economic burden on patients and the healthcare system. Affordability is an important issue for patients who have varied insurance plans with different co-paymentsCitation30,Citation31. In addition to affordability, one must also consider other health outcome measures such as treatment adherence, health-related quality-of-life [HRQoL] and clinical measures like skin clearance to determine the cost-effectiveness of treatment for different biologics. Future research focusing on both costs and HRQoL along with clinical measures would help in developing cost-effectiveness analyses for IXE and ADA therapies. This will help payers and providers make informed decisions in choosing the best therapeutic option for psoriasis.
Limitations
The study has certain limitations that should be considered while interpreting the results. The study relied on administrative claims data, which are subject to data coding limitations and data entry errors. While the Early View Database contains fully adjudicated claims, the medical component of care can take a longer time to complete the payment and, thereby, the healthcare costs could be underestimated. Rebates and patient assistance programs and commission to wholesalers are not always captured in claims. Adjustment by ICER discount factors was made but it may lead to double-discounting if the discounts have been applied in claim payments. ICER discount rates published in 2018 were used for the entire study period. And there were no discount factors for risankizumab or tildrakizumab. An average ICER rate was used to estimate discounts for these drugs. The IPTW and multivariable modeling were employed to address imbalances between patient cohorts, but residual differences may remain. For example, disease severity factors, and patient socioeconomic profile and health care environmental factors that influence resource use were not captured from claims. Results of this analysis may not be generalizable to psoriasis patients with other insurance types or without health insurance coverage.
Conclusions
In conclusion, all-cause HCRU was higher in IXE users compared to ADA users for 24 months of follow-up. All-cause and psoriasis-related costs were higher for IXE users than ADA users before and after ICER adjustment over a 2-year period. The cost differences were largely driven by higher treatment adherence associated with IXE, hence, higher index drug costs. When adjusting for both ICER and adherence rates, index drug costs were comparable. These findings may help clinicians in making informed decisions regarding prescribing psoriasis treatment for their patients and payers in making treatment access decisions for covered patients. Future studies assessing healthcare costs, psoriasis-related costs, along with treatment adherence and clinical measures, and quality-of-life measures would help in better understanding the cost-effectiveness of these drugs.
Transparency
Declaration of funding
This research was funded by Eli Lilly and Company, Indianapolis, IN.
Declaration of financial/other interests
AB has served as a scientific advisor and/or clinical study investigator for AbbVie, Abcentra, Aligos, Almirall, Amgen, Arcutis, Arena, Aslan, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, EcoR1, Eli Lilly and Company, Evommune, Forte, Galderma, Incyte, Janssen, Landos, Leo, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharma, UCB Pharma, and Vibliome. NS, CRL, and NMZ are employees of IBM Watson Health that was compensated by Eli Lilly and Company for conducting this research. NZ, SAK, RB, TR, BZ, and MM are full-time employees and stockholders of Eli Lilly and Company.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentation
A part of the study findings were presented as a poster following an abstract acceptance at the Winter Clinical Dermatology Conference (WCDC), virtually in 2021.
Supplemental Material
Download MS Word (17.4 KB)Supplemental Material
Download TIFF Image (811.6 KB)Acknowledgements
The authors thank Bilal Atiya, an employee of Eli Lilly and Company, for providing inputs during study design and critical review, and Uma Jyothi Kommoju, an employee of Eli Lilly Services India Private Limited, for providing writing support.
References
- Damiani G, Bragazzi NL, Aksut CK, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med. 2021;8:741.
- Laughter MR, Maymone MBC, Karimkhani C, et al. The burden of skin and subcutaneous diseases in the United States from 1990 to 2017. JAMA Dermatol. 2020;156(8):874–881.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72(6):961–967 e5.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–1113.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61(3):451–485.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072.
- Conti A, Damiani G, Ruggeri R, et al. Switching infliximab in psoriatic patients during COVID-19 pandemics: a real-life retrospective study comparing intra-versus interclass switching strategies. Dermatol Ther. 2021;34(5):e15088.
- Kimberly H, LH RS, Steven RF. Psoriasis treatment cost comparison: biologics versus home phototherapy. Am J Pharm Benefits. 2018;10(1):18–21.
- Wu JJ, Guerin A, Gauthier G, et al. Healthcare resource utilization, healthcare costs and dose escalation in psoriasis patients initiated on ustekinumab versus adalimumab: a retrospective claim study. J Dermatolog Treat. 2017;28(4):290–298.
- Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379–390.
- Boehncke WH, Menter A. Burden of disease: psoriasis and psoriatic arthritis. Am J Clin Dermatol. 2013;14(5):377–388.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151(6):651–658.
- Murage MJ, Gilligan AM, Tran O, et al. Ixekizumab treatment patterns and healthcare utilization and costs for patients with psoriasis. J Dermatolog Treat. 2021;32(1):56–63.
- Asahina A, Nakagawa H, Etoh T, et al. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a phase II/III randomized controlled study. J Dermatol. 2010;37(4):299–310.
- Cai L, Gu J, Zheng J, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol. 2017;31(1):89–95.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551.
- Leonardi C, Maari C, Philipp S, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: three-year results from the UNCOVER-3 study. J Am Acad Dermatol. 2018;79(5):824–830 e2.
- Reiner B. Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value: condition update: final evidence report. Boston, Massachusetts: Institute for Clinical and Economic Review, 2018.
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679.
- Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652–659.
- Greene WH. Econometric analysis. Upper Saddle River: Pearson Education India; 2003.
- Taltz [package insert]. Indianapolis: Eli Lilly and Company; 2016.
- Humira [package insert]. North Chicago, IL: Abbvie Inc.; 2002.
- Blauvelt A, Shi N, Zhu B, et al. Comparison of health care costs among patients with Psoriasis Initiating Ixekizumab, Secukinumab, or Adalimumab. J Manag Care Spec Pharm. 2019;25(12):1366–1376.
- Gordon K, Papp K, Poulin Y, et al. Long-term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open-label extension study for patients from REVEAL. J Am Acad Dermatol. 2012;66(2):241–251.
- Kamata M, Tada Y. Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: a literature review. Int J Mol Sci. 2020;21(5):1690.
- Blauvelt A, Lebwohl MG, Mabuchi T, et al. Long-term efficacy and safety of ixekizumab: a 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol. 2021;85(2):360–368.
- Driessen RJ, Bisschops LA, Adang EM, et al. The economic impact of high-need psoriasis in daily clinical practice before and after the introduction of biologics. Br J Dermatol. 2010;162(6):1324–1329.
- Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146(1):46–54.