Abstract
Objective
The objective of this study was to assess the face validity of a disease model evaluating the cost-effectiveness of ataluren for the treatment of nonsense mutation Duchenne muscular dystrophy (nmDMD).
Methods
This was a Delphi panel study comprising of physicians with first-hand experience of ataluren for the treatment of nmDMD. Consensus was sought for previously unvalidated model data, including patient health status and quality of life measured using the Health Utility Index (HUI), mortality, informal caregiving, and the expected benefit of early ataluren treatment across four states: (1) ambulatory, (2) non-ambulatory, not yet requiring ventilation support, (3) non-ambulatory, night-time ventilation support, and (4) non-ambulatory, full-time ventilation support.
Results
Nine experts from five countries participated in the Delphi panel. Consensus was obtained for all questions after three panel rounds (except for two HUI-questions concerning hand function [dexterity]). Consensus HUI-derived utilities for state (1) were 1.0000 for ataluren on top of best supportive care (BSC) and 0.7337 for BSC alone. Corresponding estimates for state (2) were 0.3179 and 0.2672, for state (3) 0.1643 and 0.0913, and for state (4) −0.0732 and −0.1163. Consensus mortality rates for states (1), (2), and (3) were 4%, 13%, and 33%, and life expectancy in state (4) was agreed to be 3 years. Panelists further agreed that two informal caregivers typically provide day-to-day care/support to patients with nmDMD, and that starting treatment with ataluren at 2 versus 5 years of age would be expected to delay loss of ambulation by an additional 2 years, and initiation of night-time and full-time ventilation support by an additional 3 years, respectively.
Limitations
The main limitation concerns the size of the Delphi panel, govern primarily by the rarity of the disease.
Conclusion
This study confirms the face validity of key clinical parameters and assumptions underlying the ataluren cost-effectiveness model.
1. Introduction
Duchenne muscular dystrophy (DMD) is a rare, X-linked recessive and universally fatal disorder with an estimated incidence of approximately 1 in every 3,500–5,000 live male births according to Parent Project Muscular Dystrophy and a recent systematic review of the current body of literatureCitation1,Citation2. DMD is caused by mutations in the DMD gene resulting in progressive muscular damage and degeneration, ultimately leading to loss of independent ambulation and serious cardiac and respiratory complicationsCitation3. In recent decades, enhancements to and harmonization of the standards of care of DMD have resulted in significant improvements to life expectancyCitation4. Yet, the burden of disease remains substantial, both on patientsCitation5, affected familiesCitation6, and societyCitation7.
In response to the extensive unmet medical need, a range of new molecular therapies have emerged for DMDCitation8. Similar to treatments of more common illnesses, upon regulatory approval, decisions of pricing and reimbursement for these orphan drugs usually comprise evidence of cost-effectiveness estimated using mathematical disease modelsCitation9. As tools within decision-analysis, the accuracy and reliability of outcomes from such economic evaluations are directly dependent on the validity of the evidence and assumptions underlying the employed disease models.
Different types of model validation are described in the literature, most involve comparing simulation outputs to numerical reference data (e.g. internal, cross, external, and predictive validity), with one exception: face validity. According to the Professional Society for Health Economics and Outcomes Research (ISPOR) Good Research Practices in Modeling Task Force, face validity is ‘the extent to which a model, its assumptions, and applications correspond to current science and evidence, as judged by people who have expertise in the problem’Citation10. Consequently, face validity is subjective: ‘people who have clinical expertise should evaluate how well each model component reflects their understanding of the pertinent medical science, available evidence, and the clinical or administrative question at issue’Citation10.
In 2014, ataluren was approved by the European Medicines Agency for the treatment of nonsense mutation DMD (nmDMD), a type of the disease constituting 10–15% of the total DMD populationCitation11. The treatment is an orally administered, small-molecule compound that promotes read-through of a nonsense mutation to produce full-length functional dystrophin protein. Ataluren is indicated for the treatment of nmDMD in ambulatory patients aged two years and older in the European Member States and Iceland, Liechtenstein, Norway, Great Britain, Northern Ireland, Kazakhstan, Israel, Republic of Korea, Brazil, Russia, and Belarus, aged five years and older in Chile and Ukraine, and pediatric male patients in Brazil.
The cost-effectiveness of ataluren has previously been estimated using a global decision-analytic model (first developed for the UK settingCitation12) and updated analyses are being prepared to align with the Health Research Collaboration United in Leading Evidence Synthesis (HERCULES) natural history model of DMDCitation13. However, similar to most models of rare diseases, key pieces of evidence required to accurately simulate affected patients and their treatment are not available (either directly or indirectly) in the literature. The objective of this Delphi panel study was to assess the face validity of clinical parameters and assumptions underlying a revised disease model evaluating the cost-effectiveness of ataluren for the treatment of nmDMD. Our aim was to strengthen the validity of current and future models in this indication, as well as the credibility of simulated results.
2. Methods
2.1. Study design
This was a Delphi panel study. The Delphi method is a well-established and frequently employed technique for consensus-building (i.e. convergence of opinions) on a specific topic using a series of questionnaires administered iteratively to a panel of expertsCitation14. In each iteration, before providing their assessment, the panelists are shown feedback from replies from previous rounds which encourages re-assessment of initial responses based on their ability to review and assess answers provided by the other Delphi participantsCitation15.
2.2. Panelists
The Delphi panel comprised of clinical experts identified from specialist/tertiary centers across Europe. To be considered eligible to participate, all experts had to meet the following inclusion criteria:
Act as the coordinating/specialist physician to patients with DMD; and
Have first-hand experience of ataluren for the treatment of nmDMD.
2.3. Delphi panel topics
Consensus among participating experts was sought for previously unvalidated key clinical parameters and assumptions underlying the ataluren cost-effectiveness model, including:
Definitions of model states (see Section Definition of disease stages) in terms of predicted forced vital capacity (pFVC);
The expected benefit of early ataluren treatment;
Crude state-specific mortality rates;
Expected state-specific medication compliance to ataluren;
State-specific involvement of informal caregivers; and
State-specific health status, health-related quality of life (HRQoL), and utilities assessed using the Health Utility Index (HUI)Citation16 and a Visual Analogue Scale (VAS).
A table listing the exact questions asked to the clinical experts as part of the Delphi panel study is provided as Supplemental Material (online).
The HUI is a widely-used generic multi-attribute classification systems encompassing 15 items, each described in five or six item-specific levels (ranging from ‘a’, indicating no/minimal negative impact on health status and a low/minimal disease burden, to ‘e’ or ‘f’, indicating a very high degree of impairment/compromise and a high/maximum disease burden). The instrument covers eight dimensions (vision, hearing, speech, emotion, pain and discomfort, ambulation, dexterity, and cognition) and has been used in previous DMD research, exhibiting good sensitivity across conventional staging of the diseaseCitation5,Citation7. The HUI currently consists of two systems, Mark II and Mark III (of which the latter is preferred as it has a more detailed descriptive system, full structural independence, and population norms availableCitation16). The HUI Mark III describes a total of 972,000 health states linked to utilities representing general population preference values ranging between −0.36 and 1Citation16 (where negative values are interpreted as health states rated worse than being dead, 0 as equal to being dead, and 1 interpreted as being in a health state of perfect health). Based on the outcomes from a previous Delphi panel studyCitation17, only questions of relevance to DMD were included for assessment (i.e. emotion, pain and discomfort, mobility, and dexterity). The VAS was shown as a continuous scale ranging from 0 to 100, where a higher value denotes better health status and higher patient HRQoL as assessed by the panelists.
2.3.1. Definition of interventions
Two interventions were considered in this study in accordance with the ataluren clinical programCitation18–21: (A) treatment with ataluren in addition to best supportive care (BSC), and (B) BSC alone. Treatment with ataluren was assumed to be initiated at a mean patient age of 5 years and continued up until and beyond loss of ambulation. BSC was defined as treatment with glucocorticoids, as well as pharmacological therapy for the management of cardiac, pulmonary, orthopedic, and gastrointestinal complications, and non-pharmacological interventions.
2.3.2. Definition of disease stages
Consensus was sought for four disease stages of nmDMD, which form part of the ataluren cost-effectiveness model based on the HERCULES natural history model of DMDCitation13:
Ambulatory;
Non-ambulatory, not yet requiring ventilation support (pFVC: ≥50%);
Non-ambulatory, requiring night-time ventilation support (pFVC: 30%–50%); and
Non-ambulatory, requiring full-time ventilation support (pFVC: <30%; or FVC <1 liter).
Due to the monotonic progression of nmDMD, panelists were instructed to provide their assessments for a 10-year-old patient for disease stage (1) (equal to the median modeled age for this state) and at the time of initiation of ventilation support for stages (3) and (4). They were also instructed to assume identical health status (i.e. equal level of physical disability and degree of impairment from nmDMD) for patients receiving ataluren in addition to BSC and those receiving only BSC at the time of ataluren treatment initiation (i.e. at 5 years of age). Finally, at the age of 10 years, patients treated with ataluren were assumed to become full-time dependent on wheelchairs for mobility – that is, transition from stage (1) to stage (2) – after a median of 8 years (i.e. at 18 years of age), compared with 2.5 years (i.e. at 12.5 years of age) for those receiving only BSCCitation22. No further differences between patients treated with ataluren and BSC were specified.
2.4. Study procedures and data collection
This Delphi panel study was executed using electronic questionnaires administered via email. As part of the initial round of the Delphi process, eligible experts were first asked to review a participant information sheet and provide informed consent, and then complete a set of background questions concerning their professional experience. Next, the panelists were asked to answer questions concerning key clinical parameters and assumptions underlying the ataluren cost-effectiveness model and assess patient health status and HRQoL. In subsequent rounds, the panelists were asked to only re-assess questions for which consensus had not been reached based on summary feedback of the most common answers from the previous iteration.
To safeguard the internal study validity, and minimize the risk of obtaining biased estimates, all experts were informed that participation was completely voluntary, that their identity would remain confidential, and that they should provide their replies (as instructed above) independently of the study sponsor. Moreover, as recommended by the ISPOR Good Research Practices in Modeling Task ForceCitation10, all panelists provided their assessment without knowing the impact on, or any outcomes from, the cost-effectiveness model analyses. In accordance with applicable regulations, Institutional Review Board approval was not sought for this Delphi panel study.
2.5. Definition of consensus
In the context of Delphi panels, there is no agreement of what constitutes consensusCitation23, and several definitions have been proposed in the literature (e.g. 80% of votes within two categories on a seven-point scale, or that 70% of Delphi subjects need to rate three or higher on a four-point Likert-type scale and the median must be ≥3.25) Citation15. In this panel, consensus for each nominal/ordinal question was pre-specified to have been achieved when at least 80% of the participating experts (rounded to nearest integer) agreed of the appropriate response level/category. Consensus for continuous outcomes was pre-specified to have been achieved when all ratings fell within a range of ±20% (e.g. ±20 points on a scale from 0 to 100), or when at least 80% of the participating experts (rounded to nearest integer) agreed to an exact value.
2.6. Statistical analysis
We summarized the distribution of replies to each HUI-question across all panel rounds and for both interventions and disease stages. HUI-derived utilities were estimated using the HUI Mark III algorithmCitation16. Additionally, for each panel round, we summarized VAS scores for each intervention and disease stage. All statistical analyses were performed in Stata 16 (StataCorp, College Station, TX, USA).
3. Results
In total, nine neuromuscular specialists, adult and pediatric neurologists, and pediatricians from five countries (i.e. Germany, Ireland, Latvia, Sweden, and the United Kingdom) participated in the Delphi panel (14 were invited, response rate: 64%). On average, panelists had 24 years of experience of their current profession (range: 15–29 years); 22 years of experience treating patients with DMD (14–28 years); and experience of ataluren for the treatment of seven patients with nmDMD (3–15 patients). Taken together, experts’ combined clinical experience of ataluren for the treatment of nmDMD comprised more than 280 patient-years.
3.1. Forced vital capacity across stages of the disease
Consensus on the definitions of model health states in terms of respiratory function was obtained in the first panel round. Specifically, the experts were in agreement that patients, on average, have a pFVC ≥50% at loss of ambulation, a pFVC ≥30% at initiation of night-time ventilation support, and a pFVC <30% (or a FVC < 1 liter) at initiation of full-time ventilation support.
3.2. The expected benefit of early ataluren treatment
After the first panel round, the experts were in agreement that patients who receive ataluren earlier in life would be expected to have a greater benefit from the treatment compared to those starting later. The consensus estimate (obtained after three panel rounds) of the expected average additional delay in time to loss of ambulation from starting treatment at 2 vs. 5 years of age was two years. The corresponding average additional delay in time to night-time and full-time ventilation support, respectively, was 3 years.
3.3. Mortality rates
The average crude mortality rate (i.e. the proportion of patients dying in each stage before progressing to the next) in disease stages (1), (2), and (3) was estimated at 4%, 13%, and 33%. The consensus estimate (obtained after three panel rounds) of the average life expectancy at the time of initiation of full-time ventilation support was 3 years.
3.4. Medication compliance to ataluren
The average expected compliance to treatment with ataluren in disease stages (1), (2), (3), and (4) was estimated at 95%, 85%, 77%, and 66%.
3.5. Informal caregiving
After the first round, the panelists were in agreement that both ambulatory and non-ambulatory patients with DMD typically have two informal caregivers – for example two parents, or one parent and one other relative – involved in their day-to-day care and support.
3.6. Patient health status and health-related quality of life
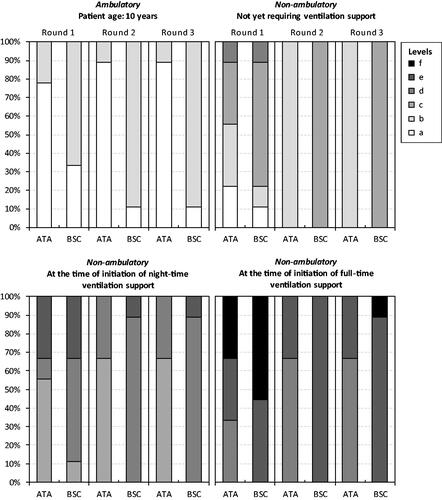
Consensus on the health status of patients treated with ataluren in addition to BSC vs. only BSC across the four disease stages were obtained after three panel rounds, with the exception of one HUI-question for stages (3) and (4). Recorded responses to the four questions from the HUI (i.e. emotion, pain and discomfort, ambulation, and dexterity) from each panel round are shown in . Consensus HUI-derived utilities for stage (1) were 1.0000 for ataluren in addition to BSC and 0.7337 for BSC alone. Corresponding estimates for stage (2) were 0.3179 and 0.2672, for stage (3) 0.1643 and 0.0913, and for stage (4) −0.0732 and −0.1163. Due to the lack of consensus for HUI-question 10 (dexterity), additional utility estimates were derived for stages (3) (0.0913 and 0.0913) and (4) (−0.1163 and −0.1163) (derived from 33% [3 of 9] experts). Consensus VAS scores for stage (1) were 80 for ataluren in addition to BSC and 62 for BSC alone. Corresponding estimates for stage (2) were 61 and 56, for stage (3) 55 and 46, and for stage (4) 46 and 38.
Figure 1. Panelists’ responses to Health Utilities Index-question 7 (emotion). Note: Ataluren in addition to best supportive care (ATA). Best supportive care (BSC). The HUI item-specific levels range from ‘a’ (indicating no/minimal negative impact on health status and a low/minimal disease burden) to ‘e’ (indicating a very high degree of impairment/compromise and a high/maximum disease burden).
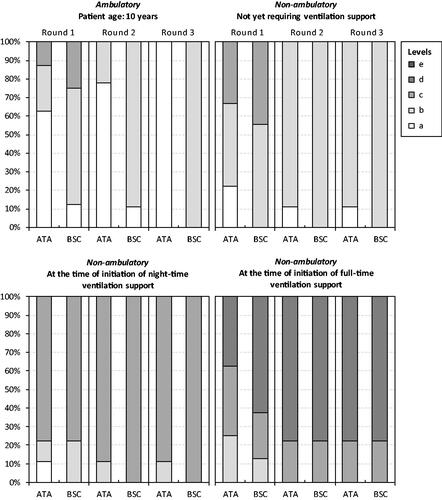
Figure 2. Panelists’ responses to Health Utilities Index-question 8 (pain and discomfort). Note: Ataluren in addition to best supportive care (ATA). Best supportive care (BSC). The HUI item-specific levels range from ‘a’ (indicating no/minimal negative impact on health status and a low/minimal disease burden) to ‘e’ (indicating a very high degree of impairment/compromise and a high/maximum disease burden).
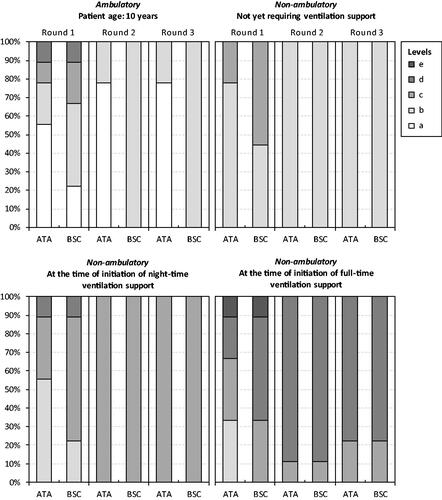
Figure 3. Panelists’ responses to Health Utilities Index-question 9 (ambulation). Note: The HUI item-specific levels range from ‘a’ (indicating no/minimal negative impact on health status and a low/minimal disease burden) to ‘f’ (indicating a very high degree of impairment/compromise and a high/maximum disease burden). Ataluren was assumed to be administered from a mean patient age of 5 years and continue up until and beyond loss of independent ambulation. Mobility in disease stages (2), (3), and (4) was ‘f’ (‘Unable to walk at all’). Ataluren in addition to best supportive care (ATA). Best supportive care (BSC).
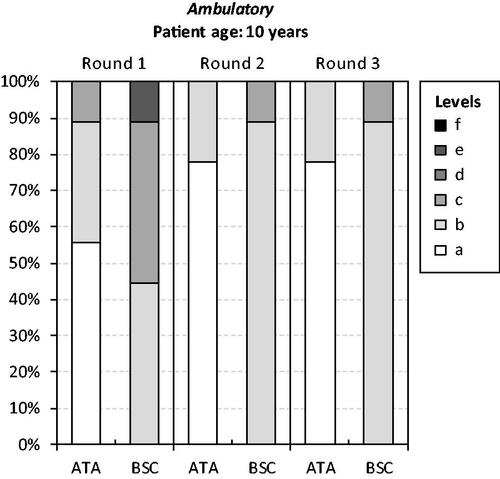
Figure 4. Panelists’ responses to Health Utilities Index-question 10 (dexterity). Note: The HUI item-specific levels range from ‘a’ (indicating no/minimal negative impact on health status and a low/minimal disease burden) to ‘f’ (indicating a very high degree of impairment/compromise and a high/maximum disease burden). Ataluren was assumed to be administered from a mean patient age of 5 years and continue up until and beyond loss of independent ambulation. Ataluren in addition to best supportive care (ATA). Best supportive care (BSC).
4. Discussion
This was a Delphi panel study evaluating the face validity of key clinical parameters and assumptions underlying a decision-analytic model evaluating the cost-effectiveness of ataluren for the treatment of nmDMD. The study outcomes show that the clinical experts, already in the first round, were in good agreement in their replies across many topics. Indeed, over the course of three panel iterations, consensus was reached for all but one question, validating a wide range of model parameters and assumptions, for most no alternative data sources are available. A similar rate of convergence of replies were noted in a previous Delphi panel in nmDMD in SwedenCitation17. Yet, considering the diversity and complexity of the panel topics, as well as the heterogenous presentation of nmDMD between patients and over time, the observed level of homogeneity in opinions is quite noteworthy.
The first topic judged by the Delphi panel concerned the definition of model health states in terms of FVC. Consensus was obtained in the first round. As part of this work, we did not seek to evaluate the selection and/or hierarchy of model states, as these have already been subject to careful assessment as part of HERCULESCitation13. That being said, based on the panel results, it is evident that the added granularity of the current compared with previous model versions – in particular the differentiation of patients after loss of ambulation – will help improve overall clinical representativeness, and also the alignment of the model structure to the known distribution of costs and utilities across the progression of the disease.
For the second topic, panelists agreed in the first round that it would be beneficial for patients to initiate treatment with ataluren earlier in life (i.e. at 2 vs. 5 years of age). By consensus, expected effectiveness ranged between 2 and 3 years of additional delay in loss of ambulation and ventilation support, respectively. For reference, these estimates are similar in magnitude to the median total delay in loss of ambulation and reaching a pFVC <50% from exposure to glucocorticoids (i.e. 3.4 yearsCitation24 and 3.1 yearsCitation25) Underscoring the utility of alternative data collection approaches in rare diseases (including Delphi panels and other expert elicitation methods), it is worth noting that to be able to detect these kind of differences in effects from early treatment in a clinical trial setting would involve follow-up of 200 children for the better part of two decades (assuming α = 0.05, β = 0.20, and a coefficient of variation [i.e. SD/mean] for age at loss of ambulation of 0.50).
The panelists judged patients treated with ataluren on top of BSC to have better health status compared with those treated with only BSC within the same disease stages, on average. There are two main explanations for this finding. First, to allow for a transparent yet robust representation of the disease, and given the scarcity of data, states in models of nmDMD are typically defined in terms of key disease milestones (i.e. ambulatory status and pulmonary function). This is also to facilitate modelling of available efficacy/effectiveness data. However, at the patient-level, these states are also characterised by a myriad of other inter-related disease symptoms, manifestations, and complications, including problems with pain, fatigue, self-care, cognitive function and attention, anxiety, emotional stress, and sadness, and sleep. Put differently, two patients of the same age residing in a specific health state might well shoulder a very different health burden. Second, the timing of reaching specific disease milestones would also be expected to have implications for the health status and HRQoL of subsequent stages of the disease. For example, we know that delaying loss of ambulation in DMD has a significant impact on age-dependent respiratory function later in lifeCitation24. Delaying loss of ambulation would also help patients take part more fully in their early adolescent life, a time period fundamental to the development and formation of, for example, self-esteem, self-identify, autonomy and independence, and social relationshipsCitation26. The latter aspect (i.e. the short- and long-term impact of the timing of loss of ambulation) deserves attention in future research. Comparing our HUI-derived utilities for BSC – ranging from 0.7337 for ambulatory patients to −0.1163 for those requiring full-time ventilation support (pFVC: <30%, or FVC <1 liter) – with previous research, our estimates are comparable to findings derived for an international cohort of patients with DMD (ranging from 0.75 for early ambulatory patients [approximately 5–7 years of age] to 0.10 for those requiring full-time ventilation support) Citation5,Citation7, as well as our previously conducted Delphi panel study in SwedenCitation17. However, it is important to keep in mind that these estimates are not directly comparable due to cross-study differences in patients and disease stages.
The panelists indicated that two caregivers typically are involved in providing the day-to-day informal care and support to patients with nmDMD. These findings are in good agreement with previous research in DMD, showing that the majority of caregivers also have support from a partner, other family member, and/or paid personal assistantCitation27, or in cases where the mother assumes the most active caregiving role, “fathers were also required to be active beyond the traditional father role”Citation28, as well as studies of this topic in other serious neuromuscular diseases, such as spinal muscular atrophy (SMA)Citation29.
Undoubtedly, data on the topics studied in this Delphi panel should ideally be obtained from well-designed observational or experimental research. However, the examined model input data are, to the best of our knowledge, not available in the scientific literature. Moreover, and as noted previously, many topics explored in the panel would not be expected to be easily studied in conventional studies, mainly due to issues pertaining to low patient numbers driven by the rarity of the disease. In fact, the data validated as part of the current study bridges several critical evidence gaps currently limiting possibilities to accurately model the disease to inform cost-effectiveness analyses and similar economic evaluations. In line with the study aim, we are therefore confident that the outcomes of this Delphi panel not only will help decision-makers appreciate the validity of outcomes from the ataluren cost-effectiveness model, but also facilitate modelling of other interventions in this indication. This, in turn, will help assure correct appraisals of the value for money of new health technologies, and thereby appropriate patient access.
The main limitation of this study concerns the size of the Delphi panel, govern primarily by the rarity of the disease. Undoubtably, a larger sample of clinical experts might have helped increase the precision of points estimates, and possibly generalizability of results. On the other hand, larger panels can introduce increased variability in replies and risk of not achieving consensus within a reasonable number of rounds, or at all. Based on our experience from implementing this and previous Delphi panels, depending on the complexity of the topics, expected rates of convergence in opinions, and timelines, we recommend to involve between 5 and 15 experts, in total. Additionally, it is worth noting that although Delphi approaches may be regarded less prone to bias from, for example, particularly dominant experts silencing the opinions of others (in, for example, round-table discussions), this method is also limited by the fact that some element of exploration is lost as statements or opinions are aggregated between rounds. Similarly, the Delphi method does not accommodate collaborative exploration of issues and arguments without necessarily concluding in a specific consensus positionCitation30.
In conclusion, the outcomes from this Delphi panel study confirms the face validity of key clinical parameters and assumptions underlying the ataluren cost-effectiveness model in nmDMD. The data provided should be helpful to inform and strengthen the validity of current and future models of nmDMD and facilitate the execution of economic evaluations of new health technologies in this patient population.
Transparency
Declaration of funding
This study was funded by PTC Therapeutics.
Declaration of financial/other relationships
EL, JJ, DO, TS, JS, US, and MCW have received support from, and/or has served as a paid consultant for, PTC Therapeutics. RZ and KB are employees of PTC Therapeutics and may own stock/options in the company. The remaining authors have no conflicts of interest.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
EL, RZ, and KB designed the study. EL coordinated the study and analysed the data. AMC, JJ, DO, TS, JS, US, MT, MCW, and TW participated in the Delphi panel. EL led the interpretation of findings with input from the other authors. EL drafted the manuscript. All authors critically reviewed the manuscript for important intellectual content, and approved the final manuscript version as submitted.
Supplemental Material
Download MS Word (18.7 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Ellis JA, Vroom E, Muntoni F. 195th ENMC international workshop: newborn screening for Duchenne muscular dystrophy 14-16th December, 2012, Naarden, The Netherlands. Neuromuscul Disord. 2013;23(8):808–689.
- Ryder S, Leadley RM, Armstrong N, et al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017;12(1):79.
- Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687–695.
- Landfeldt E, Thompson R, Sejersen T, et al. Life expectancy at birth in Duchenne muscular dystrophy: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35(7):643–653.
- Landfeldt E, Lindgren P, Bell CF, et al. Health-related quality of life in patients with Duchenne muscular dystrophy: a multi-national, cross-sectional study. Dev Med Child Neurol. 2016;58(5):508–515.
- Landfeldt E, Edström J, Buccella F, et al. Duchenne muscular dystrophy and caregiver burden: a systematic review. Dev Med Child Neurol. 2018;60(10):987–996.
- Landfeldt E, Lindgren P, Bell CF, et al. The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology. 2014;83(6):529–536.
- Mackenzie SJ, Nicolau S, Connolly AM, et al. Therapeutic approaches for Duchenne muscular dystrophy: old and new. Semin Pediatr Neurol. 2021;37:100877.
- Gammie T, Lu CY, Babar ZU. Access to orphan drugs: a comprehensive review of legislations, regulations and policies in 35 countries. PLOS One. 2015;10(10):e0140002.
- Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force-7. Value Health. 2012;15(6):843–850.
- Pichavant C, Aartsma-Rus A, Clemens PR, et al. Current status of pharmaceutical and genetic therapeutic approaches to treat DMD. Mol Ther. 2011;19(5):830–840.
- National Institute for Health and Care Excellence. NICE guidance for ataluren. [cited 2018 May 1]. Available from: https://www.nice.org.uk/guidance.
- Project HERCULES. [cited 2021 Dec 11]. Available from: https://hercules.duchenneuk.org.
- Linstone H, Turoff M. The Delphi method: techniques and applications. Reading, MA. Addison-Wesley; 1975.
- Hsu CC, Sanford BA. The Delphi technique: making sense of consensus. Pract Assess Res Evaluation. 2007;12(10):1–8.
- Horsman J, Furlong W, Feeny D, et al. The health utilities index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54.
- Landfeldt E, Lindberg C, Sejersen T. Improvements in health status and utility associated with ataluren for the treatment of nonsense mutation Duchenne muscular dystrophy. Muscle Nerve. 2020;61(3):363–368.
- Finkel RS, Flanigan KM, Wong B, et al. Phase 2a study of ataluren-mediated dystrophin production in patients with nonsense mutation Duchenne muscular dystrophy. PLOS One. 2013;8(12):e81302.
- Bushby K, Finkel R, Wong B, et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve. 2014;50(4):477–487.
- McDonald CM, Campbell C, Torricelli RE, et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10101):1489–1498.
- McDonald CM, Muntoni F, Penematsa V, et al. Ataluren delays loss of ambulation and respiratory decline in nonsense mutation Duchenne muscular dystrophy patients. J Comp Eff Res. 2022;11(3):139-155.
- Mercuri E, Muntoni F, Buccella F, et al. Age at loss of ambulation in patients with DMD from the STRIDE registry and the CINRG Natural history study: a matched cohort analysis. Neuromuscular Disorders. 2021;31(Supplement 1):S92-S92.
- Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003;41(4):376–382.
- McDonald CM, Henricson EK, Abresch RT, et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet. 2018;391(10119):451–461.
- Trucco F, Domingos JP, Tay CG, et al. Cardiorespiratory progression over 5 years and role of corticosteroids in Duchenne muscular dystrophy: a single-site retrospective longitudinal study. Chest. 2020;158(4):1606–1616.
- Eiser C, Morse R. Quality-of-life measures in chronic diseases of childhood. Health Technol Assess. 2001;5(4):1–157.
- Williams K, Davidson I, Rance M, et al. Symptoms and impacts of ambulatory nonsense mutation Duchenne muscular dystrophy: a qualitative study and the development of a patient-centred conceptual model. J Patient Rep Outcomes. 2021;5(1):75.
- Donnelly CM, Quinlivan RM, Herron A, et al. A systematic review and qualitative synthesis of the experiences of parents of individuals living with Duchenne muscular dystrophy. Disabil Rehabil. 2022;1–14. DOI:10.1080/09638288.2022.2060336
- McMillan HJ, Gerber B, Cowling T, et al. Burden of spinal muscular atrophy (SMA) on patients and caregivers in Canada. J Neuromuscul Dis. 2021;8(4):553–568.
- Ramos GF, Kuiper S, Dompeling E, et al. Structuring and validating a cost-effectiveness model of primary asthma prevention amongst children. BMC Med Res Methodol. 2011;11:150.
