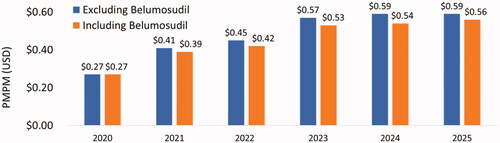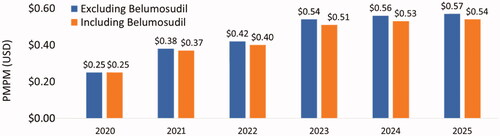Abstract
Aims
To assess the impact of belumosudil on the cost of care in chronic graft-versus-host disease (cGVHD) patients who have failed at least two prior lines of systemic therapy using a budget impact model.
Methods
A budget impact model with a 5-year time horizon was constructed in Microsoft Excel. The base case model uses the US prevalence rate of 3 L/4L + cGVHD patients from literature and secondary sources, with the potential for user-defined inputs, including model perspectives. The model includes data for two perspectives: the national US population and a hypothetical US private payer health insurance plan with 10 million (Mil) members. Additional model inputs include market share of cGVHD treatments, their associated adverse event rates, and healthcare resource utilization.
Results
The potential annual budget impact for the US national and payer plans was evaluated for cGVHD patients. Based on belumosudil utilization increasing to 55% in 3 L and 4 L + by 2026, cost savings of ∼5.5% and 6.7% ($128.8 and $4.9 Mil USD) were observed from national and payer perspectives, respectively. Cost savings in 2026 were derived from fewer AEs ($108.4 and $3.9 Mil USD, for national and payer perspectives; e.g. neutropenia, and thrombocytopenia) and reduced HCRU ($65.1 and $2.3 Mil USD, for national and payer perspectives; e.g. emergency room visits, ICU stays, etc.).
Limitations
Results from the model were dependent on the available data inputs and assumptions. Real-world values may differ from the assumed performance of treatments, market growth, and treatment dosing and duration.
Conclusion
The model results suggest that the introduction of belumosudil to treat cGVHD would be associated with substantial cost savings when evaluating a scenario with versus without belumosudil from a US payer perspective.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative treatment for numerous hematological malignancies. Chronic graft-versus-host disease (cGVHD), a common and clinically significant complication, can limit the effectiveness of allogenic HCT and has an incidence rate of up to 50% of HCT patientsCitation1. United States (US) claims analyses suggest roughly 14,000 patients are currently living with cGVHD, and approximately 5,000 new patients are diagnosed each yearCitation2.
cGVHD is a multi-system, immune-mediated, inflammatory, and fibrotic disease in which immune cells from an HCT attack the recipient, resulting in significant morbidity and mortality. Five-year survival rates of patients with chronic GVHD range from 58–82%Citation3. Quality-of-life (QoL) is also negatively impacted by cGVHD. The National Institutes of Health (NIH) Consensus Criteria state that the severity of cGVHD is significantly related to impairment in QoL independent of other comorbidities, donor sources for transplantation, and socio-demographic variables. The condition can be characterized by a combination of tissue inflammation and fibrosis manifesting across multiple organ systems and frequently involves, but is not limited to, the skin, mucosa, and lungs. cGVHD-induced fibrosis leads to chronic disability, especially when affecting joints or extensive areas of the skin. Widespread sclerotic skin manifestations caused by inflammation and fibrosis are associated with poor survivalCitation4. In post-HCT patients, cGVHD is the leading cause of non-relapse mortality more than 2 years after allogeneic transplantation.
Treatment options for cGVHD are available; however, many have drawbacks that limit their long-term effectiveness. Systemic corticosteroids are the mainstay of first line treatment for cGVHD, but a significant fraction of patients fail systemic therapies or become steroid refractory. Approximately 50–70% of patients with cGVHD require secondary treatment within 2 years after initial systemic treatmentCitation5. While there are many second-line standard-of-care options available (e.g. mTOR inhibitors, calcineurin inhibitors, extracorporeal photopheresis, mycophenolate mofetil, steroid combination therapy, ibrutinib, and ruxolitinib) these therapies are associated with increasing immunosuppression, side-effects, and there is no consensus on which secondary cGVHD therapy is the most advantageousCitation2,Citation6. Current treatment options may also jeopardize patient recovery because of an increased risk of infections, cytopenias, and second primary malignancies. Due to these obstacles, cGVHD patients utilize a high proportion of resources within the US healthcare system, and an estimated $27 billion in annual earnings have been lost due to the inability of these patients to return to the workforceCitation7.
Belumosudil is a selective Rho-associated coiled-coil kinase 2 (ROCK2) inhibitor and immunomodulator that is approved for the treatment of cGVHD for adult and pediatric patients 12 years and older after failure of two or more prior lines of systemic therapy (i.e. 3 L/4L+). National Comprehensive Cancer Network (NCCN) guidelines have included belumosudil as a recommended category 2A option for the treatment of patients with steroid refractory cGVHDCitation8. Belumosudil has been well tolerated in clinical trials, with a low incidence of grade ≥3 adverse events (AEs) (including cytopenias and infections), which may lead to increased compliance to treatment and lower absolute costsCitation9,Citation10. A budget impact model was created to estimate the impact of increasing utilization of belumosudil in 3 L/4L + cGVHD using a US national population or individual payer population perspectives.
Methods
Budget impact model design
A 5-year budget impact model was developed using Microsoft Excel to estimate the annual budget impact of the adoption of belumosudil for the treatment of adult and pediatric patients 12 years and older after failure of two or more prior lines of systemic therapy (i.e. 3 L/4L+) for a hypothetical 10-million-member US payer plan (i.e. base-case scenario). The model also offered a US national perspective, to examine the annual budget impact for all prevalent cases of 3 L/4L + cGVHD. Using established literature and secondary sources, US cGVHD prevalence rates were incorporated, and the following therapies for 3 L/4L + cGVHD were included: ibrutinib; ruxolitinib; and a standard-of-care market basketCitation2,Citation11–16. Standard-of-care treatments included calcineurin inhibitors, steroids, extracorporeal photopheresis, mTOR inhibitors, or mycophenolate mofetil. Estimates for the total number of diagnosed cGVHD patients and the total number of patients on 3 L/4L + were derived from the starting 10-million-member US payer population and used annual percentages of newly-performed allogeneic HCTs from the CIBMTR Annual Report 2019 and the CIBMTR Transplant Activity Report 2019Citation13. This was then combined with the prevalence and incidence of cGVHD derived from a prior claims analysis and other published literature to obtain a total of 539 cGVHD diagnosed patientsCitation15,Citation16. Data from the prior claims analysis is then used to identify the proportion of treated cGVHD patients on 3 L/4L+. The patient estimates for the national perspective were derived from the same calculations only using a starting population of approximately 331 million people per the US CensusCitation12. The base case market share assumption begins with 76% of the patients on standard of care, 10% on ibrutinib, and 14% on ruxolitinib for 3 L treatment. For 4 L, it is assumed that 71% are on standard of care, 7% are on ibrutinib, and 22% are on ruxolitinib. In both lines, belumosudil has no market share as the base case is prior to launch.
Based on the lack of studies assessing belumosudil versus other therapies, similar efficacy to existing treatments was assumed. Treatment allocations were based on current and projected market share. In the base case, belumosudil treatment was once daily (QD) per the FDA-approved labelCitation17. However, also per the label, patients receiving belumosudil concomitantly with a strong CYP3A inducer or proton pump inhibitor require twice daily (BID) dosing, so a user-defined setting for BID dosing was incorporated. Patients were assumed to be fully adherent to their respective line of therapy, and no administration costs were assumed for any treatments.
Annual treatment drug costs (sourced from Redbook) were calculated using the expected treatment duration (days) based on prescribing informationCitation18. An annual price increase of 7% was applied to treatment with ibrutinib, ruxolitinib, and belumosudil. A 2% annual price increase was applied to standard-of-care. A treatment discount of 1% annually was assumed from the wholesale acquisition cost (WAC) for all treatments.
Costs associated with healthcare resource utilization and the treatment of grade 3/4 (i.e. severe or life-threatening) adverse events (AEs) were calculated. Grade 3/4 AEs and their rates in cGVHD were sourced from prescribing information, clinical trials, or published literatureCitation19–21. AEs were monetized using Medicare Diagnosis Related Groups costs, with conversion to commercial plan costs utilizing a ratio from the Congressional Budget OfficeCitation22–24. Healthcare visits, including clinic, physician office, outpatient, inpatient, emergency room, and intensive care unit settings, were based on prior claims analyses. Health care resource utilization (HCRU) and costs per healthcare visit for both Medicare and commercial populations were also based on prior claims analyses. This analysis used US administrative claims data to examine ibrutinib and ruxolitinib use in cGVHD patients and captured outcomes such as treatment persistence, cost of care, and healthcare resource utilization (i.e. care setting and frequency of visits)Citation25.
The overall percentage and absolute change in annual budget, per member per month (PMPM) savings, annual budget by treatment share, annual budget by cost category, and annual cost per patient were calculated. Sensitivity analyses were conducted by adjusting model parameters ±10% and ±25% to determine impacts on absolute cost. Adjusted parameters included the number of patients eligible for belumosudil in 2026, the proportion of patients receiving belumosudil with BID dosing, treatment cost of belumosudil, treatment cost of ibrutinib, treatment cost of ruxolitinib, AE costs, AE occurrence rates for belumosudil, AE occurrence rates for ibrutinib, and AE occurrence rates for ruxolitinib. Additional scenario analyses were performed based on market share adjustments, including a scenario in which belumosudil share increased only due to decreases in branded therapies (i.e. ruxolitinib and/or ibrutinib).
Results
Base case scenario
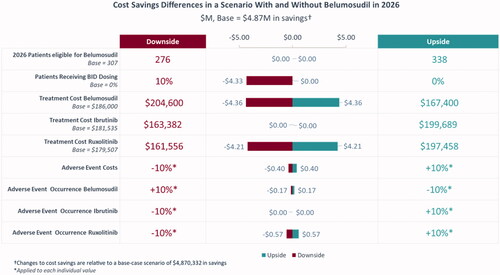
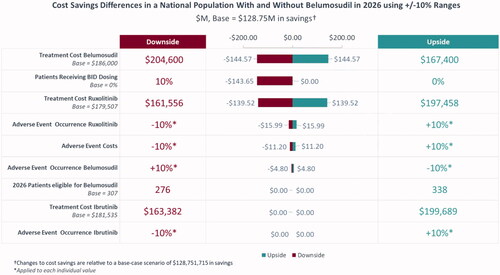
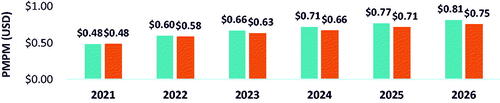
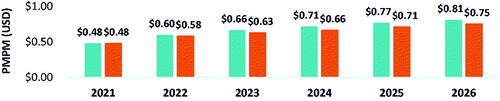
Based on the base case scenario, in which there were 307 patients eligible for belumosudil (i.e. treated on 3 L and 4 L+) by 2026 and belumosudil market utilization increased to 55% in 3 L and 4 L + by 2026, the projected annual budget impact for a hypothetical 10-million-member US payer perspective represented a saving of $4,870,332 (6.7%) in absolute cost () and PMPM cost savings of $0.03 () led by savings in AEs ($3,866,987) and HCRU ($2,322,634). From the national perspective, there was a projected annual cost saving of $128,751,715 (5.5%) () and PMPM cost savings of $0.03 () led by savings in AEs ($108,406,330) and HCRU ($65,112,249). Savings in annual budget were projected to be realized in the first year after baseline (i.e. 2022) with PMPM cost savings of $0.02 from the hypothetical 10-million-member US payer perspective and $0.01 in savings from the national perspective.
Table 1. Annual budget impact and absolute cost change – payer population.
Table 2. Annual budget impact and absolute cost change – National population.
Sensitivity analyses were conducted to assess the parameters with the largest impact on the model in both the payer and national populations. Based on individual adjustments of ±10% to multiple parameters, the proportion of patients receiving BID belumosudil dosing, the treatment cost of belumosudil, and the treatment cost of ruxolitinib were identified as having the highest impact on absolute cost ( and ). Similar results were observed when adjustments of ±25% were used for parameters (Supplementary Figures S1 and S2).
Alternate scenarios
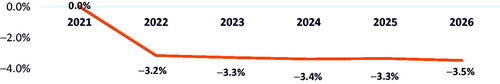
In the scenario where belumosudil share was taken from only ibrutinib and ruxolitinib, by 2026, the model estimated a 7.1% reduction in a 10-million-member plan’s budget () and a PMPM saving of $0.06 versus a market without belumosudil (). Percentage cost offsets due to reductions in AE and HCRU costs with belumosudil adoption were 5.8% and 3.5%, respectively.
Figure 5. Budget percent change in 2026 with adoption of belumosudil with ibrutinib and ruxolitinib reduction.
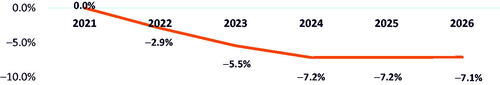
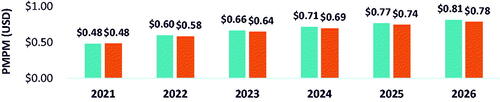
When belumosudil share was taken from ruxolitinib only, an estimated reduction in budget of 7.1% () and saving of $0.06 PMPM () would be observed by 2026, largely due to cost reductions of 5.8% and 3.5% in AEs and HCRU, respectively.
Figure 7. Budget percent change in 2026 with adoption of belumosudil with ruxolitinib reduction only.
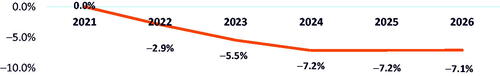
If belumosudil share was derived from ibrutinib only, similar results were observed, with a 3.5% reduction in budget () and $0.03 in PMPM savings by 2026 (). AE and HCRU cost reductions versus a scenario without belumosudil were 2.8% and 1.7%, respectively.
Discussion
A budget impact model of a hypothetical 10-million-member payer perspective or of the US national perspective was generated to project the potential budget impact of introducing belumosudil for the treatment of cGVHD for adult and pediatric patients 12 years and older after failure of two or more prior lines of systemic therapy (i.e. 3 L/4L+). This model is also consistent with NCCN guidelines that support belumosudil as a treatment option for patients with steroid refractory cGVHD (category 2A recommendation)Citation8.
The budget impact model demonstrated significant absolute cost savings starting in the first year that continued throughout the 5-year projection period. Analyses of various market share scenarios among branded agents demonstrated consistent cost savings with belumosudil and may better reflect the eventual real-world utilization bof elumosudil. These cost savings were led by reductions in severe AEs and HCRU compared to ruxolitinib and ibrutinib, including reductions in thrombocytopenia and infectionsCitation9,Citation10,Citation19,Citation26,Citation27. Belumosudil is also able to potentially address the fibrotic processes for lung disease, which can further decrease patient exposure to severe AEsCitation9. Lower AE rates translated to fewer hospital visits, inpatient stays, and subsequent reductions in overall HCRU. Notably, although the model base case indicates that belumosudil adoption will be cost-saving overall, the PMPM saving is $0.03. Although this saving may seem minimal and restricted to a small population of patients, it also suggests alternative scenarios in the model that incrementally increase the cost of care with belumosudil may still keep the cost of belumosudil adoption cost neutral versus existing standard of care.
The cost of branded therapies in cGVHD accounts for a significant portion of total treatment cost. Sensitivity analyses to determine the key variables impacting absolute cost confirmed that the treatment cost of belumosudil, the treatment cost of ruxolitinib, and the proportion of patients receiving BID dosing of belumosudil were the top influencers of absolute cost. The calculated WAC per patient, per year for branded treatment options currently available in the market can reach as high as $179,507 for ruxolitinib and $181,535 for ibrutinib. Similarly, belumosudil is priced at $232,500 per patient per year of treatment with QD dosingCitation18. The FDA label requires BID dosing in patients receiving strong CYP3A inducers or proton pump inhibitors, as these have the potential to impact belumosudil metabolism. The proportion of real-world patients who would receive BID dosing for belumosudil is currently unknown. Due to the similarity of cost between the branded agents and belumosudil and the presumption of similar efficacy, it was anticipated that belumosudil market share would likely increase due to reductions in ibrutinib and/or ruxolitinib use, as modeled in scenario analyses. The impact of drug cost is also reflected in the impact of BID dosing of belumosudil. Although the FDA label requires BID dosing in patients receiving strong CYP3A inducers or proton pump inhibitors due to the potential to impact belumosudil metabolism, dosing in the pivotal trials demonstrating belumosudil efficacy was QD. Notably, the proportion of real-world patients who will receive BID dosing for belumosudil is unknown.
This study was subject to several limitations. With respect to the model view itself, the model is restricted to a 5-year time horizon to reflect the average enrollment period for members of US commercial insurance plans, which may be less reflective of other coverage scenarios, such as the US Medicare population. The 5-year time horizon may also be less generalizable outside the US, where longer time horizons are more often considered. Several model inputs are also limiting. First, the results were dependent on the available data inputs and assumptions. Factors such as the performance of treatments, market growth, and treatment dosing and duration were based on clinical trials and secondary literature and may differ from the real-world. WAC costs from Redbook were used as a recent source of standardized drug pricing (versus 2019 ASP costs from Medicare Part D files), but they may more closely reflect commercial plans versus Medicare plans. Additionally, only direct costs were considered, and no assumptions were made for indirect costs incurred by patients. Second, the comparative efficacy of belumosudil versus other cGVHD therapies is unknown; the belumosudil, ibrutinib, and ruxolitinib clinical trial programs used open-label studies as it was considered unethical to use a comparator which a patient likely already failed. Third, patients were assumed to incur a one-time AE cost if they experienced a grade 3/4 AE, and costs for lower grade AEs were not included in the model. Fourth, the degree of treatment discontinuation was not included in this model. However, analyses have shown first year discontinuation rates for ibrutinib and ruxolitinib to be as high as 50% and 41%, respectivelyCitation25. The prior analyses also examined a time period before the approval of belumosudil, when there were few options for subsequent lines of therapy. Therefore, it is possible that having belumosudil as an additional option may result in an increase in discontinuation rates for ruxolitinib and ibrutinib. Further uncertainties around discontinuation include the timing (i.e. discontinuation is not necessarily linear over the year), subsequent line of therapy (based on exhaustion of standard of care options), and impacts on efficacy. Therefore, drug cost reductions due to discontinuation were not incorporated into the model due to the requirement for additional assumptions made beyond available evidence. Finally, documented AEs and HCRU for certain cGVHD therapies may not have occurred/been caused by the cGVHD treatment.
Conclusions
The introduction of belumosudil as a therapy for 3 L/4L + cGVHD patients in our model resulted in substantial cost savings for US health plans as a result of the safety profile. Belumosudil may provide an alternative cost-saving option to existing cGVHD treatments. Additional research is needed to characterize the real-world profile of belumosudil including true market growth, AE and HCRU rates, and the proportion of patients requiring BID dosing.
Transparency
Declaration of funding
This study was funded by Kadmon Corporation LLC, New York, a Sanofi Company.
Declaration of financial/other interests
CB is a member of the scientific advisory committees for BMS, CRISPR, Autolus, Nikarta, Mana, and Novartis. JRS, SD, and BM are employees of Trinity Life Sciences, which was contracted for these analyses by Kadmon Corporation, a Sanofi Company. JRS also holds equity in Trinity Life Sciences. JI and HT are employees of Kadmon Corporation, a Sanofi Company, with stock/stock options.
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Previous presentations
These data were previously presented at the Academy of Managed Care Pharmacy Nexus 2021 Annual Meeting and American Society of Hematology 2021 Annual Meeting & Exposition.
Supplemental Material
Download MS Word (91.9 KB)Acknowledgements
The authors would like to acknowledge Iona Bartek for manuscript writing support.
References
- Arora M, Cutler CS, Jagasia MH, et al. Late acute and chronic graft-versus-Host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(3):449–455.
- Bachier CR, Aggarwal SK, Hennegan K, et al. Epidemiology and treatment of chronic graft-versus-host disease post-allogeneic hematopoietic cell transplantation: a US claims analysis. Transplant Cell Ther. 2021;27(6):504.e1–e6.
- Goerner M, Gooley T, Flowers ME, et al. Morbidity and mortality of chronic GVHD after hematopoietic stem cell transplantation from HLA-identical siblings for patients with aplastic or refractory anemias. Biol Blood Marrow Transplant. 2002;8(1):47–56.
- Jagasia MH, Greinix HT, Arora M, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e1.
- Flowers ME, Storer B, Carpenter P, et al. Treatment change as a predictor of outcome among patients with classic chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(12):1380–1384.
- Wolff D, Schleuning M, von Harsdorf S, et al. Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-Host disease. Biol Blood Marrow Transplant. 2011;17(1):1–17.
- Jones C, Fernandez L, Weimersheimer P, et al. Estimating the burden of cost in chronic graft-versus-host disease: a human Capital approach. J Health Econ Outcome Res. 2016;4(2)06/01:113–118.
- Saad A, de Lima M, Anand S, et al. Hematopoietic cell transplantation, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(5):599–634.
- Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar study. Blood. 2021;138(22):2278–2289.
- Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic Graft-Versus-Host disease. J Clin Oncol. 2021;39(17):1888–1898.
- Bureau USC. Data from: American Community Survey Demographic and Housing Estimates. 2018.
- 2019 U.S. Population Estimates Continue to Show the Nation’s Growth Is Slowing. 2019. https://www.census.gov/newsroom/press-releases/2019/popest-nation.html.
- (CIBMTR) CfIBMTR. 2019 Annual Report. 2019. https://www.cibmtr.org/About/AdminReports/Documents/2019CIBMTRAnnualReport.pdf.
- National Marrow Donor Program acftCWBYCTPottUSDoHaHS, Health Resources and Services Administration, Healthcare Systems Bureau. Transplant Activity Report. 2019. https://bloodstemcell.hrsa.gov/data/donation-and-transplantation-statistics/transplant-activity-report.
- Filipovich AH, Weisdorf D, Pavletic S, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956.
- Jagasia M, Giglia J, Chinratanalab W, et al. Incidence and outcome of chronic graft-versus-host disease using national institutes of health consensus criteria. Biol Blood Marrow Transplant. 2007;13(10):1207–1215.
- Rezurock Highlights of Prescribing Information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214783s000lbl.pdf.
- Data from: RED BOOK Online. IBM Micromedex [database online]. 2021.
- Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385(3):228–238.
- Jakafi Highlights of Prescribing Information. 2021. https://www.jakafi.com/pdf/prescribing-information.pdf.
- Imbruvica Highlights of Prescribing Information. 2020. https://www.imbruvica.com/files/prescribing-information.pdf.
- Report to the Congress: Medicare Payment Policy, March 2020. 2020.
- An Analysis of Private-Sector Prices for Hospital Admissions. 2017.
- (CMS) CfMMS. Data from: Inpatient Charge Data FY 2017. 2017. Deposited November 27. 2019.
- Bachier C, Eiznhamer D, Milgroom A, et al. Costs and adverse events associated with ibrutinib or ruxolitinib in chronic Graft-Versus-Host disease. Blood. 2020;136(Supplement 1):14–15.
- Abedin S, McKenna E, Chhabra S, et al. Efficacy, toxicity, and infectious complications in ruxolitinib-treated patients with corticosteroid-refractory graft-versus-host disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(8):1689–1694.
- Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130(21):2243–2250.