?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims
Acute respiratory tract infections (ARTIs) are common in hematopoietic stem cell transplantation (HSCT) recipients, however, data is limited regarding epidemiology and economic burden of ARTI in HSCT recipients in Japan. We evaluated the incidence of ARTI in HSCT recipients, associated economic burden, and ARTI-related treatments post-HSCT.
Materials and methods
Patients receiving HSCT between July 2017 and December 2018, and those enrolled in the JMDC Claims Database for ≥6 months before index month (month when latest medical procedure code of HSCT recorded) were included. The outcomes included demographics, ARTI incidence, healthcare resource utilization (HCRU), direct costs, and ARTI-related treatments.
Results
In 330 analyzed patients, the ARTI incidence rate was 85.5% during total follow-up, consisting of post-HSCT hospitalization of mean 2.1 months and post-discharge periods of mean 17.6 months (post-HSCT hospitalization: 44.8%; post-discharge: 77.6%). For ARTI vs non-ARTI patients during post-HSCT hospitalization, length of hospitalization was significantly longer (mean [SD] months; 2.40 [1.73] vs 1.84 [1.09]; p = 0.0004), and median cost was significantly higher (JPY; 6,250,120.00 vs 4,774,570.00; p = 0.0096). The cost of outpatient visits during post-discharge periods, drug-related and non-drug-related costs of outpatient visits were generally higher for ARTI vs non-ARTI patients. In ARTI vs non-ARTI patients, utilization of any symptom relievers (decongestants, antitussives, and antipyretics), bronchodilators, immunoglobulin G, antibiotics, antivirals, and oxygen supply were numerically higher during post-HSCT hospitalization and post-discharge periods. The proportion of patients and mean prescription days for immunosuppressants during post-HSCT hospitalization were higher in ARTI vs non-ARTI patients.
Limitations
This administrative claims study lacks clinical data and contains only direct medical costs. Patients were retained if they had at least 1 month of enrollment post-HSCT.
Conclusions
In HSCT recipients, ARTI leads to substantial incremental HCRU and direct costs for management in real-world settings in Japan.
PLAIN LANGUAGE SUMMARY
People receiving hematopoietic stem cell transplantation (HSCT) commonly suffer from acute respiratory infections (ARTIs). The real-world data on its incidence and economic impact in Japan is limited. In this study, using the JMDC Claims Database 330 HSCT recipients were identified during July 2017 and December 2018. Of these patients, 85.5% developed ARTI either during post-HSCT hospitalization (44.8%, within mean 2.1 months) or post-discharge period (77.6%, within mean 17.6 months). Patients with ARTI had longer hospital stays (2.40 months vs 1.84 months) and higher in-patient treatment costs (6,250,120.00 JPY vs 4,774,570.00 JPY) than those without ARTI. The costs associated with out-patient treatment, both drug-related and non-drug-related, were also higher for ARTI patients than non-ARTI patients. The use of medicines for stuffy nose (decongestants), dry cough (antitussives), and fever (antipyretics), and other medicines to treat respiratory infections (such as bronchodilators, immunoglobulin G, antibiotics, antivirals, and oxygen supply) was generally high with ARTI patients both during post-HSCT hospitalization and during post-discharge periods. The use of immunosuppressants was also more in patients who acquired ARTI as compared with non-ARTI patients during post-HSCT hospitalization. This study demonstrates the significant impact of ARTI in terms of economic and healthcare resource utilization in HSCT recipients in Japan.
Introduction
Hematopoietic stem cell transplantation (HSCT) is a critical therapy tool widely used in the treatment of several malignant and non-malignant hematologic disordersCitation1. While the outcomes for patients undergoing HSCT have improved over the past few decades, complications due to immunosuppression and treatment-related toxicities limit the HSCT success. Pulmonary complications occur in approximately one-third of the HSCT recipients, and remain a leading cause of morbidity with mortality in up to 50% of casesCitation2,Citation3. Bacterial, viral, and fungal infections significantly affect the HSCT recipients, of which, respiratory viral infections (including respiratory syncytial virus, influenza, parainfluenza, rhinovirus, enteroviruses and adenoviruses) are associated with high morbidity and mortalityCitation4–7.
Due to the prolonged immunosuppression, acute respiratory tract infections (ARTIs) are common after HSCT and may cause respiratory failure leading to intensive care unit admission and mechanical ventilationCitation8,Citation9. Respiratory viruses cause upper respiratory infection (URI), which may progress to lower respiratory infections (LRI) or severe pneumoniaCitation6. The disease progression from URI to LRI contributes to disease severity and may occur in 30–40% of HSCT recipients with a mortality rate ranging from 10–50% recipientsCitation10. In Japanese HSCT recipients, a high frequency of fatal pulmonary complications are reportedCitation11.
Hospitalizations for HSCT without complications are less expensive as compared with the average HSCT costCitation12, whereas severe post-HSCT complications contribute to high overall costsCitation13. Epidemiological data and economic burden of ARTI in HSCT recipients are very limited, especially in Japan. The current study is designed to evaluate the incidence of ARTI in HSCT recipients, illustrate the economic burden of ARTI after HSCT, and provide the epidemiological and demographic data and ARTI-related treatments in Japanese patients undergoing HSCT.
Patients and methods
Study design
This was a retrospective evaluation of data from the JMDC Claims Database (JMDC Inc.)Citation14. The JMDC data source comprises health insurance claims data, medical examination data, as well as ledger information of all members of multiple contracted health insurance associations since 2005. The JMDC data includes patient-level demographic information, inpatient and outpatient, data including diagnosis, procedures, and prescriptions as dispensed claims information of all insured people in Japan. The information on the medical care was aggregated by month in the database.
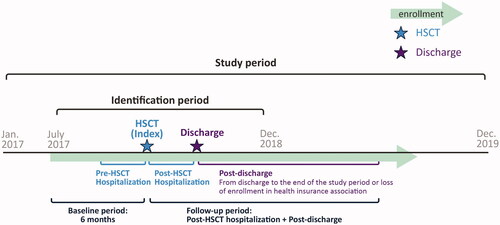
depicts the study scheme. Patients who received HSCT between July 2017 and December 2018, defined as the identification period, were included in the study. The last month of HSCT procedure was defined as index month. The baseline period was defined as 6 months prior to the index month. The post-HSCT hospitalization period was considered from HSCT (index month) to the discharge month, defined as the last month of the hospitalization period (i.e. continuous issue of in-hospital/diagnosis procedure combination [DPC] claim). However, if outpatient claim was issued after the index month, the patient was considered discharged at the previous month of first outpatient claim. This assumption is due to the database characteristics of medical care data aggregation by month. The post-discharge period was considered from the month after discharge to the end of observation, defined as either end of the study period (December 2019) or loss of enrollment in health insurance association. The overall follow-up period included post-HSCT hospitalization and post-discharge periods.
Patient population and study cohorts
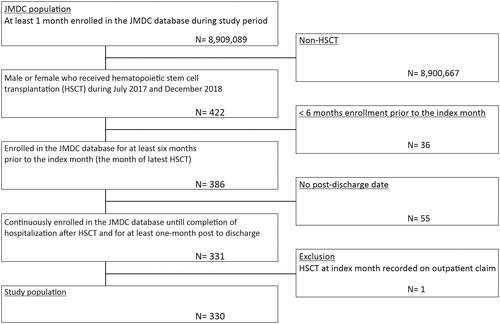
Patients receiving HSCT including bone marrow transplantation, peripheral stem cell transplantation, cord blood transplantation, autotransplantation, and allotransplantation, as defined by the medical procedure code (Supplementary Table S1) during the identification period, and those who were enrolled in the JMDC database for at least 6 months prior to the index month, i.e. the month of latest HSCT, were included in the study. Patients had to remain enrolled in the JMDC database until completion of the hospitalization after HSCT and for at least 1 month post-discharge to be included in the analysis. Patients with HSCT at index month who were recorded on outpatient claim were excluded. The patient confidentiality was maintained as the patients’ data were anonymized and no patient identifiers were used, hence, the study is exempt from review by institutional review board.
For this study, patients with HSCT were divided into two main cohorts: (i) ARTI patients and (ii) non-ARTI patients. Each ARTI patient experienced at least one ARTI incidence during the post-HSCT hospitalization, post-discharge, or follow-up periods (combining post-HSCT hospitalization and post-discharge periods). The ARTI was defined by the diagnosis of ICD-10 codes of J00–J22. The ARTI incidence was identified by treatment start date recorded in claims. Each month, ARTI patients were classified into URI only and LRI. The URI only included acute URIs (ICD 10 code: J00–JU06) and influenza without LRI (J09, J10, J111, and J118). The LRI included pneumonia (J110, J12–J16, and J18) and other acute LRIs (J20–J22). The non-ARTI patients were defined as patients without ARTI incidence during post-HSCT hospitalization, post-discharge, and follow-up periods each.
Study outcomes
Demographic and other characteristics of HSCT recipients
The demographic characteristics, age at index month, diagnosis at index month, conditioning regimens at index month and 1 month prior, type of HSCT, details of index month and calendar month at discharge, comorbidities, use of corticosteroids at baseline, and use of immunosuppressants during post-HSCT hospitalization were investigated. Data are presented separately for the post-HSCT hospitalization and post-discharge periods.
Incidence of ARTI
The ARTI incidence rate after HSCT was calculated for the follow-up period and, consequently, separately for post-HSCT hospitalization and post-discharge periods, using ARTI patients as numerator and HSCT recipients (study population) as denominator, as follows:
Furthermore, ARTI rates for each period were also calculated based on person years (PY) and person months (PM), as below:
* One patient who experienced two infections was counted as “two cases”.
The percentage of ARTI in each month was calculated as per the above equations, only HSCT patients who enrolled in the database in the respective month were used as the denominator.
Healthcare resource utilization (HCRU) and direct cost
The HCRU and direct cost were calculated for the post-HSCT hospitalization period and every month post-discharge. For the post-HSCT hospitalization period, the length of hospitalization in months, cost of post-HSCT hospitalization, and the drug related and non-drug related costs were considered. The cost of post-HSCT hospitalization involved the 10× sum of total point in inpatient/DPC claims issued in the post-HSCT hospitalization period.
Considering the expected non-uniformity in the post-discharge period, the HCRU and direct cost in the post-discharge period were calculated by utilizing the number of outpatient visits (times), cost of outpatient visits, and drug and non-drug related costs for each month. All costs were expressed in Japanese Yen (JPY). The value in United States Dollars (USD) with a conversion rate of purchasing power parity of hospital services from the 2017 round of the Eurostat-OECD PPP program as 78.3 JPY = 1 USD in brackets is shown for referenceCitation15.
Treatment for ARTI
The proportion of patients who received different medications including fluids and medical procedures in ARTI vs non-ARTI groups were assessed for both post-HSCT hospitalization and post-discharge periods. The World Health Organization Anatomical Therapeutic Chemical (ATC) codes for medications (Table S2), fluids (Table S3) and immunosuppressants (Table S4) are provided as supplementary materials.
Statistical analysis
Frequencies and percentages were presented for categorical variables; mean, standard deviation, and median were calculated for continuous variables. The percentages and 95% CIs were provided for ARTI rate, ARTI rate based on person years or months, and monthly ARTI rate. Descriptive statistics were presented for demographic characteristics, HCRU, direct cost, and treatments for each of ARTI and non-ARTI patients. Additionally, multiple comparison analyses for ARTI, URI only, LRI, and non-ARTI patients were performed. Analysis of variance (ANOVA) was conducted to compare the means of three groups as URI only, LRI, and non-ARTI for continuous variables, excluding related costs. Kruskal-Wallis test as the nonparametric method for multiple comparison was conducted for variables related to costs, since the distribution of each cost that tails to the right is assumed. Statistical analyses were performed using SAS version 9.4 (TS1M6).
Results
A total of 422 patients received HSCT between July 2017 and December 2018, of which 330 were considered for the final analysis ().
Patients' demographics and characteristics
Post-HSCT hospitalization period
The mean age of ARTI patients was significantly higher compared with non-ARTI patients (p = 0.0175). For ARTI vs non-ARTI patients, there were no significant differences in gender distribution, any conditioning regimens at index month and 1 month prior, any types of HSCT, the proportion of patients receiving HSCT at index month and month at discharge, and any comorbidities except asthma (p = 0.0462). At index month, diagnosis of leukemia was significantly higher in ARTI vs non-ARTI patients during post-HSCT hospitalization (p = 0.0385), whereas malignant lymphoma, plasma cell dyscrasia, and solid tumors were not. The use of a cytarabine-based conditioning regimen was significantly lower in URI only compared to LRI (p = 0.0434). The proportion of patients using immunosuppressants was numerically higher in ARTI vs non-ARTI patients (p = 0.3559); the mean prescription days for immunosuppressants were significantly higher in ARTI vs non-ARTI patients (p = 0.0003). The use of mycophenolate mofetil + tacrolimus hydrate was significantly higher in ARTI vs non-ARTI (p = 0.0027), URI only vs LRI vs non-ARTI (p = 0.0045), and URI only vs non-ARTI (p = 0.0022). Compared with non-ARTI patients, the use of corticosteroids at baseline was significantly lower in ARTI (p = 0.0328) and URI only patients (p = 0.0338) ( and Supplementary Table S5).
Table 1. Patients' demographics and characteristics of ARTI and non-ARTI patients during post-HSCT hospitalization.
Post-discharge period
The mean age of patients was significantly lower for ARTI vs non-ARTI patients (p = 0.0138), but there was no significant difference in gender distribution, any diagnosis at index month, any conditioning regimens at index month and 1 month prior, any types of HSCT, the proportion of patients receiving HSCT at index month and month at discharge, and any comorbidities between the groups. The use of fludarabine-based conditioning regimen was significantly lower in URI only compared to LRI (p = 0.0069). The use of corticosteroid at baseline, use of immunosuppressants post-discharge and mean prescription days were not significantly different for ARTI vs non-ARTI patients; similar trends were observed for URI only vs non-ARTI, LRI vs non-ARTI, and URI only vs LRI patients. The use of anti-human thymocyte immunoglobulin, rabbit + tacrolimus hydrate was significantly lower in URI vs non-ARTI patients (p = 0.0347) (Supplementary Table S6).
ARTI incidence rate after HSCT
The ARTI incidence rate was 85.5, 44.8, and 77.6% during the total follow-up, post-HSCT hospitalization, and post-discharge periods, respectively. The ARTI incidence rate per PY was 2.0 in the total follow-up period (mean 19.7 months) and 1.8 in the post-discharge period (mean 17.6 months). Similarly, the ARTI incidence rate per PM was 0.2 during the total follow-up period, 0.3 during the post-HSCT hospitalization period, and 0.1 during the post-discharge period (). In allogeneic, autologous, and cord blood transplantation recipients, a similar incidence rate was observed (Supplementary Table S7).
Table 2. ARTI incidence rate after HSCT over the follow-up period.
The ARTI incidence rate was 37% at index month and 21.9% at the first month (Month 1) after HSCT during hospitalization. At index month, the URI only incidence rate was 16.4% and the LRI incidence rate was 20.6%. The incidence of URI only and LRI were reported until months 6 and 7, respectively (). Similar values were found in allogeneic, autologous, and cord blood transplantation recipients (Supplementary Table S8).
Table 3. ARTI incidence rate in each month during post-HSCT hospitalization.
During the post-discharge period, the ARTI incidence rate was 17.6% at month 1 and 18.8% at month 30. Similarly, the incidence of URI only (Month 1: 9.4%; Month 30: 12.5%) and LRI (Month 1: 8.2%; Month 30: 6.3%) were reported until month 30 (Table S9). In allogeneic, autologous, and cord blood transplantation recipients, the incidence rate at month 1 was 18.4, 14.5, and 22.5% respectively. The incidence of URI only at month 1 was 9.6, 8.3, and 1.3% and that of LRI was 8.8, 6.2, and 11.3% in allogeneic, autologous, and cord blood transplantation recipients, accordingly (Supplementary Table S10).
Healthcare resource utilization and direct cost
Post-HSCT hospitalization period
The mean length of hospitalization was significantly longer for ARTI patients as compared with non-ARTI patients (p = 0.0004) during the post-HSCT hospitalization period (). Similar trends for significantly longer length of post-HSCT hospitalization were observed for LRI vs non-ARTI patients (p < 0.0001) and for LRI vs URI only patients (p = 0.0459), whereas it was not significant for URI only vs non-ARTI patients (Supplementary Table S11).
Table 4. Healthcare resource utilization and direct cost during post-HSCT hospitalization period.
The median cost (JPY; PPPUSD) of post-HSCT hospitalization in ARTI patients was significantly higher as compared with non-ARTI patients (6,250,120.00 vs 4,774,570.00, p = 0.0096; 79,822.73 vs 60,977.91 PPPUSD); similar results were observed for LRI vs non-ARTI patients (p = 0.0011) and for LRI vs URI only patients (p = 0.0244). The median drug-related (2,213,574.02 vs 1,751,519.92, p = 0.0065; 28,270.42 vs 22,369.35 PPPUSD) and non-drug-related (3,750,023.95 vs 2,839,802.66, p = 0.0143; 47,893.03 vs 36,268.23 PPPUSD) costs were significantly higher for ARTI patients as compared with non-ARTI patients (). Significantly higher drug-related (p = 0.0007) and non-drug related costs (p = 0.0018) were observed in LRI vs non-ARTI patients. Similarly, the drug-related (p = 0.0307) and non-drug related costs (p = 0.0508) were higher for LRI vs URI only patients. There was no statistically significant difference between URI only vs non-ARTI patients for all different costs assessed above (Supplementary Table S11).
When looking at allogeneic and cord blood transplantation, a significantly longer length of hospitalization and higher cost of post-HSCT hospitalization in ARTI patients as compared with non-ARTI patients were observed. On the other hand, those of autologous transplantation recipients did not. Costs of post-HSCT hospitalization in allogeneic (7,479,840.00 JPY or 95,527.97 PPPUSD) and cord blood transplantation recipients (10,488,960.00 JPY or 133,958.62 PPPUSD) were higher than those of patients with autologous transplantation (2,543,975.00 JPY or 32,490.10 PPPUSD). Similarly, a longer mean length of hospitalization (months) was observed in allogeneic and cord blood transplantation recipients (2.84 and 3.41, respectively) as compared with the autologous group (1.45) (Supplementary Table S12).
Post-discharge period
The number of outpatient visits (times), cost of outpatient visit, drug-related and non-drug-related costs of outpatient visits were generally higher for ARTI patients as compared with non-ARTI patients during the post-discharge period; similar trends were observed for LRI or URI only patients when compared with non-ARTI patients (Supplementary Table S13). The number of outpatient visits of patients with allogeneic, autologous, and cord blood transplantation was always higher in ARTI patients as compared with non-ARTI patients while the cost of outpatient visits was variable (Supplementary Table S14).
ARTI-related treatments in HSCT recipients
Post-HSCT hospitalization period
The utilization of different ARTI-related medications including symptom relievers, antipyretics, bronchodilators, immunoglobulin G, antibiotics, and antivirals were numerically higher in ARTI vs non-ARTI patients. A significantly higher usage of antitussives was observed in ARTI vs non-ARTI patients (p = 0.0037), and LRI vs non-ARTI patients (p = 0.0016). The use of antifungal agents was significantly lower in URI only patients as compared with non-ARTI (p = 0.0159) and LRI patients (p = 0.0029). The utilization of medical procedures such as ventilator and oxygen supply were numerically higher in ARTI and LRI patients as compared with non-ARTI patients ( and Supplementary Table S15).
Table 5. ARTI-related treatments in HSCT recipients during post-HSCT hospitalization period.
Post-discharge period
The utilization of any ARTI related medications after discharge was higher in ARTI patients as compared with non-ARTI patients (p < 0.0001). In ARTI vs non-ARTI patients, the use of antitussives, antipyretics, bronchodilators, antibiotics, and antivirals was significantly higher (p < 0.0001 for all). The use of antitussives, antipyretics, bronchodilators, antibiotics, and antivirals was significantly higher in LRI vs non-ARTI patients (p < 0.0001 for all). The use of antitussives (p < 0.0001) and antipyretics (p = 0.0015) was significantly higher in URI only vs non-ARTI patients. A numerically higher proportion of ARTI and LRI as compared with non-ARTI patients received oxygen supply (Supplementary Table S16).
Discussion
In HSCT recipients, there is scarcity of data on the rate of cumulative respiratory tract infections (RTIs) including viral, bacterial, and any other etiologies, and the majority of the studies have demonstrated data on respiratory viral infectionsCitation16,Citation17. In our study, we evaluated the incidence of ARTIs of any of these etiologies in HSCT recipients. Among the 330 patients included in this study, over 85% had ARTI during the total follow-up period (2.1 and 17.6 months for post- HSCT hospitalization and post-discharge period, respectively). A study by Sim et al.Citation17 reported the incidence of viral RTIs at 14.7% in patients who received allogeneic HSCT. The lower incidence of RTIs in this study could be attributed to the fact that the episodes of RTIs were considered till only 100 days after HSCT and only viral infection episodes were counted, whereas in our study episodes of any RTIs were considered for longer mean follow-up period of 1.6 years. In our study, the average prescription days for use of immunosuppressants was significantly higher in patients who acquired infections as compared with non-ARTI patients. It is known that the use of immunosuppressants increases the risk of acquiring opportunistic infections in HSCT recipientsCitation18; it is more likely that allogeneic transplantation recipients with acute/chronic graft-versus-host disease need more intensive immunosuppression, hence are more likely to acquire respiratory tract infections.
In our analysis, during the post-HSCT hospitalization period, the ARTI incidence rate was lower at 44.8% as compared with 77.6% reported during the post-discharge period with a mean follow-up period of 19.7 months (2.1 and 17.6 months for post-HSCT hospitalization and post-discharge period, respectively). Similarly, other studies have demonstrated that respiratory infection and pulmonary complications were high post-discharge and were common reasons for readmission to hospital in Japanese patientsCitation19 and patients in EgyptCitation20. Marinelli et al.Citation21 reported viral RTIs in 75.8% of patients more than 100 days post-HSCT.
The URIs are more common than LRIs in HSCT recipients and the progression of URI to LRI indicates the disease severity. Sim et al.Citation17 reported that 58.5% of post-HSCT recipients initially presented with URIs, of which 28.9% of patients developed LRIs. Marinelli et al.Citation21 reported the incidence of LRI at 45.6% in HSCT recipients. In our study, the incidence of URI only and LRI was 16.4 and 20.6% at the index month, with the maximum monthly incidence of 33.3% (Month 6) and 75.0% (Month 7) during the post-HSCT hospitalization period, and of 12.5% (Month 30) and 13% (Month 25) during the post-discharge period, respectively. However, less than one-third of the patients remained in the post-HSCT hospitalization period from Month 2 and the post-discharge period from Month 22 and as such these findings should be interpreted with caution.
The development of infectious disease has previously been reported as one of the predictors for length of hospitalization in HSCT recipientsCitation22,Citation23. Furthermore, it has been reported that increased length of hospital stay due to the transplant complications, including infections, is a driver for an increase in the hospital costCitation22. In our study also, the average length of hospitalization was significantly higher in ARTI vs non-ARTI patients.
In our study, the cost of post-HSCT hospitalization was significantly higher in patients who developed ARTI as compared with non-ARTI patients. Also, patients with LRIs had a significantly higher cost of post-HSCT hospitalization and HCRU vs patients with URIs and non-ARTIs. The drug and non-drug related cost were also higher for ARTI patients during post-HSCT hospitalization and post-discharge period, which indicate a significant economic impact of ARTI management for HSCT recipients in Japan. Previously published studies have also reported infections as one of the factors that contributes to higher cost in HSCT recipientsCitation24–26. In addition, the cost of post-HSCT hospitalization in our study was higher in allogeneic and cord blood transplantation recipients as compared to autologous transplantation recipients. Graft-versus-host disease after the allogeneic transplantation, both acute and chronic, has been reported to be associated with significant HCRU and costsCitation27,Citation28.
Compared with non-ARTI patients, the patients with ARTIs had a higher utilization of the medications including symptom relievers, antipyretics, bronchodilators, immunoglobulin G (IgG), antibiotics, and antivirals during the post-HSCT hospitalization and post-discharge periods. In the study by Ghantoji et al.Citation25, the use of intravenous IgG was a strong predictor for higher cost for RTIs in HSCT recipients. Our findings show antiviral utilization at 96.6% during the post-HSCT hospitalization period and 94.9% during the post-discharge period. In the study by Sim et al.Citation17, antiviral therapy was received by 21.5% of patients but this low percentage could be attributed to the very small number of patients (n = 14) in the study. In addition, patients in our study received antivirals as a potential prevention measure per the Japanese guidelinesCitation29. Besides, strategies like early interception for symptomatic infection before initiating antivirals and regular diagnostics such as immunoassays and chest scans to monitor patients’ susceptibility for RTIs might have long-term cost implicationsCitation10.
This study is conducted using the data from the JMDC claims database, which includes approximately 7.3 million insured persons (as of April 2020) in Japan. Even if a patient transfers hospital or uses multiple facilities, the data can be tracked. This study and the database used have several limitations. The laboratory values, clinical assessments, and safety were not included in this database. The reason for change in patients’ treatments was not recorded. As information on outpatient visit was aggregated by month, in the case of more than one visit in the month, the visit date and the prescribed treatment could not be distinguished by each diagnosis. Patients were retained only if they had at least 1 month of enrollment post-HSCT, which may have caused selection bias. During the post-discharge period, this study evaluated HCRU and direct cost for outpatient visit only, which may have caused underestimation. Reported results are as per the relevant patients available in the JMDC databases, meeting the defined inclusion/exclusion criteria in our study, and may not be generalizable to the entire patient population.
Conclusion
This study illustrates the significant impact of ARTI in terms of economic and healthcare resource utilization in HSCT recipients in Japan. The respiratory infections lead to overall incremental costs in HSCT recipients with regards to the inpatient management costs including hospitalization, prescription costs, and the outpatient management costs. Advancement in prevention and treatment, for example, vaccine and antiviral, will enhance an optimal management of respiratory infections. Some HSCT recipients may be less likely to respond to vaccination and prophylactic usage of antivirals (pre-exposure prophylaxis) are expected to be the alternative options. While further evaluation is needed, these strategies have the potential to minimize the incidence of such infections and reduce the associated economic burden on the healthcare system.
Transparency
Declaration of funding
KW received funding from Janssen Pharmaceutical K.K for this study. The funder did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of financial/other relationships
KW and YN are employees of Janssen Pharmaceutical K.K.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
The Deputy Editor in Chief helped with adjudicating the final decision on this paper.
Author contributions
KW conceived and designed the study, and supervised analysis. KW and YN interpreted the results and substantially contributed to the critical revision of manuscript, with KW writing the first draft. All the authors consented to the final version of the manuscript. All authors met ICMJE criteria and all those who fulfilled these criteria are listed as authors.
Supplemental Material
Download MS Word (242.3 KB)Acknowledgements
The authors thank Priyanka Sharma (SIRO Clinpharm Pvt. Ltd.) for providing writing assistance and Uma Kundu (SIRO Clinpharm Pvt. Ltd.) for logistical support in preparing this manuscript.
Data availability statement
The data that support the findings of this study were derived from JMDC Claims Database (https://www.jmdc.co.jp/en/). Data access request for research is available at https://www.jmdc.co.jp/en/inquiry. Requestors need to accept the terms and conditions of the data request and may need to pay the corresponding data access fee.
References
- Iida M, Kodera Y, Dodds A, Registry Committee of the Asia-Pacific Blood and Marrow Transplantation Group (APBMT), et al. Advances in hematopoietic stem cell transplantation in the Asia-Pacific region: the second report from APBMT 2005-2015. Bone Marrow Transplant. 2019;54(12):1973–1986.
- Peña E, Souza CA, Escuissato DL, et al. Noninfectious pulmonary complications after hematopoietic stem cell transplantation: practical approach to imaging diagnosis. Radiographics. 2014;34(3):663–683.
- Chi AK, Soubani AO, White AC, et al. An update on pulmonary complications of hematopoietic stem cell transplantation. Chest. 2013;144(6):1913–1922.
- Sahin U, Toprak SK, Atilla PA, et al. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J Infect Chemother. 2016;22(8):505–514.
- Boonyaratanakornkit J, Vivek M, Xie H, et al. Predictive value of respiratory viral detection in the upper respiratory tract for infection of the lower respiratory tract with hematopoietic stem cell transplantation. J Infect Dis. 2020;221(3):379–388.
- Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11(10):781–796.
- Chemaly RF, Rathod DB, Couch R. Respiratory viruses. In: Safdar A, editor. Principles and practice of cancer infectious diseases. Current Clinical Oncology. Totowa, NJ: Humana Press; 2011.
- Rajapreyar P, Kopp W, Randolph A. Acute respiratory failure and management. In: Duncan C, Talano JA, McArthur J, editors. Critical care of the pediatric immunocompromised hematology/oncology patient. Cham: Springer; 2019.
- Atilla E, Sahin D, Atilla PA, et al. Upper respiratory viral infections in patients with haematological malignancies after allogeneic haematopoietic stem cell transplantation: a retrospective study. Antivir Ther. 2018;23(6):523–527.
- Pochon C, Voigt S. Respiratory virus infections in hematopoietic cell transplant recipients. Front Microbiol. 2018;9(3294):3294.
- Chen-Yoshikawa TF, Sugimoto S, Shiraishi T, et al. Prognostic factors in lung transplantation after hematopoietic stem cell transplantation. Transplantation. 2018;102(1):154–161.
- Jones JA, Qazilbash MH, Shih YC, et al. In-hospital complications of autologous hematopoietic stem cell transplantation for lymphoid malignancies: clinical and economic outcomes from the nationwide inpatient sample. Cancer. 2008;112(5):1096–1105.
- Saito AM, Cutler C, Zahrieh D, et al. Costs of allogeneic hematopoietic cell transplantation with high-dose regimens. Biol Blood Marrow Transplant. 2008;14(2):197–207.
- Chakravarty SD, Abell J, Leone-Perkins M, et al. A novel qualitative study assessing Patient-Reported outcome measures among people living with psoriatic arthritis or ankylosing spondylitis. Rheumatol Ther. 2021;8(1):609–620.
- OECD.Stat. Organisation For Economic Co-Operation and Development; 2022 [cited 2022 May 25]. Available from: https://stats.oecd.org/.
- Mikulska M, Del Bono V, Gandolfo N, et al. Epidemiology of viral respiratory tract infections in an outpatient haematology facility. Ann Hematol. 2014; 93(4):669–676.
- Sim SA, Leung VKY, Ritchie D, et al. Viral respiratory tract infections in allogeneic hematopoietic stem cell transplantation recipients in the era of molecular testing. Biol Blood Marrow Transplant. 2018;24(7):1490–1496.
- Cho S-Y, Lee H-J, Lee D-G. Infectious complications after hematopoietic stem cell transplantation: current status and future perspectives in Korea. Korean J Intern Med. 2018;33(2):256–276.
- Seto A, Atsuta Y, Kawashima N, et al. Impact of hospital length of stay on the risk of readmission and overall survival after allogeneic stem cell transplantation. Int J Hematol. 2018;108(3):290–297.
- Maher OM, Silva JG, Huh WW, et al. Etiologies and impact of readmission rates in the first 180 days after hematopoietic stem cell transplantation in children, adolescents, and young adults. Pediatr Hematol Oncol. 2017;39(8):609–613.
- Marinelli T, Wee LYA, Rowe E, et al. Respiratory viruses cause late morbidity in recipients of hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020;26(4):782–788.
- Godara A, Siddiqui NS, Munigala S, et al. Length of stay and hospital costs for patients undergoing allogeneic stem-cell transplantation. JCO Oncol Pract. 2021;17(3):e355–e368.
- Sundaramurthi T, Sundaramurthi T. Predictors of length of hospital stay and readmission in hematologic stem cell transplant recipients [Doctoral dissertation]. Baltimore: University of Maryland; 2015 [cited 2021 Jul 23]. Available from https://archive.hshsl.umaryland.edu/handle/10713/4606.;en_US.
- Yong MK, Tio SY, Valentine J, et al. The economic and health utilization cost of clinically significant cytomegalovirus infection following allogeneic hematopoietic stem cell transplantation. Blood. 2019;134(Supplement_1):3437–3437.
- Ghantoji SS, Karanth S, El Haddad L, et al. Clinical and economic burden of respiratory viral infections in hematopoietic stem cell transplant recipients: the MD Anderson experience. Open Forum Infect Dis. 2017;4(suppl_1):S319–S319.
- Ueno R, Nishimura S, Fujimoto G, et al. The clinical and economic burden of cytomegalovirus management post allogeneic hematopoietic stem cell transplantation in Japan – a retrospective database study. Curr Med Res Opin. 2019;35(12):2089–2096.
- Schain F, Batyrbekova N, Liwing J, et al. Real-world study of direct medical and indirect costs and time spent in healthcare in patients with chronic graft versus host disease. Eur J Health Econ. 2021;22(1):169–180.
- Yu J, Lal L, Anderson A, et al. Healthcare resource utilization and costs associated with acute graft-versus-host disease following allogeneic hematopoietic cell transplantation. Support Care Cancer. 2020;28(11):5491–5499.
- Taylor AL, Wright JT. Jr. Should ethnicity serve as the basis for clinical trial design? Importance of race/ethnicity in clinical trials: lessons from the African-American heart failure trial (A-HeFT), the African-American study of kidney disease and hypertension (AASK), and the antihypertensive and Lipid-Lowering treatment to prevent heart attack trial (ALLHAT). Circulation. 2005;112(23):3654–3660.