Abstract
Objective
To evaluate the costs and benefits associated with the use of abobotulinumtoxinA (aboBoNT-A) and onabotulinumtoxinA (onaBoNT-A) for lower limb spasticity in children, upper and lower limb spasticity in adults, and cervical dystonia in adults.
Methods
This pharmacoeconomic analysis compared aboBoNT-A with onaBoNT-A. A decision tree model with a 1-year time horizon was conducted from a UK National Health Service (NHS) perspective using data from a variety of sources: randomized controlled trials (RCTs), network meta-analyses (NMAs), observational studies, and a physician survey investigating treatment patterns and resource utilization. Four patient populations were included: pediatric patients with lower limb spasticity (PLL), and adults with upper limb spasticity (AUL), lower limb spasticity (ALL), and cervical dystonia (CD). Outcomes included costs, quality-adjusted life years (QALYs) gained, cost per responder, and incremental cost per QALY gained. The effectiveness of each treatment was evaluated as a response to treatment. The base case assumption was that all patients in the model continued to receive botulinum toxin type A (BoNT-A) treatments at regular intervals regardless of treatment response status. Scenario analysis evaluated the impact of discontinuing BoNT-A for patients without a response to the first injection.
Results
The model found that aboBoNT-A resulted in greater quality-of-life and lower costs compared with onaBoNT-A for the management of spasticity and CD in all included indications. Across populations, cost savings ranged from £304 to £3,963 and QALYs gained ranged from 0.010 to 0.02 over a 1-year time horizon. Results were robust to scenario analyses and were driven by the impact of treatment response on health-related quality-of-life.
Conclusions
AboBoNT-A was associated with higher treatment response, improved quality-of-life, and reduced costs in spasticity and CD versus onaBoNT-A. These findings could help deliver more effective and efficient healthcare in the NHS.
PLAIN LANGUAGE SUMMARY
The objective of this study was to compare the costs and health outcomes associated with abobotulinumtoxinA (aboBoNT-A; Dysport) and onabotulinumtoxinA (onaBoNT-A; Botox) for treating children and adults with a variety of conditions related to limb spasticity and cervical dystonia. Therapies such as aboBoNT-A and onaBoNT-A have been shown to reduce spasticity, deformity, pain, and cervical dystonia symptoms. They can also improve function, movement, and self-care abilities. We estimated the treatment costs for patients with spasticity and patients with cervical dystonia receiving aboBoNT-A and onaBoNT-A in the UK. We also estimated other health-related costs that patients were expected to incur while receiving these treatments, as well as their quality of life.
For each indication (spasticity in the upper and lower limbs in adults and children, cervical dystonia in adults), research studies were identified to estimate the likelihood of patient response for aboBoNT-A and onaBoNT-A. Survey studies were assessed to understand use of health services and costs for patients who respond to therapy vs. those who do not. We estimated total costs over one year and expected quality of life for patients. Costs included the costs of aboBoNT-A and onaBoNT-A treatments, as well as other health services.
In all identified studies, the likelihood of response was higher for aboBoNT-A than for onaBoNT-A. This was associated with reduced need for other health services (and therefore lower costs), and better quality of life for patients receiving aboBoNT-A. In addition, the cost per year of aboBoNT-A treatment was lower than onaBoNT-A treatment for all indications. Therefore, treatment with aboBoNT-A was consistently associated with lower costs and better quality of life. This outcome is referred to as “economically dominant,” meaning that from a health economic perspective, aboBoNT-A would be preferred to onaBoNT-A for treating patients with spasticity or cervical dystonia.
Introduction
Spasticity involves muscle hypertonia which can be painful, result in complications, and decrease the ability to perform activities of daily living such as self-care and quality-of-lifeCitation1,Citation2. It is a complex condition to manage as it can cause muscle contracture and deformity, potentially limiting function and movementCitation2–4. Spasticity can affect the lower limbs, neck, and upper limbs. Cervical dystonia (CD), or spasmodic torticollis, is a painful condition that involves involuntary contraction of neck muscles which causes uncontrollable head tilting and twistingCitation5.
Managing spasticity and CD may include physical and occupational therapy, as well as pharmacotherapy, with a mainstay of treatment being injectable botulinum toxin type A (BoNT-A) therapiesCitation1,Citation5,Citation6. BoNT-A has been shown to reduce spasticity, deformity, pain, and CD symptoms, and improve function, movement, and self-care abilitiesCitation4,Citation6,Citation7. BoNT-A injections approved for use and routinely used for the treatment of spasticity and CD indications in the UK include abobotulinumtoxinA (aboBoNT-A; Dysport, Ipsen, Paris)Citation8 and onabotulinumtoxinA (onaBoNT-A; Botox, Allergan, Dublin)Citation9.
The mechanism of action of both of these BoNT-A treatments are similar, but there are differences in approved indications, clinical profile (maximum benefit, onset of action, and duration of effect)Citation10, and annual treatment costsCitation11. AboBoNT-A is manufactured by Ipsen and indicated for symptomatic treatment of focal spasticity in the upper limbs, lower limbs (affecting the ankle joint due to stroke or traumatic brain injury), and CD in adults; and the upper limbs and lower limbs (dynamic equinus foot deformity) in children (≥2 years old) with cerebral palsyCitation8. OnaBoNT-A is manufactured by Allergan Inc. and indicated for symptomatic treatment of focal spasticity in the upper limbs (wrist and/or hand), lower limbs (causing ankle and foot disability), and CD in adults; and the lower limbs (dynamic equinus foot deformity) in children (≥2 years old) with cerebral palsy in the UKCitation9.
There is a paucity of trials that directly compare BoNT-A treatments, and published comparisons mostly consist of indirect comparisons and network meta-analysesCitation11–16. When faced with multiple therapeutic alternatives, cost-effectiveness analysis can be used to determine the value of each choice, using a common unit of comparison or “effectiveness”. In spasticity, economic evaluations have been conducted using the quality-adjusted life year (QALY)Citation17 as well as the achievement of a response to treatmentCitation18. The objective of this manuscript is to describe the development and results of a model that evaluated the costs and benefits [from the UK National Health Service (NHS) and personal social services (PSS) perspective] associated with the use of aboBoNT-A and onaBoNT-A for treatment of adult upper limb spasticity (AUL), adult lower limb spasticity (ALL), CD in adults, and pediatric lower limb spasticity (PLL).
Methods
Model structure
The economic evaluation was conducted using a decision tree model with a 1-year time horizon, with treatment efficacy characterized by the percent of patients who were deemed responders to therapy. The model was comprised of two mutually exclusive health states defined by response vs. non-response to therapy; the rationale for this structure was based on the BoNT-A mechanism of action: injections reduce the muscle contraction associated with spasticity, enhancing the ability of a patient to achieve treatment goals with physical therapy, such as reduction of pain, improved range of motion, improved function, or othersCitation19,Citation20, and patients who achieve their treatment goals can be considered as responders to therapy.
A 1-year time horizon was selected given that there are limited data available to inform longer-term outcomes of BoNT-A therapy, specifically whether there would be any variation in ongoing persistence of treatment effect and comparative response rates by BoNT-A. In the absence of such data the focus of this analysis was on the short-term. In general, studies of comparative efficacy of BoNT-A tend to be short-term, evaluating a single injection of BoNT-A. To date, the ULIS-III study is the only longitudinal observational study to include multiple BoNT-A products, but its follow-up duration was limited to 2 yearsCitation21. A longer time horizon would serve to amplify observed first-year differences but would not be anticipated a priori to alter interpretation or relative trends and, given that mortality is of limited relevance as an endpoint for managing symptoms of spasticity or CD, and in particular for assessing difference by BoNT-A treatment, the 1-year horizon was anticipated to be sufficient in describing comparative economic outcomes.
A schematic model diagram is illustrated in . A variety of outcome measures exist in spasticity and CD. In this analysis, treatment response and non-response was defined by the Modified Ashworth Scale (MAS), Goal Attainment Scale (GAS), Physician’s Global Assessment (PGA), Disability Assessment Scale (DAS), or Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) criteria, depending on data availability. Descriptions of each measure and definitions of treatment response are reported in Supplementary Table S1.
Figure 1. Schematic model structure. Abbreviations: QALY, quality-adjusted life-year; QoL, quality of life.
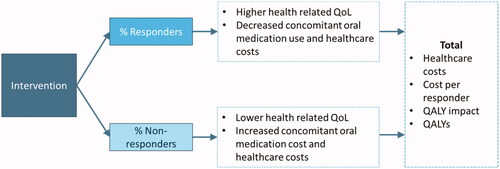
For analyses of upper and lower limb spasticity, the model defines response based on MAS or GAS, which are the two most common clinical measuresCitation22. Both measures provide an ordinal assessment of treatment response and evaluate different aspects of muscle tone and spasticity. The MAS measures resistance to passive movement by an investigator, and the GAS measures progress towards the patient’s goals for treatment. Each measure has different merits as potential outcomes within cost-effectiveness analyses. The MAS is the most widely used outcome measure in clinical trials and is well validated, but it does not necessarily correlate directly with health-related quality of life (HRQoL) as a theoretical construct. The GAS is a functional measure with a direct relationship to the lived patient experience and activities of daily living, but its subjective nature and variability in the assessment approach do not easily facilitate comparisons between patients or groups.
The severity of CD is characterized in the model using the TWSTRS scoreCitation23. The TWSTRS is commonly used in clinical trials and prospective studies as it allows a more extensive assessment of functional features of CD. While the complexity of the TWSTRS score may limit its use in routine clinical practice, available data from the clinical literature provides details of both response rates and the link between response rates and utility values, allowing for its use in characterizing outcomes within the model.
Population
The economic evaluation considered four separate patient populations with spasticity or CD: children (2–18 years old) with lower limb spasticity (PLL), and adults (19 years and older) with AUL, ALL, or CD. Pediatric upper limb spasticity was not included as this analysis has been conducted and publishedCitation17.
Comparators
Comparators included in this economic evaluation were aboBoNT-A and onaBoNT-A. Details of indications and doses (noting that both aboBoNT-A and onaBoNT-A doses are measured in Units (U), which are not interchangeable across products) for aboBoNT-A and onaBoNT-A are given in Supplementary Table S2.
Perspective
The analysis was conducted from the perspective of the UK National Health Service (NHS) and Personal Social Services (PSS) as per the NHS Reference CaseCitation24.
Discount rate
An annual discount rate was not applicable due to the 1-year time horizon of the model.
Treatment effectiveness
The effectiveness of each comparator was evaluated as a response to treatment. Treatment response rates are summarized in . The sample sizes reported in the table were used to calculate standard errors.
Table 1. Model inputs for each population.
The choice of outcome measure to define treatment response for each population in the model was based on the availability of comparative data. All studies comparing onaBoNT-A and aboBoNT-A within the respective populations of interest and reporting outcomes defined as the proportion responding to therapy were considered.
The optimal data source in this setting was a priori defined to be prospective observational studies, for which outcomes including response rate could be observed in a real-world setting. For indications where such studies were not available, network meta analyses (NMA) syntheses of randomised controlled trial (RCT) evidence were instead utilised. For AUL and CD, respectively, prospective observational studies were known to authors (ULIS-III in AUL, which included an outcome of GAS responseCitation21, and INTEREST IN CD1, which included an outcome of TWSTRS responseCitation25). A systematic literature review (SLR) was conducted to assess whether any additional observational studies for any of the indications of interest were available. The search strategy for the SLR is included in Supplementary Table S3. A total of 1,183 abstracts were identified and reviewed by two independent reviewers; besides the already-identified ULIS-III and INTEREST IN CD studies, no further eligible observational studies were identified. Thus, a synthesis of RCT evidence was utilized for ALL and PLL, with further details of specific sources listed below.
AUL
Treatment effectiveness in the AUL indication (characterized by GAS) was obtained from an international prospective observational study conducted to assess the impact of BoNT-A on upper limb spasticity in adults (ULIS III)Citation26.
ALL
Treatment efficacy in the ALL indication (characterized by MAS) was obtained from a systematic review and network meta-analysis in post-stroke ALLCitation27.
PLL
Treatment efficacy in the PLL indication (characterized by MAS, with GAS considered in sensitivity analysis) was obtained from an SLR and NMA of RCTs conducted in children (≤18 years) with spastic symptoms who were receiving BoNT-A therapyCitation13.
Alternative treatment efficacy estimates were based on individual studies identified in the SLRCitation13 and used in a recent cost-effectiveness analysis based on two RCTs that reported GAS outcomes in sufficient detail to allow a side-by-side comparison. One was a phase III clinical trial by Bjornson et al.Citation28 that evaluated the efficacy of onaBoNT-A, and the other was a study conducted by Delgado et al.Citation29 to evaluate the efficacy of aboBoNT-A.
CD
Treatment efficacy in the CD indication (characterized by TWSTRS) was obtained from the INTEREST IN CD1 prospective observational study of adult patients with idiopathic CD treated with aboBoNT-A and onaBoNT-A at 38 centers across Europe and Australia (NCT00833196)Citation25. Two definitions of response were considered within this study (both requiring ≥25% improvement on TWSTRS). The “alternative definition” further incorporated avoidance of Grade 3 AEs into the response definition and found a larger statistically significant benefit for aboBoNT-A vs. onaBoNT-A than the primary definition. In the analysis presented here, the lower response benefit associated with the primary definition was conservatively utilized.
Treatment persistence
The base case assumption was that all patients in the model continued to receive BoNT-A treatments at regular intervals regardless of treatment response status. A scenario analysis evaluated the impact of discontinuing BoNT-A for patients without a response to the first injection.
Adverse events
Adverse events (AEs) were not included in the model as low incidences have been reported for both aboBoNT-A and onaBoNT-A and, in comparative analysis, no differences are anticipated between alternative BoNT-A productsCitation28–30. However, oral anti-spasticity therapies given as alternatives to BoNT-As tend to be associated with greater toxicity, and the adverse event implications of increased concomitant use of these therapies in non-responders were explored.
Mortality
Mortality was not considered in the model due to the short time horizon and the lack of data to inform estimates of differences in mortality between BoNT-A.
Resource use and costs
The model included costs and resources associated with treatment acquisition and administration, healthcare appointments, and concomitant oral medications. Resources and unit costs were collected from a UK NHS and social services perspective and reported in 2019 British pounds. The sources used to obtain each estimate are discussed in detail below.
BoNT-a treatment
Frequency and administration
For the economic evaluation, comparative observational data was the preferred source of injection intervals, whenever available. The ULIS-III study followed AUL patients for up to 2 years and provided mean injection intervals for aboBoNT-A and onaBoNT-ACitation18. Average dose intervals were calculated across all cycles and weighted by the number of patients injected in each cycle (). For all other indications, real-world data on dose intervals was not available. In the absence of comparative data, it was assumed that 12-week injection intervals would be equivalent across BoNT-As.
In the UK, BoNT-A must be administered in clinic by a health care provider; the total cost of each dose includes the cost of an outpatient neurology follow-up attendance obtained from NHS National Tariff: £116 for adults (relevant to both spasticity and CD) and £189 for childrenCitation31.
Doses and list price
Doses used in the economic evaluation were based on doses reported in the studies used to inform treatment response rates. For AUL, these were the average and standard errors observed in the ULIS-III studyCitation18, while for CD the median dose was published and this was used as a proxy for the mean, assuming that the standard deviation (SD) was equivalent to 20% of the meanCitation25. For ALL and PLL, in the absence of real-world data, the doses used in pivotal trials were assumed. In probabilistic analysis, these doses were varied by assuming a common ratio of SD to mean as was observed for AUL.
The modeled doses for each population are presented in . Children received weight-based doses per affected limb according to the characteristics of a hypothetical patient population based on the PLL trial populationCitation32.
AboBoNT-A and onaBoNT-A are available in different vial sizes at different costs (). The public list prices for each available vial size were obtained from the British National FormularyCitation33. The cost of treatment was calculated as the minimum number of vials required to achieve the required dose for each patient. The base case model included the cost of treatment wastage; the impact of excluding treatment wastage was explored in scenario analysis.
Table 2. List price for aboBoNT-A and onaBoNT-A.
Health services
A survey was administered to a geographically representative sample of UK clinicians with experience treating AUL (n = 11), PLL (n = 12), and CD (n = 12) to inform healthcare resource use and cost estimates for this cost-utility analysisCitation22.
The resulting healthcare resource use data used in the model were the median and range of reported averages, for BoNT-A responders and non-responders (Supplementary Tables S4–S6).
In the economic evaluation, the average annual cost per patient associated with each resource item identified in the resource use survey was calculated using unit costs obtained from the NHS Reference Costs 2017/18Citation34 and inflated to 2018/19 costs using the Hospital & Community Health Services Pay & Prices IndexCitation35.
The survey did not include ALL. For the economic evaluation, it was assumed that healthcare costs for ALL would be the same as those for AUL.
In the economic model, uncertainty was incorporated into these results as follows:
Costs for each category within each indication were characterized by the reported mean and standard error, assuming that the reported ranges represented 95% confidence intervals and hence 2 × 1.96 standard errors.
For responders, these means and SDs were used directly to populate beta distributions via the method of moments and generate probabilistic values.
For non-responders, the process was repeated but for the incremental difference with responders rather than the absolute cost; structuring things this way allows for the correlation between responder and non-responder estimates to be retained across probabilistic iterations (e.g. while it is plausible that the difference between the two groups will vary probabilistically, it is not expected that non-responders would have lower costs than responders, which is a phenomenon that could occur if the costs were varied independently).
Oral medications
The survey of UK physicians conducted to inform this economic evaluation found that oral medication use was almost 50% less among BoNT-A treatment responders compared with non-responders for spasticity conditions and CDCitation22. Oral medications prescribed by two or more physician survey respondents for the treatment of patients with AUL, PLL, and CD are listed in Supplementary Tables S7–S9. Recommended average daily doses for each oral medication were obtained from the physician survey, multiplied by the unit cost of each medication obtained from the British National Formulary 2017 and inflated to 2018/19 costs using the Hospital & Community Health Services Pay & Prices IndexCitation35. Medians and ranges represented the distribution of responses across physician respondents.
Quality-of-life
Utility weights for treatment responders and non-responders were obtained from the published literature, as described below, by indication.
AUL
Utility values for the AUL indication were obtained from a study designed to evaluate the relationship between disability, HRQoL, and caregiver burden in patients with upper limb post-stroke spasticityCitation36, in a post-hoc analysis of a large, prospective, multicenter, open-label study of post-stroke spasticity in the upper extremity with a 12-month follow-upCitation37.
For the “responder” utility in the model, an average of utility values associated with “No disability” and “mild disability” states from Doan et al.Citation36 was used. Non-responder utility was the average utility associated with “moderate disability” and “severe disability”.
ALL
Utility values for the ALL indication were informed by an aboBoNT-A post-hoc analysis of an RCT and an open-label extension phase that collected walking speed data and patient responses to the EQ-5D-5L at weeks 1, 4, 12, 16, 20, and 24 following the initial treatmentCitation38. A random effects panel data analysis was completed to estimate the utility decrement associated with patients moving from one walking speed derived health state to another. Utility values for patients who were “household walkers” (0.5400), “limited community ambulators” (0.4918), and “community ambulators” (0.4049) were reported. We assumed that a “response” was equivalent to patients moving from one walking speed category to the next one (which can be conservative, depending on the magnitude of improvement). Values for household walkers and limited community ambulators were used to inform utilities for responders and non-responders, respectively.
PLL
Base case utilities for responders and non-responders with PLL were assumed to be the same as for ALLCitation38. For testing in scenario analyses, health state utilities were consistent with those used in a prior modelCitation39, in which Pediatric Quality of Life Inventory (PEDql) results were transformed to EQ-5D via a published algorithmCitation40. The PEDql data were obtained from clinical trial data for aboBoNT-A vs. placebo, pooled across treatments, and stratified by GAS responseCitation29. A mixed-effects regression model was fit to obtain utilities associated with GAS response states. The resulting utility values were 0.82 (standard error 0.0091) associated with response and 0.78 (standard error 0.0117) associated with non-response.
CD
Utility values for the CD indication were obtained from a prospective open-label study of cranial and cervical dystonia treated long-term with BoNT-A therapyCitation41. The EQ-5D utility index at baseline and first BoNT-A follow-up were assumed to map to utility values for treatment non-responders and responders, respectively (i.e. baseline to non-responders, and post-BoNT-A injection to responders).
Adverse events of oral medications
In addition to the cost savings associated with decreased use of oral medications, the probability of adverse events associated with the use of oral medications may also be reduced. In this analysis, adverse events were included for supplemental oral medications and not the primary medications under investigation (BoNT-As). This decision was made because BoNT-A use is associated with low rates of adverse events (regardless of type)Citation28–30, whereas oral medication use is associated with key adverse eventsCitation42. Moreover, adverse events associated with oral medication use are believed to vary depending on the type of BoNT-A used concomitantly, as each type of BoNT-A has a different efficacy and thus a differential need for supplemental oral medication useCitation22. Adverse events common to oral spasticity medications include fatigue, lethargy, dizziness, weakness, and constipation. The Cost-Effectiveness Analysis (CEA) RegistryCitation43 was searched for disutility values related to adverse events associated with oral anti-spasticity medications.
The values judged to be most relevant to the current model (Supplementary Table S10) were principally based on two studies:
Matza et al.Citation44 used the time trade-off method with 200 people from the general population and 200 people with migraines in the UK, to estimate utilities associated with specific treatment-related adverse events, including a number of events identified as being associated with oral medications used in spasticity and CD.
Sullivan et al.Citation45 reported a UK-based catalogue of EQ-5D index scores for a wide variety of chronic conditions that can be used to estimate QALYs in cost-effectiveness analyses in the UK.
These sources were not specific to spasticity or CD populations (for which published adverse event disutilities were not identified), but as the estimates are intended to be associated with experiencing the adverse events rather than specific to spasticity or CD, the values identified were deemed justifiable for use in spasticity and CD patients.
In probabilistic analysis, an analogous approach was taken to modeling rates of oral medication use and corresponding adverse events, as was described for overall health care resource use and the difference between responders and non-responders, specifically:
Point estimates were calculated for responders and non-responders, respectively, based on the per cent oral medication use by response status and the per cent AE rates by oral medication use.
Upper and lower bounds for responders and non-responders, respectively, were calculated based on the range of reported values for per cent oral medication use in the UK physician survey; these ranges were assumed to represent 95% confidence intervals, and the corresponding standard errors were calculated.
The method of moments was used to generate probabilistic estimates of per cent AE incidence for responders and non-responders, respectively.
This was multiplied by the responder and non-responder rates for each BoNT-A therapy to get an overall distribution by therapy.
Uncertainty
A 5,000-iteration probabilistic analysis was run to characterize variability in outcomes based on stochastic uncertainty in input variables.
Scenario analyses were conducted by changing relevant model parameters to reflect alternative assumptions regarding the following:
Adverse event disutilities (included, excluded);
Treatment wastage included vs. excluded;
Number of injections received by non-responders (one only vs. multiple injections); and
Data source for healthcare costs.
The healthcare data cost source analysis was relevant for adult indications only. An alternative source was used to inform healthcare costs and the difference between responders vs. non-responders. In a study by Ward et al.Citation46 comparing resource utilization and cost for patients receiving BoNT-A therapy vs. oral therapy in post-stroke spasticity, expert panel survey results suggested a larger incremental difference than the survey results. Current UK unit costs were applied to the resource use estimates reported in this study, and were assessed as alternative values for ALL and AUL.
Results
Base case
AboBoNT-A was found to result in greater quality-of-life and lower costs compared with onaBoNT-A for the management of the condition in all included indications. Across the four populations, estimated cost savings were £304 for AUL, £394 for ALL, £3,963 for PLL, and £616 for CD. The magnitude of cost savings varied across indications, reflecting a variety of differences specific to each indication (e.g. average cost per dose, length of dosing interval and potential treatment variation across dosing intervals, relative distribution of costs between BoNT-As vs. other background medical costs) (). Incremental QALYs associated with aboBoNT-A were 0.02 for AUL, 0.01 for ALL, 0.02 for PLL, and 0.02 for CD over the 1-year time horizon. Results were robust across several scenario analyses and were driven by the impact of treatment response on HRQoL. Full results across indications are provided in . Based on estimated results with respect to incremental cost savings and health outcome benefits, aboBoNT-A was found to be an economically dominant therapy relative to onaBoNT-A across all included populations.
Table 3. Base case deterministic results.
Scenario analyses
The impact of three assumptions was tested in scenario analysis: exclusion of treatment wastage, no follow-up injections for non-responders to the initial injection, and exclusion of disutility of AEs for oral medications. Across all populations, assuming no follow-up injections for non-responders in ALL resulted in an increased incremental cost for aboBoNT-A compared with onaBoNT-A. Across all other scenarios, the conclusions of the model were unchanged relative to the base case. The results of the scenario analyses are presented in .
Table 4. Scenario analysis results.
Probabilistic sensitivity analysis
For each indication, a 5,000-iteration probabilistic sensitivity analysis (PSA) run was conducted. Across indications, results were robust in the PSA, with iterations centred around the base-case value (Supplementary Figure S1). For a willingness-to-pay threshold of £20,000, aboBoNT-A was found to be cost-effective, as characterized by net benefit in 100% of iterations for all indications. AboBoNT-A was dominant (less expensive and more effective in 100% of iterations for AUL, 99% of iterations for ALL, 97% of iterations for PLL, and 97% of iterations for CD).
Discussion
This study synthesized a variety of data sources describing clinical effectiveness, healthcare resource use, and costs associated with the treatment of spasticity and CD in the UK. Based on clinical evidence supporting trends to higher treatment response rates for aboBoNT-A, and a lower acquisition cost for aboBoNT-A relative to onaBoNT-A, economic analysis suggests that aboBoNT-A is likely to be economically dominant for the treatment of spasticity and CD in the UK. Across four populations in the base case, cost savings ranged from £302 to £1,085 and QALYs gained ranged from 0.01–0.03 over a 1-year time horizon. Based on dosing schedules, differences in drug costs were more pronounced for the PLL indication vs. adult indications considered here. Although the model only considered a 1-year time horizon, for individuals who continue to respond over time, these estimates would compound over a longer time horizon. Results were robust to several scenario analyses and were driven by the impact of treatment response on HRQoL. In pediatric indications, results were most sensitive to inclusion vs. exclusion of medication wastage, as for smaller bodyweights, excess product when rounding up to the nearest vial size can be an important contributor to treatment costs.
For adult spasticity indications, results were most sensitive to use of the Ward et al.Citation46 healthcare costs vs. the UK resource use survey. The Ward et al.Citation46 study reported costs that were lower overall, and also more sensitive to response rates, resulting in an increased relative differential between aboBoNT-A and onaBoNT-A results given the higher response rates. Treatment response was found to have a limited impact on resource use, attributable to the similar treatment for responders and non-responders described by UK clinicians, who stated that medical visits often occur at fixed schedules, and improved ability to respond to physical therapy with BoNT-A response may encourage additional physiotherapy sessionsCitation22. At the same time, using the outcomes of the UK survey in the model was conservative in the context of other resource use studies, as Ward et al.Citation46 reported a 12% decrease in healthcare costs for patients receiving BoNT-As vs. oral therapies, compared to <1% difference between responders vs. non-responders to BoNT-As for AUL and PLL indications in the UK resource use study. The scenario analysis conducted to account for this uncertainty resulted in higher savings with aboBoNT-A when the alternative resource use source was used. Resource utilization estimates were based on survey results and published literature from a geographically representative sampleCitation30. For CD, results were similar across all scenario analyses conducted (no treatment wastage, non-responders receive one injection only, AE disutilities excluded).
To our knowledge, this is the first modeling study to compare aboBoNT-A and onaBoNT-A across all indicated populations considered here (ALL, AUL, PLL, CD) for the management of spasticity and CD, but our findings are consistent with those of other economic studies in these indications. In real-world longitudinal studies in adults and children with limb spasticity and adults with CD, aboBoNT-A has been reported to be similar or more effective and less costly than onaBoNT-ACitation47–51. In a Canadian cost-effectiveness modeling study of children with lower leg spasticity, aboBoNT-A improved quality-of-life and reduced costs compared with onaBoNT-ACitation39. In a Russian Markov cohort model, aboBoNT-A was found to be the most cost-effective option compared to either onaBoNT-A or standard of care therapies aloneCitation52. In the UK, BoNT-A was found to be cost-effective compared with oral therapy for the treatment of post-stroke spasticity in adultsCitation46. In the independent administrative health care claims analysis, the calculated annual treatment cost per treated patient with CD or limb spasticity was lowest on aboNoNTA-A compared to other products assessed, including onaBoNT-A, which was most expensiveCitation49.
While clinical studies could only be included if they reported sufficient data, specifically, response rates by BoNT-A therapy on an appropriate scale, other publications describing observational studies and/or direct comparisons of BoNT-As were identified. Where appropriate data points were not reported for incorporation into this analysis, results were consistent in trending toward a numeric benefit for aboBoNT-A relative to onaBoNT-A. For CD, an observational study published by the INTEREST IN CD study group was utilized, which included a single injection only; comparative response rates have been used previously in economic analysesCitation53. A second observational study by the INTEREST IN CD study group (INTEREST IN CD2)Citation54 was conducted over multiple injections, although the primary outcomes and published results for this study are based on results combined across BoNT-As and thus did not provide data to inform comparative response rates.
The inputs into this analysis draw from a range of study designs (observational real-world evidence, systematic literature review, and network meta-analyses) across indications, all of which found numeric and/or statistical benefits for aboBoNT-A vs. onaBoNT-ACitation13,Citation26,Citation27,Citation53. In addition to the studies directly incorporated into the analysis, further evidence of effectiveness (while not directly providing response rates as needed for the current analysis) supports this trendCitation55,Citation56. Thus, while these studies could not be directly incorporated due to the nature of efficacy endpoints reported, the conclusions drawn were similar.
The model has several strengths. First, the underlying sources of effectiveness inputs were based on SLRs (for ALL and PLL), which are comprehensive and based on good research practices, or large real-life studies (for AUL and CD) reporting effectiveness outcomes for both aboBoNT-A and onaBoNT-A. The outcome of a binary response indicator is based on scales deemed appropriate for each respective indication (based on the clinical features of the scales and availability of published outcomes). Whenever input sources were limited or heterogenous, a conservative approach was taken. For instance, a 1-year time horizon, although limited in the context of a chronic condition, was opted for in order to limit extrapolations, and accounting for previous cost-effectiveness modeling yields more conservative outcomes for aboBoNT-A vs. onaBoNT-A than over a longer time horizonCitation39. The outcomes were consistent across the indications and based on respective input sources. Probabilistic sensitivity analyses and one-way scenario analyses demonstrated the robustness of base case findings.
A challenge of generalizing the results of this model to the real world is that there is substantial dose variation in BoNT-A treatments in clinical practice and debate regarding optimal dose conversion across BoNT-AsCitation57. Dosing assumptions in this model were based on the underlying input sources on efficacy/effectiveness (ULIS-III) and INTEREST IN CD1 in AUL and CD, respectively, as real-world studies reporting data for both aboBoNT-A and onaBoNT-A. For the PLL and ALL indications, real-world studies were not available, and doses used in pivotal trials were assumed. In the absence of variability data for these indications, it was assumed that the ratio of variability to mean observed in the ULIS-III study would apply. As such, some variability was incorporated but data were not available for indication-specific validation. A broader evidence review in CD has found notable variability in dosing according to patient needs, individual practices and other considerationsCitation58. Additional limitations include the fact that a simplified binary response vs. non-response structure was applied, and the time horizon was limited to 1 year, while the indications under consideration are chronic in nature. The 1-year time horizon was selected due to the limited availability of long-term data, to avoid the need for strong assumptions about extrapolation. A Canadian cost-effectiveness analysis that considered scenarios with 2-year and 12-year time horizonsCitation22, respectively, found cost-effectiveness results to more strongly favor aboBoNT-A with a longer time horizon, suggesting that the 1-year horizon used here was likely conservative to aboBoNT-A. In addition, limited data were available to link utility and response, which necessitated some combination of multiple scales for measuring response and subsequently linking it to HRQoL. This analysis focused on health outcomes of QALYs, as is typical in economic modeling analyses, as well as binary response indicators. The latter was the core metric for treatment effectiveness in the model, and potentially a more clinically meaningful and intuitive complement to the economics-focused QALY metric. While QALYs are a useful metric for comparing a variety of disparate health states across multiple diseases, utility measures may not fully capture all nuances of spasticity and CD, or the results of BoNT-A treatment response. Similarly, while response serves as a global measure of achieving treatment benefits, it does not describe the specific impact on HRQoL to spasticity and CD patients in detail. A key challenge in the analysis was identifying robust data across BoNT-A therapies across indications for quantitative inclusion in the model, and QALYs and responder status were deemed the most relevant and well-reported outcomes. As future data emerge on additional scales for measuring treatment response, and/or longer-term outcomes (e.g. mortality in post-stroke patientsCitation59), expanded economic analysis to incorporate such data would be warranted.
Conclusion
The presented cost-effectiveness model is based on evidence regarding treatment effectiveness, health-related quality-of-life, drug acquisition costs, and health services costs, across base case, sensitivity, and scenario analyses. Overall, the outcomes of the model suggest that aboBoNT-A can be associated with cost-savings and increased QALYs compared to onaBoNT-A (i.e. economically dominant) across all considered indications (ALL, AUL, PLL, CD) in spasticity and CD. Adoption of aboBoNT-A in the UK NHS has the potential to improve the quality-of-life for patients while reducing health care expenditure.
Transparency
Declaration of funding
This study was sponsored by Ipsen.
Declaration of financial/other interests
ND is an employee of Ipsen, the study sponsor, and this work was carried out in the course of her employment. KJ is an employee of Broadstreet HEOR who was contracted by Ipsen to support this work. JW was an employee of Ipsen, the study sponsor, at the time this work was conducted, and this work was carried out in the course of his employment.
A reviewer on this manuscript has disclosed that they received honoraria and fellowship support from Ipsen. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Prior publication
Some of the findings reported here will be presented in poster form at the International Neurotoxins Association’s 2022 TOXINS congress to be held 27–30 July 2022, in New Orleans, LA.
Supplemental Material
Download MS Word (76.9 KB)Supplemental Material
Download MS Word (380.4 KB)Acknowledgements
The authors thank the study participants, their families and caregivers, and the study investigators for generating clinical data which could be used in this analysis.
References
- Mugglestone M, Eunson P, Murphy M. Spasticity in children and young people with non-progressive brain disorders: summary of NICE guidance. BMJ. 2012;345:e4845.
- Olver J, Esquenazi A, Fung VS, et al. Botulinum toxin assessment, intervention and aftercare for lower limb disorders of movement and muscle tone in adults: international consensus statement. Eur J Neurol. 2010;17(Suppl 2):57–73.
- Rawicki B, Sheean G, Fung VS, et al. Botulinum toxin assessment, intervention and aftercare for paediatric and adult niche indications including pain: international consensus statement. Eur J Neurol. 2010;17(Suppl 2):122–134.
- Sheean G, Lannin NA, Turner-Stokes L, et al. Botulinum toxin assessment, intervention and after-care for upper limb hypertonicity in adults: international consensus statement. Eur J Neurol. 2010;17(Suppl 2):74–93.
- Mayo Clinic. Cervical dystonia. 2021. [cited 2021 Jun 22]. https://www.mayoclinic.org/diseases-conditions/cervical-dystonia/symptoms-causes/syc-20354123?lang=en.
- Yelnik AP, Simon O, Bensmail D, et al. Drug treatments for spasticity. Ann Phys Rehabil Med. 2009;52(10):746–756.
- Brin MF, Comella CL, Jankovic J, et al. Long-term treatment with botulinum toxin type a in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord. 2008;23(10):1353–1360.
- Dysport powder for solution for injection. 2020. [cited 2020 Oct 12]. https://www.medicines.org.uk/emc/product/7261/smpc.
- Botox powder for solution for injection. 2020. [cited 2020 Oct 12]. https://www.medicines.org.uk/emc/product/859/smpc.
- Brockmann K, Schweitzer K, Beck G, et al. Comparison of different preparations of botulinum toxin a in the treatment of cervical dystonia. Neurol Asia. 2012;17(2):115–119.
- Jacinto J, Ashford S, Fheodoroff K, et al. Real-life data on the time to retreatment with botulinum toxin a in upper limb spasticity management. World Congress for NeuroRehabilitation, 2020; October 7–11 Virtual Congress.
- Danchenko N, Delgado D, Haeussler K, et al. PND10 indirect treatment comparison of botulinum toxins a for the treatment of pediatric upper limb spasticity. Value Health. 2020;23(Suppl 1):S260.
- Guyot P, Kalyvas C, Mamane C, et al. Botulinum toxins type A (BoNT-A) in the management of lower limb spasticity in children: a systematic literature review and bayesian network Meta-analysis. J Child Neurol. 2019;34(7):371–381.
- Andringa A, van de Port I, van Wegen E, et al. Effectiveness of botulinum toxin treatment for upper limb spasticity poststroke over different ICF domains: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2019;100(9):1703–1725.
- Baker J, Pereira G. The efficacy of botulinum toxin a for spasticity and pain in adults: a systematic review and meta-analysis using the grades of recommendation, assessment, development and evaluation approach. Clin Rehabil. 2013;27(12):1084–1096.
- Fu X, Wang Y, Wang C, et al. A mixed treatment comparison on efficacy and safety of treatments for spasticity caused by multiple sclerosis: a systematic review and network Meta-analysis. Clin Rehabil. 2018;32(6):713–721.
- Danchenko N, Johnston KM, Haeussler K, et al. Comparative efficacy, safety, and cost-effectiveness of abobotulinumtoxinA and onabotulinumtoxinA in children with upper limb spasticity: a systematic literature review, indirect treatment comparison, and economic evaluation. J Med Econ. 2021;24(1):949–961.
- Turner-Stokes L, Ashforda S, Jacintod J, et al. Economic outcomes in real-world use of botulinum toxin-A products for adult patients with upper limb spasticity: a UK perspective. Paper presented at: TOXINS 2021; January 16–17, 2021.
- Schwabe AL. Botulinum roxin in the treatment of pediatric upper limb spasticity. Semin Plast Surg. 2016;30(1):24–28.
- Turner-Stokes L, Ashford S, Fheodoroff K, et al. The GAS-eous tool: goal attainment scaling - evaluation of outcome for upper-limb spasticity. 2013. https://www.kcl.ac.uk/cicelysaunders/resources/tools/gas-eous
- Turner-Stokes L, Jacinto J, Fheodoroff K, et al. Longitudinal goal attainment with integrated upper limb spasticity management including repeat injections of botulinum toxin A: findings from the prospective, observational upper limb international spasticity (ULIS-III) cohort study. J Rehabil Med. 2021;53(2):jrm00157.
- Johnston KM, Danchenko N, Lundkvist J. PND34 resource use related to cervical dystonia, pediatric lower limb spasticity and adult upper limb spasticity in the UK: a physician questionnaire. Value Health. 2020;23(Suppl 1):S265.
- Jost WH, Hefter H, Stenner A, et al. Rating scales for cervical dystonia: a critical evaluation of tools for outcome assessment of botulinum toxin therapy. J Neural Transm. 2013;120(3):487–496.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013. https://www.nice.org.uk/process/pmg9/chapter/the-reference-case.
- Misra VP, Ehler E, Zakine B, et al. Factors influencing response to botulinum toxin type a in patients with idiopathic cervical dystonia: results from an international observational study. BMJ Open. 2012;2(3):e000881.
- Jacinto J, Ashford S, Fheodoroff K, et al. Factors associated with favorable response in real-world use of botulinum toxin a products for adult patients with upper limb spasticity. TOXINS 2022; July 27–30, 2022, New Orleans, LA.
- Schnitzler A, Danchenko N, Lundkvist J, et al. PND13 network meta-analysis for botulinum toxin a for the treatment of adult lower limb spasticity (ALLS) in post-stroke population. Value Health. 2020;23:S260.
- Bjornson K, Hays R, Graubert C, et al. Botulinum toxin for spasticity in children with cerebral palsy: a comprehensive evaluation. Pediatrics. 2007;120(1):49–58.
- Delgado MR, Tilton A, Russman B, et al. AbobotulinumtoxinA for equinus foot deformity in cerebral palsy: a randomized controlled trial. Pediatrics. 2016;137(2):1–9.
- Fehlings D, Narayanan U, Andersen J, et al. Botulinum toxin-A use in paediatric hypertonia: Canadian practice patterns. Can J Neurol Sci. 2012;39(4):508–515.
- NHS England. National Tariff 2019/20: Non-mandatory prices. 2019. https://www.england.nhs.uk/publication/national-tariff-payment-system-documents-annexes-and-supporting-documents/
- Gracies J-M, Francisco G, Jech R, et al. The effect of guided self-rehabilitation contracts combined with simultaneous injections of abobotulinumtoxinA into upper and lower limbs on voluntary movements in adults with spastic hemiparesis. IPMDS International Congress; Sept 24, 2019, Nice, France.
- British National Formulary [Internet]. British Medical Association and Royal Pharmaceutical Society of Great Britain. 2020. [cited 2020 Jan 10]. https://bnf.nice.org.uk/.
- NHS Improvement. 2017/18 Reference costs: national schedule of reference costs. 2020.
- Curtis L, Burns A. Unit costs of health and social care 2019. Canterbury (UK): University of Kent; 2019.
- Doan QV, Brashear A, Gillard PJ, et al. Relationship between disability and health-related quality of life and caregiver burden in patients with upper limb poststroke spasticity. Pm R. 2012;4(1):4–10.
- Elovic EP, Brashear A, Kaelin D, et al. Repeated treatments with botulinum toxin type a produce sustained decreases in the limitations associated with focal upper-limb poststroke spasticity for caregivers and patients. Arch Phys Med Rehabil. 2008;89(5):799–806.
- Moore P. Cost-effectiveness analysis on dysport: a focus on early intervention to improve barefoot walking speed. Value Health. 2021;24:S160.
- Deighton A, Johnston K, Dabirvaziri P, et al. Acute migraine medication and medication overuse headache: a systematic literature review. Paper presented at: American Headache Society Annual Scientific Meeting 2020, Virtual.
- Khan KA, Petrou S, Rivero-Arias O, et al. Mapping EQ-5D utility scores from the PedsQL generic core scales. Pharmacoeconomics. 2014;32(7):693–706.
- Hilker R, Schischniaschvili M, Ghaemi M, et al. Health related quality of life is improved by botulinum neurotoxin type a in long term treated patients with focal dystonia. J Neurol Neurosurg Psychiatry. 2001;71(2):193–199.
- Chang E, Ghosh N, Yanni D, et al. A review of spasticity treatments: pharmacological and interventional approaches. Crit Rev Phys Rehabil Med. 2013;25(1–2):11–22.
- CEA Registry. 2020. [cited 2020 Sep 8]. http://healtheconomicsdev.tuftsmedicalcenter.org/cear2/search/search.aspx.
- Matza LS, Deger KA, Vo P, et al. Health state utilities associated with attributes of migraine preventive treatments based on patient and general population preferences. Qual Life Res. 2019;28(9):2359–2372.
- Sullivan PW, Slejko JF, Mark J, et al. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31(6):800–804.
- Ward A, Roberts G, Warner J, et al. Cost-effectiveness of botulinum toxin type a in the treatment of post-stroke spasticity. J Rehabil Med. 2005;37(4):252–257.
- Al-Mazraawy B. Potential cost savings were identified across the Yale Health System when primarily using DysportVR vs. BotoxVR. 53rd Annual ASHP Midyear Clinical Meeting; 2018, Anaheim, CA.
- Dursun N, Akarsu M, Gokbel T, et al. Switching from onabotulinumtoxin-A to abobotulinumtoxin-A in children with cerebral palsy treated for spasticity: a retrospective safety and efficacy evaluation. J Rehabil Med. 2019;51(5):390–394.
- Eckwright D, Burke J, Gleason P. Real-world botulinum toxin (BT) utilization and treatment cost for cervical dystonia (CD) and limb spasticity (LS). AMCP Managed Care & Specialty Pharmacy Nexus Meeting; October 29–November 1, 2019, National Harbour, MD.
- Tapias G, Garcia-Romero M, Crespo C, et al. Cost-minimization analysis in the treatment of spasticity in children with cerebral palsy with botulinum toxin type A: an observational, longitudinal, retrospective study. Farm Hosp. 2016;40(5):412–426.
- Tedroff K, Befrits G, Tedroff CJ, et al. To switch from botox to dysport in children with CP, a real world, dose conversion, costeffectiveness study. Eur J Paediatr Neurol. 2018;22(3):412–418.
- Ugrekhelidze DT, Kulikov AY. Pharmacoeconomic analysis of different types of treatment for spastic forms of cerebral palsy. Pharmacoeconom T&P. 2015;3(3):71–78.
- Misra VP, Danchenko N, Maisonobe P, et al. Economic evaluation of abobotulinumtoxinA vs onabotulinumtoxinA in real-life clinical management of cervical dystonia. J Clin Mov Disord. 2020;7(2):2–10.
- Misra VP, Colosimo C, Charles D, et al. INTEREST iN CD2, a global patient-centred study of long-term cervical dystonia treatment with botulinum toxin. J Neurol. 2018;265(2):402–409.
- Ranoux D, Gury C, Fondarai J, et al. Respective potencies of Botox and Dysport: a double blind, randomised, crossover study in cervical dystonia. J Neurol Neurosurg Psychiatry. 2022;72(4):459–462.
- Han Y, Stevens AL, Dashtipour K, et al. A mixed treatment comparison to compare the efficacy and safety of botulinum toxin treatments for cervical dystonia. J Neurol. 2016;263(4):772–780.
- Scaglione F. Conversion ratio between botox®, dysport®, and xeomin® in clinical practice. Toxins. 2016;8(3):65.
- Evidente VG, Pappert EJ. Botulinum toxin therapy for cervical dystonia: the science of dosing. Tremor Other Hyperkinet Mov. 2014;4(0):273.
- Danchenko N, Whalen J, Burchakova M, et al. POSA121 modelling long-term outcomes and mortality risk for post-stroke spasticity patients on abobotulinumtoxina treatment and rehabilitation therapy. Value in Health. 2022;25(1):S57.
