Abstract
Introduction
A direct transfer to angiosuite (DTAS) protocol has shown to be effective and safe by shortening in-hospital workflows and encouraging long-term outcome benefits. To implement DTAS at a new facility, a large organizational effort is necessary. We performed a cost-utility analysis and budget impact analysis (BIA) of the operation of a new angiosuite, primarily dedicated to stroke patients, that allows facilities to approximate the cost implications of utilizing a DTAS pathway.
Methods
Sixty-one patients who underwent endovascular treatment (EVT) following DTAS were matched for baseline variables to 117 patients who underwent a conventional imaging protocol at a hospital in Catalonia, Spain. An economic model, based on actual data from these patients, was developed to assess the short- and long-term clinical and economic implications of DTAS. In the BIA, the DTAS scenario was gradually implemented for 20% of patients each year until reaching a plateau at 80% of patients in the DTAS pathway. Initial investment and additional organizational costs, €4 million, were taken into consideration to compare the budget impact of the DTAS scenario with no organizational changes over five years.
Results
DTAS was associated with better patient functional independence rates (mRS 0–2: 50.9% vs. 41.0%) and a quality-adjusted life-years gain of 0.82 per patient. Despite the additional initial investment, DTAS development was associated with an estimated 10.2% reduction (€14.7 million) of the total costs (€144.5 million). Cost savings were mainly due to long-term associated costs related to patient disability (€13.2 million).
Limitations
The study relies on data obtained from a single-center, and therefore it may be difficult to generalize the findings
Conclusions
Our economic model predicts that the implementation of a DTAS program is cost-effective compared with no organizational changes. Our model also predicts better clinical outcomes for patients in terms of functional independence and quality-adjusted life years.
Introduction
Globally, stroke is a public health concern as a prominent cause of long-term disability and early mortality. Specifically in Spain, the estimated annual incidence rate of stroke is 141 cases per 100,00 inhabitantsCitation1. Due to the ever-growing elderly population and prevalence of risk factors for stroke, the rate of incidence will, most likely, continue to increase over time. However, there are steps healthcare providers can take to both improve patient outcomes and reduce costs incurred by stroke patients. In this study, we will discuss one such economic option for acute ischemic stroke due to large vessel occlusion (LVO).
The standard of care for acute ischemic stroke due to LVO was established via landmark clinical trials demonstrating the safety and efficacy of endovascular treatment (EVT)Citation2–4. These studies identified that the time from symptom onset to reperfusion is one of the leading predictors of positive clinical outcomes in patients who undergo EVTCitation5,Citation6. Every 30-min delay in achieving reperfusion reduces the probability of functional independence by 10%–15%Citation7.
An institutional Direct Transfer to Angiosuite (DTAS) protocol aims to shorten the duration of the in-hospital workflowCitation8–14. DTAS is based on ruling out the presence of an intracranial hemorrhage or a large established infarct core by utilizing flat-panel computed tomography (CT) early on during the patient evaluationCitation15–17. Through each DTAS institutional protocol that includes minor differential features, DTAS has consistently been shown to be safe and effective at improving in-hospital delays and patient clinical outcomes. In some comprehensive stroke centers, DTAS has reduced door-to-groin-puncture time to 16 min, with reductions ranging from 22 to 59 min, without increasing safety concerns, and has shown encouraging long-term, beneficial patient outcomesCitation9,Citation13,Citation18.
However, implementing a DTAS protocol into daily clinical practice requires the immediate availability of the interventional team and the angiography suite. Organizational efforts and economic investments may be required to ensure that DTAS can be performed when necessary.
This study compares the cost-utility of the DTAS pathway to the traditional Direct to CT suite (DTCT) for suspected LVO patients. We also explored the budgetary impact of gradually implementing a DTAS protocol to apply 24 h a day, seven days a week (24/7) to 80% of the EVTs, compared with a no-change scenario in which all patients follow the traditional pathway based on the conventional CT-scan selection upon arrival (DTCT).
Methods
Model structure
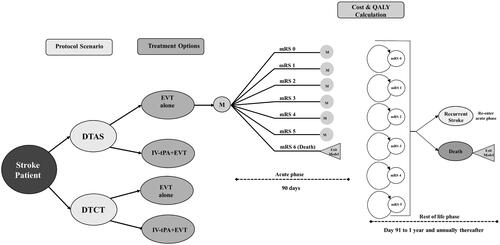
The modeling approach and main structure for our cost-utility study started with a decision tree structure that separated patients into two different populations according to treatment pathways (DTCT vs. DTAS). Patients were further separated into two treatment options in each pathway (EVT alone versus intravenous thrombolysis (IV-tPA)+EVT)).
A Markov model was also used based on a cost-effectiveness model comparing IV-tPA + EVT with IV-tPA alone in the United KingdomCitation19 and then adapted in SpainCitation20, ItalyCitation21, and AustraliaCitation22. The Markov model was used to estimate health outcomes based on the modified Rankin Scale (mRS), costs, and Quality-Adjusted Life-Years (QALYs) associated with each endpoint of the decision tree ().
The Markov structure included two different phases. The first is an acute-subacute phase from stroke onset to 90 days. The second is a rest-of-life phase from day 91 until the end of the patient’s life. The first cycle of the rest-of-life phase was 275 days, afterwards, each subsequent cycle in the rest-of-life phase represented one year.
During the acute-subacute phase, patients were assigned an mRS score representative of their degree of disability. An mRS score of zero indicated no symptoms, a score of five indicated severe disability (e.g. bedridden, requiring constant nursing care), and an mRS score of six represented patient death. These scores were assigned to patients based on the treatment they received in their corresponding protocol scenario and treatment pathway. Patients maintained the same mRS score unless they experienced a recurrent stroke or exited the model due to death. When a recurrent stroke occurred, patients reentered the acute-subacute phase and were assigned an mRS score of equal or higher value compared with their prior score. All treatment effects were considered to occur during the acute-subacute phase. A half-cycle correction was applied to account for patients who transitioned midway through a cycle.
All costs in this study were calculated from the National Health Service (NHS) perspective and presented in Euros, considering a lifetime horizon. Costs were also inflated to reflect the currency value in 2021. Costs and health outcomes were discounted at an annual rate of 3%, in line with the Spanish guidelines for health economic evaluationsCitation23. This study was developed following the Consolidated Health Economic Evaluation Reporting Standards (CHEERS)Citation24.
We also conducted a Budget Impact Analysis (BIA) that compared the DTCT pathway versus a combination of the DTCT + DTAS pathway. The gradual transition from DTCT to DTCT + DTAS was modeled at an annual rate of 20%, based on our center’s experience and learning curve. Thus, after one year 20% of patients were in a DTAS pathway, after two years 40% were in a DTAS pathway, and so on and so forth until a plateau at 80% of patients in a DTAS pathway. This steady transition from one pathway to another model a hospital’s gradual transition to a new protocol. The model plateaus at 80% because not all stroke patients would be eligible for the DTAS pathway. A five-year timeframe was used in the BIA to reflect the steady transition between pathways
Model outcomes
In the model, lifetime health outcomes and costs per pathway for a hypothetical cohort of 1000 patients were calculated. The main outcomes were the calculated Incremental Cost-Utility Ratio (ICUR), the quotient of the incremental costs, and QALYs. The DTAS protocol's net economic value, expressed as the net monetary benefit (NMB), was calculated considering a willingness-to-pay (WTP) threshold of €25,000/QALY in accordance with the latest guidelines for health economic evaluations in SpainCitation25,Citation26.
Patient cohort
At our center (Vall d’Hebron University Hospital) about 300 patients undergo EVT each year. Approximately 80.0% were potential DTAS candidates based on the time from stroke onset to hospital arrival (<6 h), stroke severity (National Institute Health Stroke Scale, NIHSS ≥10), and pre-stroke functional status (mRS ≤2)Citation12. Thus, we considered 300 patients as the number of EVT performed in the center annually for BIA.
Clinical effectiveness
DTAS has consistently been shown to shorten the in-hospital workflow (i.e. door-to-groin-puncture time) with varying degrees of success for observed clinical outcomesCitation2–4,Citation14. For this study, the individual data of 178 patients treated in 2018 were analyzed to obtain the clinical outcomes that were used in the model. Among them, 61 patients followed DTAS and 117 patients underwent a conventional imaging protocol (DTCT) before EVT. Both groups were matched using a propensity score approach to ensure the absence of significant bias and that the baseline characteristics of the two study groups are balanced. DTAS patients were propensity scored matched at a 1:2 ratio to DTCT patients. The propensity scores were estimated based on pre-morbid mRS, onset-to-door time and NIHSS score. A comparison of baseline characteristics between the two groups was conducted using the Pearson χ2 test or the Fisher exact test for categorical variables and the t-test or the Mann–Whitney test for continuous variables. Statistical significance was determined by a probability value of <0.05 for all tests. A multivariate analysis was conducted to confirm the direct relationship between DTAS and functional outcome, and the result is reported as adjusted odds ratios (aOR). Both groups were similar based on NIHSS stroke severity, time from symptom onset to hospital arrival, and age (Supplement Material Table A1).
Health outcomes were quantified using QALYs to weigh the life-years gained by their utility value during those years. The utility values ranged from zero (death) to one (optimal health). Negative values were possible and represented states of health that are considered worse than death. These values are based on the Oxford Vascular Study, with utilities that ranged from −0.054 to 0.935 ()Citation27. The probability of experiencing a recurrent stroke was applied in two different periods of the rest-of-life phase: from 91 days to one year and from one year onwards based on previously published dataCitation28.
Table 1. Model parameters from published literature.
Mortality
To reflect death unrelated to stroke during the rest-of-life phase, age and gender-specific mortality rates were applied to patients in each cohort according to published rates for the autonomous Catalonian community (Supplement, Table A2)Citation29. Due to the higher rates of death observed in patients after a stroke, mortality rates were adjusted by incorporating a disease-related risk of death scaled by mRS score ()Citation30.
Resource cost
The cost of resources used for treatment and care was broken down into three categories: acute hospital costs, long-term costs, and investments.
Acute hospital costs
Acute care costs were determined using patient cost data obtained directly from the hospital’s billing system. These costs included: the hospital stay, laboratory exams, devices, and procedure costs. In addition, any costs incurred after the patient was discharged until 90 days post-stroke were also included in the acute hospital costs category.
In the BIA, the total acute cost for the combined pathway (DTCT + DTAS) was calculated for each new patient cohort modeled, representing a different proportion of patients switching from DTCT to DTAS annually for the first five years.
Long-term costs
Long-term costs were calculated based on a Spanish cost-effectiveness study comparing EVT + IV-tPA vs IV-tPA aloneCitation20. This study provided the annual costs for nursing and residential care based on mRS score. As the costs are presented for a full year, the cost equivalent to the first 90 days post-stroke was discounted to reflect the cost of the post-acute phase (91 days to one year of the first Markov cycle). Thereafter, the full annual cost was used in each subsequent cycle for the rest of the patient’s lifetime. These costs were additionally adjusted to reflect the Euro’s rate of inflation in 2021.
Investments
To implement the DTAS pathway, a dedicated angiography suite and staff reorganization would be required. The full investment was factored in as part of the costs carried out at the beginning of the first year and its related annual costs were computed progressively. The total cost of this investment was calculated to be €4 million, including the cost of the new angiograph (€2.1 million), suite building (€300,000), and maintenance fees (€90,000 per year). An additional €700,000 was considered for staff investments. In the BIA we assumed that even after full implementation of the angiography suite, up to 80% of EVT patients would fit the DTAS criteria.
Sensitivity analysis
The model uncertainty was controlled by performing two different sensitivity analyses (SAs), deterministic and probabilistic. A deterministic SA was performed to examine the cost effects of a one-way variation of key model input parameters such as age, health utilities, mRS score in the acute-subacute phase, risk of recurrent stroke, risk of death, and costs. A probabilistic SA was undertaken to account for variability in outcomes due to the statistical uncertainty of the model inputs. The probabilistic SA consisted of simultaneous variations of all relevant parameters according to a distribution function previously assigned (). These variations were modeled by running 10,000 Monte Carlo simulations.
Results
Based on data from patients who underwent EVT at our center in 2018, we calculated the costs of running the DTAS and DTCT pathways. These data included 61 DTAS patients and 117 DTCT patients who all underwent EVT. The baseline clinical characteristics and comorbidities of these two groups did not differ in terms of stroke severity, time from symptom onset to hospital arrival, and age (Supplement Material Table A1). The mean age of stroke onset of the full patient cohort was 73.61 years old (standard deviation of 11.0).
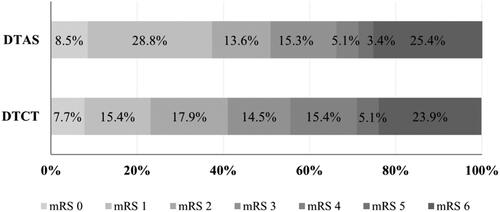
Median door-to-groin-puncture or door-to-groin time (DTG) was shorter in the DTAS group 19 (interquartile range (IQR) 15–23) min versus 73 (IQR 39–91) min; p < .01. At 90 days, the DTAS group presented a higher rate of good functional clinical outcomes (mRS 0–2: 50.0% vs. 40.2%; p =.03) without additional safety issues. After adjusting by potential confounders, DTAS was independently associated with a good functional outcome (aOR 1.32 (95% CI 1.02–2.56), p = .038). The mean acute care costs per mRS score ranged from €14,640 (mRS 0) to €30,470 (mRS 5) where the highest costs were observed in patients with levels of higher disability (). In terms of possible improved clinical outcomes after 10 years, the progressive DTCT to DTAS model scenario was projected to result in better cumulative functional independence rates (mRS 0–2: 50.9% DTAS vs. 41.0% DTCT, ).
Table 2. Results of calculated clinical outcomes and acute hospital cost analysis.
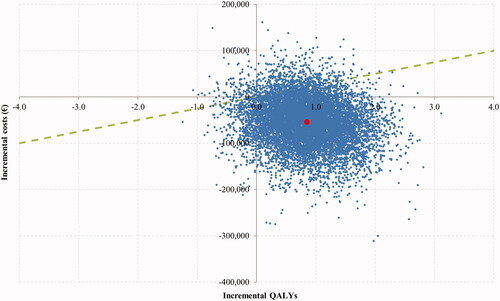
In the base case scenario, the DTAS pathway was projected to result in better effectiveness and lower costs than the DTCT pathway over the patient’s lifetime. The model suggests that a savings of €51,505 per patient could be achieved by transferring patients directly to the angiosuite (€184,919 DTCT vs. €133,413 DTAS). The calculated number needed to treat (NNT) per mRS score at 90 days (shift analysis) was 2.90 and the NNT per additional independent patient at 90 days was 10.89. Moreover, DTAS was associated with a superior QALY gain of 0.82 QALYs per patient (5.19 vs. 4.37). A net monetary benefit of €72,056 per patient was obtained at a €25,000/QALY willingness to pay threshold ().
Table 3. Cost-utility analysis results.
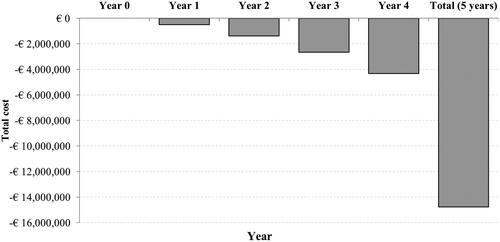
Per the BIA, after five years of gradual implementation, the DTAS pathway was estimated to incur a 10.2% cost reduction (€14.7 million) (). The total cumulative cost of the DTCT pathway was estimated to be €144.5 million and the DTAS pathway was projected to cost €129.5 million over five years. The cost savings of the DTAS protocol were mainly due to long-term associated costs related to patient disability and, to a lesser extent, in-hospital costs. DTAS’s long-term associated costs were €13.2 million; an 11.6% reduction from the total for DTCT. In addition, the in-hospital costs were projected to be €36.2 million for DTAS and €37.7 million for DTCT. Given all these factors, the initial investment for the angiosuite was projected to be recovered after three years.
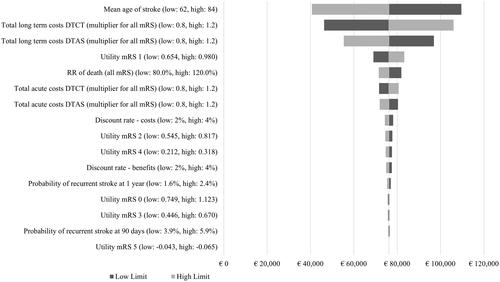
The deterministic SA suggested that the parameters with the highest impact on the economic results were long-term care costs, mean patient age, acute hospital costs, and utility values, as described in the tornado diagram (). The NMB ranged from €40,694 to €109,584.
The probabilistic SA showed DTAS was dominant at €25,000 per QALY. The DTAS pathway yielded better health outcomes while providing cost savings in 87.2% of the 10,000 simulations performed and was cost-effective in 93.2% of the iterations (). When the maximum willingness to pay threshold was used (€60,000/QALY), the DTAS pathway was cost-effective in 95.6% of the iterations.
Discussion
Management of acute ischemic stroke patients has constantly evolved over the last 25 years with different strategies employed to shorten workflows and time to reperfusionCitation31. DTAS represents a new step in this direction, with confirmed benefits in terms of time savings and promising clinical results. This study demonstrated the investment necessary to develop a scenario in which selected stroke patients could undergo a predominantly DTAS-focused protocol on arrival and the healthcare system would be compensated three years after the initial investment. The cost savings with DTAS would primarily be due to the associated reduction in acute and long-term costs for stroke patient care. Moreover, our results point to better clinical outcomes for EVT patients with a DTAS protocol making it also a cost-effective strategy.
The development of a DTAS scenario was associated with better functional outcomes for stroke patients. According to our findings, 2.9 patients would need to be treated using the DTAS protocol to reduce the probability of disability, as measured by mRS score. Compared with the current gold standard medical treatment, we calculated that 2.6 thrombectomies would need to be performed for marked outcome improvementCitation2. Moreover, the model showed a gain of 0.82 QALYs per patient and lower associated costs leading to a total savings of €51,505 per patient. Deterministic and probabilistic sensitivity analysis results demonstrated the robustness of the model and revealed that the cost-effectiveness of DTAS is consistent in the majority of calculated simulations.
Multiple clinical investigations have demonstrated the potential benefit of a DTAS protocol. One such study, the ANGIOCAT trial (NCT04001738), a prospective, open, randomized clinical trial, has clearly demonstrated the clinical benefits of a DTAS protocol in terms of time and cost savings. These findings could shortly be corroborated by a second ongoing randomized clinical trial the DIRECTANGIO study (NCT03969511). Thus, DTAS could become a more widely used pathway with a high proportion of suspected stroke patients primarily transferred and imaged in the angiography suite. As a caveat, patients participating in the DTAS scenario could potentially include “false-positive” candidates (intracranial hemorrhage and non-LVO ischemic stroke); however, the risk of a false-positive is worth the benefit associated with earlier diagnosis of stroke patients admitted after symptom onsetCitation18. Therefore, to maximize the clinical benefits of DTAS, the angiosuite and corresponding team need to be immediately available upon patient arrival at the facility. Existing angiosuites in large volume centers usually have busy schedules and a large number of elective cases. Hence, implementing DTAS might be challenging for hospitals initially, and could induce delays and cancelations of non-urgent cases at first. Once the 24/7 onsite staff becomes more experienced and a scheduling protocol becomes established, this concern should diminish. Initial investment to implement a dedicated DTAS angiosuite, and its associated running costs, is necessary to obtain all potential benefits and reduce collateral dysfunctions. According to our model, this investment of roughly €4 million could be fully compensated by cost savings in three years the total cost of care is considered. Our findings could help inform hospitals’ decision-making process when considering new investments to improve the endovascular treatment of stroke.
Some of the major strengths of our study are the acute and subacute economic and clinical data directly obtained from real-world patients, and not based on clinical trial publications performed out of routine daily practiceCitation19,Citation20. A limitation of our study, however, is these data were obtained from a single center in Catalonia, Spain. Thus, our findings could be difficult to generalize for global use due to center-specific clinical and organizational characteristics, and variations in the DTAS protocol. In addition, these real-world data are from a premier center in Europe. Such timelines and quick patient improvement may be difficult for less experienced staff to replicate. Furthermore, our economic results are influenced by the organization of the public healthcare system in Catalonia, Spain. In Catalonia, the acute and hospitalization costs lie within the public system, whereas long-term costs are shared between the government and patients and/or caregivers. Therefore, the economic results may vary depending on the healthcare organization; for example, assuming only a hospital perspective for the analysis may prolong the time required for the investment to reach a breakeven point. Such is the nature of all model-based cost-effectiveness studies though. The accuracy with which one can apply these conclusions is based on institutions’ similarity to the model system. Furthermore, although the use of propensity score matching, there was still a residual imbalance between cohorts in the study. Although propensity-score matching and regression were used, residual confounding cannot be entirely excluded.
A DTAS pathway could demonstrate an even greater benefit if we had factored in the mitigation of the economic burden on society as well. The indirect cost on society due to patients’ productivity losses as a result of early mortality or morbidity could also be a benefit of DTAS. In this study, however, we did not calculate that economic burden. The mean age in our patient cohort was 73 years old, and thus beyond the average retirement age (67 years old) in Spain. Based on regional statistics, it seems that a reduced proportion of elderly people would remain in the labor market after retirement age, and therefore the economic impact for this group might be underestimated. Furthermore, incorporating informal caregiving costs into economic analysis could also increase economic benefits. The majority of informal caregiving is provided by unpaid family members, which leads to a considerable burden from an economic standpoint. This is, however, one of the most overlooked components in efforts to estimate the indirect cost of stroke, and our study is no exception.
Conclusion
The development of a DTAS protocol in a high-volume comprehensive stroke center was modeled to be a highly cost-efficient measure. Cost reductions compared with a DTCT protocol were observed at all levels. The prevailing source of cost-utility predicted by our model is due to long-term costs associated with patient disability reduction. In addition, our model predicted the potential for clinical benefits in terms of better patient functional independence and QALYs. Although these results are promising, they should be validated and confirmed in the context of other healthcare systems, hospitals, and clinical trials.
Transparency
Declaration of funding
This study was sponsored by Medtronic.
Declaration of financial relationships
MR receives payment from Philips as Co-principal investigator of the WE TRUST study, and he has a consulting agreement with Medtronic, Stryker, Cerenovus, CVAid, Methinks, Anaconda Biomed, and Apta Targets. The other authors report no conflict. VSR, PGM and JW are Medtronic employees.
Author contributions
MR, AVJ: contributed to the design, and data collection. MR, VSR, PGM, JW: contributed to data analysis, interpretation, drafting, review, and revision of the manuscript. AT, MR, CAM: reviewed and revised the manuscript.
Acknowledgements
None reported.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethics approval
The study protocol was approved by the ethics committee of Vall d’Hebron Institut de Recerca, Barcelona, Spain (PR(AG)156/2018).
Supplemental Material
Download MS Word (17.8 KB)Data availability statement
The manuscript and supplement included all relevant data.
References
- Vega T, Zurriaga O, Ramos JM, et al. Stroke in Spain: epidemiologic incidence and patterns; a health sentinel network study. J Stroke Cerebrovasc Dis. 2009;18(1):11–16.
- Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):E344–E418.
- Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J NeuroIntervent Surg. 2019;11(6):535–538.
- Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279–1288.
- Jahan R, Saver JL, Schwamm LH, et al. Association between time to treatment with endovascular reperfusion therapy and outcomes in patients with acute ischemic stroke treated in clinical practice. JAMA. 2019;322(3):252–263.
- Ribo M, Molina CA, Cobo E, et al. Association between time to reperfusion and outcome is primarily driven by the time from imaging to reperfusion. Stroke. 2016;47(4):999–1004.
- Squire BT, Tamayo-Sarver JH, Rashi P, et al. Effect of prehospital cardiac catheterization lab activation on door-to-balloon time, mortality, and false-positive activation. Prehosp Emerg Care. 2014;18(1):1–8.
- Jadhav AP, Kenmuir CL, Aghaebrahim A, et al. Interfacility transfer directly to the neuroangiography suite in acute ischemic stroke patients undergoing thrombectomy. Stroke. 2017;48(7):1884–1889.
- Psychogios MN, Behme D, Schregel K, et al. One-Stop management of acute stroke patients: minimizing door-to-reperfusion times. Stroke. 2017;48(11):3152–3155.
- Ribo M, Boned S, Rubiera M, et al. Direct transfer to angiosuite to reduce door-to-puncture time in thrombectomy for acute stroke. J NeuroIntervent Surg. 2018;10(3):221–224.
- Mendez B, Requena M, Aires A, et al. Direct transfer to angio-suite to reduce workflow times and increase favorable clinical outcome. Stroke. 2018;49(11):2723–2727.
- Bouslama M, Haussen DC, Grossberg JA, et al. Flat-panel detector CT assessment in stroke to reduce times to intra-arterial treatment: a study of multiphase computed tomography angiography in the angiography suite to bypass conventional imaging. Int J Stroke. 2021;16(1):63–72.
- Psychogios MN, Maier IL, Tsogkas I, et al. One-Stop management of 230 consecutive acute stroke patients: report of procedural times and clinical outcome. J Clin Med. 2019;8(12):2185.
- Maier IL, Leyhe JR, Tsogkas I, et al. Diagnosing early ischemic changes with the latest-generation flat detector CT: a comparative study with multidetector CT. AJNR Am J Neuroradiol. 2018;39(5):881–886.
- Leyhe JR, Tsogkas I, Hesse AC, et al. Latest generation of flat detector CT as a peri-interventional diagnostic tool: a comparative study with multidetector CT. J Neurointerv Surg. 2017;9(12):1253–1257.
- Sarraj A, Goyal N, Chen M, et al. Direct to angiography vs repeated imaging approaches in transferred patients undergoing endovascular thrombectomy. JAMA Neurol. 2021;78(8):916–926.
- Requena M, Olive M, Garcia-Tornel A, et al. Time matters: adjusted analysis of the influence of direct transfer to angiography-suite protocol in functional outcome. Stroke. 2020;51(6):1766–1771.
- Lobotesis K, Veltkamp R, Carpenter IH, et al. Cost-effectiveness of stent-retriever thrombectomy in combination with IV t-PA compared with IV t-PA alone for acute ischemic stroke in the UK. J Med Econ. 2016;19(8):785–794.
- de Andres-Nogales F, Alvarez M, de Miquel MA, et al. Cost-effectiveness of mechanical thrombectomy using stent retriever after intravenous tissue plasminogen activator compared with intravenous tissue plasminogen activator alone in the treatment of acute ischaemic stroke due to large vessel occlusion in Spain. Eur Stroke J. 2017;2(3):272–284.
- Ruggeri M, Basile M, Zini A, et al. Cost-effectiveness analysis of mechanical thrombectomy with stent retriever in the treatment of acute ischemic stroke in Italy. J Med Econ. 2018;21(9):902–911.
- Al-Senani F, Al-Johani M, Salawati M, et al. A national economic and clinical model for ischemic stroke care development in Saudi Arabia: a call for change. Int J Stroke. 2019;14(8):835–842.
- Lopez Bastida J, Oliva J, Antonanzas F, et al. [A proposed guideline for economic evaluation of health technologies]. Gac Sanit. 2010;24(2):154–170.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Int J Technol Assess Health Care. 2013;29(2):117–122.
- Vallejo-Torres L, Garcia-Lorenzo B, Castilla I, et al. On the estimation of the cost-effectiveness threshold: why, what, how? Value Health. 2016;19(5):558–566.
- Sacristan JA, Oliva J, Campillo-Artero C, et al. [What is an efficient health intervention in Spain in 2020?]. Gac Sanit. 2020;34(2):189–193.
- Luengo-Fernandez R, Gray AM, Bull L, et al. Quality of life after TIA and stroke: ten-year results of the oxford vascular study. Neurology. 2013;81(18):1588–1595.
- Mohan KM, Wolfe CD, Rudd AG, et al. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42(5):1489–1494.
- Instituto Nacional de Estadisticas; Resultados nacionales, por comunidades autónomas y provincias, Tablas de mortalidad por año, sexo, edad y funciones. Series desde 1991–2019. 2019. Available from: https://www.ine.es
- Slot KB, Berge E, Sandercock P, et al. Causes of death by level of dependency at 6 months after ischemic stroke in 3 large cohorts. Stroke. 2009;40(5):1585–1589.
- Steen Carlsson K, Andsberg G, Petersson J, et al. Long-term cost-effectiveness of thrombectomy for acute ischaemic stroke in real life: an analysis based on data from the Swedish Stroke Register (riksstroke). Int J Stroke. 2017;12(8):802–814.