Abstract
Aims
A third of non-valvular atrial fibrillation (NVAF) patients are non-adherent to direct oral anticoagulants (DOACs). Estimates of the economic value of full adherence and the cost of two types of adherence improving interventions are important to healthcare planners and decision-makers.
Methods
A cost-utility analysis estimated the impact of non-adherence over a 20-year horizon, for a patient cohort with a mean age of 77 years, based on data from the Stockholm Healthcare database of NVAF patients with incident stroke between 2011 and 2018. Adherence was defined using a medication possession ratio (MPR) cut-off of 90%; primary outcomes were the number of ischemic strokes and associated incremental cost–utility ratio.
Results
Hypothetical comparisons between cohorts of 1,000 patients with varying non-adherence levels and full adherence (MPR >90%) predicted an additional number of strokes ranging from 117 (MPR = 81–90%) to 866 (MPR <60%), and years of life lost ranging from 177 (MPR = 81– 90%) to 1,318 (MPR < 60%; discounted at 3%). Chronic disease co-management intervention occurring during each DOAC prescription renewal and patient education intervention at DOAC initiation will be cost-saving to the health system if its cost is below SEK 143 and SEK 4,655, and cost-effective if below SEK 858 and SEK 28,665, respectively.
Conclusion
Adherence improving interventions for NVAF patients on DOACs such as chronic disease co-management and patient education can be cost-saving and cost-effective, within a range of costs that appear reasonable to the Swedish healthcare system.
PLAIN LANGUAGE SUMMARY
Atrial fibrillation (AF) is the most common type of chronic cardiac arrhythmia and a major risk factor for ischemic stroke (IS). The objective of this study was to compare the costs and health outcomes associated with adherence to direct oral anticoagulant (DOAC) therapy in Sweden. The study also aimed to demonstrate the potential benefits of developing interventions to improve DOAC adherence. DOAC therapy (DOACs; apixaban, dabigatran, edoxaban, and rivaroxaban) has been approved in Europe for the prevention of stroke in adult patients with AF. It has been demonstrated to provide warfarin-similar reductions in stroke risk in NVAF patients, with reductions in mortality and intracranial hemorrhage. However, non-adherence to DOAC medication prevents patients and healthcare systems from fully benefiting from DOAC therapy, resulting in a lower benefit than those seen in randomized controlled trials. DOAC non-adherence is where AF patients deviate from the DOAC treatment regimen as prescribed by health providers. This study suggested that non-adherence to DOAC therapy has a substantial impact on ischemic stroke risk and significant additional healthcare system costs. Patient education and chronic disease co-management (two types of DOAC adherence improving intervention) can be cost-saving and cost-effective within a range of costs that appear reasonable to the Swedish healthcare system. Healthcare policy-makers should prioritize initiatives aimed at improving DOAC adherence in order to improve outcomes in AF.
Introduction
Atrial fibrillation (AF) is the most common type of chronic cardiac arrhythmia and a major risk factor for ischemic stroke (IS). AF affects 37.5 million individuals worldwide (2020), with its prevalence expected to double by 2030Citation1. The risk of IS in AF patients is approximately 5-times greater than for a person with a normal heart rhythmCitation2. In Sweden, the prevalence of AF is estimated to be at least 3% of the total adult population (≥20 years)Citation2, with the proportion of AF among IS patients reported to be 33%Citation2. AF also poses a substantial economic burden on the Swedish healthcare system. The cost of AF attributable to stroke is approximately €274 million (inflated to 2022 costs)Citation3.
In recent years, four direct anticoagulant therapies (DOACs; apixaban, dabigatran, edoxaban, and rivaroxaban) have been approved in Europe for the prevention of stroke in adult patients with non-valvular atrial fibrillation (NVAF). DOACs have been shown to provide warfarin-similar reductions in stroke risk in NVAF patients, with reductions in mortality and intracranial hemorrhage (ICH)Citation4–7. Since their introduction, clinical guidelines have adopted DOACs as the mainstay of current management for stroke prevention due to their ease of use, limited monitoring requirements, and negligible interactions with other drugsCitation8.
However, medication non-adherence prevents patients and healthcare systems from fully benefiting from DOAC therapy, resulting in lower efficacy estimates than those observed in randomized controlled trials (RCTs)Citation9. In a recent systematic review of DOACs in the real-world setting, around a third of patients were non-adherent (MPR ≤ 80%), which was associated with a heightened risk of stroke (HR = 1.39; 95% CI = 1.06–1.81)Citation10.
Currently, there is a paucity of literature describing the clinical and economic impacts of DOAC non-adherence for stroke prevention. Consequently, the possible health gains and cost savings when DOAC adherence is potentially improved may be unclear to payers.
This analysis reports the results of a cost-effectiveness study to establish the clinical and economic impact of full and partial adherence to DOAC therapy in Sweden. It is intended to demonstrate the potential benefits of developing interventions to improve treatment adherence.
Methods
Model structure
A cost–utility analysis to assess the impact of adherence to DOACs was conducted from a Swedish healthcare system perspective over a 20-year time horizon and a discount rate of 3% as per Swedish guidelinesCitation11.
A Markov model structure with a 3-month cycle length was developed in Microsoft Excel for the cost-utility analysis. Patients were modeled to occupy one of the following health states at any given time: “stable AF”, “ischemic stroke” (acute and post), “intracranial hemorrhage” (acute and post) and “dead” (). Each health state was associated with a cost and a utility value. The structure of the model was based on the natural history of NVAF, previous cost-effectiveness studies of stroke prevention in AFCitation12, and expert consultation.
Figure 1. Model structure (cycle length duration: 3 months; time horizon: 20 years; baseline age of entry: 77 years old).
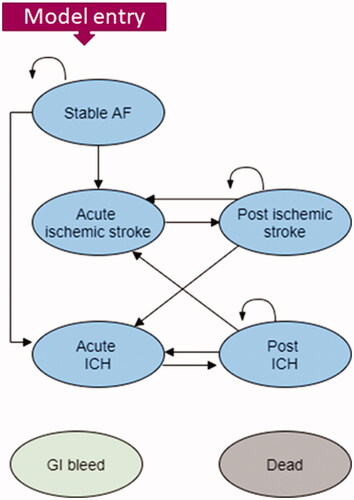
Patients entered the model with stable AF, a mean age of 77 years, and receiving treatment with a DOAC. Whilst on treatment, patients could remain in the “Stable AF” health state or experience an IS or ICH. If patients experienced an IS, they would initially move to the “acute IS” health state which would incur management costs. Following this, patients would transition to a “post IS” health state. Similarly for ICH events, patients would move to an initial “acute-ICH” health state followed by a “post-ICH” state in the following model cycles. In the post-IS health state patients could experience another acute IS or an acute ICH. Conversely, those in a post-ICH health state could experience another acute IS or an acute ICH.
Across all health states, patients could experience either a “gastrointestinal (GI) bleed” or move to the absorbing “Dead” state. The long-term consequences of events were considered until death.
Input data for the model was identified by a targeted literature review. Costs in Swedish Kronor (SEK) were converted to Euros via the 2021 exchange rate (Euro 1 = 10.3263 SEK, August 20) from the European Central BankCitation13.
Model inputs and data sources
Inputs used in the model are presented in .
Table 1. Summary of model definitions for adherence and persistence.
Table 2. Summary of model clinical inputs.
Table 3. Summary of model cost inputs.
Table 4. Summary of model utility values.
Clinical parameters
Clinical parameters included in the model were identified by a targeted literature review. Data pertaining to DOAC persistence and adherence rates came from a publication by Komen et al.,Citation14 a study which analyzed Swedish data on 21,028 AF patients from the Stockholm Healthcare database who were treated with DOACs during the period 2011–2018. The following definitions were applied (). Adherence to treatment was based upon the medication possession rate (MPR) in persistent DOAC users using the following categories: MPR >95%, 95–91%, 90–81%, 80–71%, 70–61%, and <61%. Patients were defined as being persistent by whether they had collected a DOAC prescription within 91 days of the end of supply from a prior prescription. Definitions of adherence which were applied in the model are outlined in .
Probability for stroke, adjusted for levels of non-adherence, was obtained from the Komen et al.Citation14 publication, which used a regression model not assuming any shape of the relationship between these two variables (as opposed to a linear dose–response relationship). It was assumed DOACs affect the severity of ICH rather than its incidenceCitation15, therefore a constant risk probability was assumed for ICH events. This meant no additional ICH risk for non-adherent or non-persistent patients.
The probability of death in the model was derived from two separate contributors to overall mortality: background and stroke/ICH related mortality. Background mortality was based on Swedish-specific life tables (last updated: December 2020) published by the World Health OrganizationCitation20, with average age and gender characteristics obtained from the Komen et al.Citation14 study. Stroke and ICH-related mortality estimates were obtained from another Komen et al.Citation15 publication utilizing data from the Stockholm Healthcare database. The base case analysis assumes a constant ICH mortality risk, irrespective of adherence to DOAC treatment. A summary of the model’s clinical inputs is provided in .
Healthcare resource utilization and costs
Costs and resource use in the model were based on the perspective of the Swedish healthcare system and included two main categories: health state costs and drug acquisition costs (). In the case of health state costs, these were based on ongoing disease management for AF and the costs associated with managing a stroke or bleeding related event (ICH or GI bleed). The drug acquisition costs were based on apixaban only since most patients (75%) in the Komen et al.Citation14 study received a prescription for apixaban. Cost data were obtained from the literature and expressed in terms of Swedish Krona (SEK). Where appropriate, costs were inflated to 2021 values using the Swedish Consumer Price IndexCitation21.
Health-related outcomes
Utility data was identified through a targeted review of the literature with source data based on Swedish populations used where available (). The primary data sources for health state utility values in the model were two long-term observational studies exploring the quality-of-life impact of stroke from Sweden (AF patients)Citation19 and England (general stroke patients)Citation18. For GI bleeds, a standard disutility rate of 0.1 was applied for the duration of one model cycle.
Base-case analysis
Primary model outcomes were total costs, events (IS, ICH, and GI bleeds), life years (LY), and quality-adjusted life years (QALY). The base-case analyses compared fully adherent (>95% MPR) DOAC users with all other MPR categories, as well as persistent DOAC users with non-persistent DOAC users.
Scenario analysis
The cost of the two types of adherence improving interventions: patient education at DOAC initiation (1), and chronic disease co-management at prescription renewal (medication reminders, pharmacist consultation) occurring at each cycle (2) was determined by scenario analysis. This was to ascertain the value required for these interventions to be cost-saving and cost-effective for the Swedish health system. For both interventions, it was assumed the intervention increased the proportion of fully adherent patients by five percentage points from 81 to 86%. The uneven distribution of DOAC patients across different levels of adherence, as presented by Komen et al., was considered by presenting weighted average intervention costs.
Sensitivity analysis
Two types of sensitivity analysis were performed: one-way sensitivity analysis (OWSA) and probabilistic sensitivity analysis (PSA).
In the OWSA, the model input parameters such as health state costs, drug costs, utility values, and mortality rates were varied to identify which parameters had the greatest impact on model results. A list of all parameters and their ranges is available in the Supplementary Appendix (Table S1).
For the PSA, input parameters were randomly sampled from their assigned distribution during 1,000 simulations. The results from the PSA were summarized using cost-effectiveness acceptability curves (CEAC) across a wide range of willingness-to-pay (WTP) thresholds.
Ethical considerations
Given that the analysis was based on data from previously published articles, this study was exempt from the need for approval by Ethical Review Boards.
Results
Base case
The base-case cost-effectiveness analysis showed that lower adherence rates to NOAC treatment have a large clinical and economic impact. As the MPR percentage declined, the number of IS events per 1,000 patients was doubled in the MPR 71–80% cohort and quadrupled in the lowest adherence level (MPR < 61%) when comparing with the fully adherent group (235 events, 521 events, and 1,101 events, respectively). The increase in events substantially increased the associated treatment costs. Patients in the lowest adherence cohort (MPR < 61%) were associated with a marked reduction in patients’ LYs by 1,318 as well as QALYs by 1,318. For this group, there were also substantially increased health care costs of SEK 142,502,959 (€13,800,002) per 1,000 patients. Most of the total cost increase was explained with the cost of IS. For detailed results across the different levels of adherence (MPR categories), see .
Table 5. Summary of base-case results.
In terms of the overall incremental cost-effectiveness ratio results, fully adherent DOAC users were dominant (i.e. more effective and less costly) versus lower adherent (MPR) comparisons.
Scenario analysis
Scenario analysis results showed that putative interventions assumed to increase the proportion of fully adherent patients from 81 to 86% were dominant (i.e. would prevent strokes and save costs). For intervention one (educational intervention) the maximum cost per patient to be cost-saving and cost-effective was SEK 4,665 and SEK 28,665, respectively. For intervention two (chronic disease co-management) the maximum cost per patient to be cost-saving and cost-effective was SEK 143 and SEK 858, respectively.
Sensitivity analysis
OWSA results showed that the cost-effectiveness outcomes were consistent with the model base case. The most sensitive parameters were patient age and discount rate (for both costs and QALYs), however, all results in the analysis remained dominant. For the PSA, fully adherent DOAC use is highly likely to be cost-effective at various WTP thresholds (Supplementary Appendix, Figure S1).
Discussion
It has been well established that DOACs reduce AF-related stroke substantially, achieving at least equal efficacy when compared with vitamin K antagonists, with a reduction in mortality and ICH. However, the effectiveness of DOACs is limited by poor adherence, with the economic impact not widely evaluated. Considering the shorter half-life of DOACs when compared to other oral anticoagulants, the level of adherence and associated reductions in efficacy become particularly important.
The results from our analysis demonstrate a strong relationship between different DOAC adherence levels (using MPR) and the number of IS events, which contribute to increased mortality and reduced quality-of-life (expressed in LYs and QALYs, respectively).
We also showed that not only are there substantial negative clinical outcomes associated with poor adherence, but also considerable economic consequences. IS has a high morbidity and mortality rate, with patients often requiring long-term care following a stroke and living a substantially decreased quality-of-life. Our analysis found that management of IS-related events is the main driver of costs and resource use in the NVAF population, representing 56% of costs in fully adherent/persistent DOAC users. Lower adherence rates have the greatest impact on IS-related costs, with 80.7% of total healthcare spending being attributed to IS costs in patients with MPR < 61%.
There is a clear need to improve DOAC adherence to reduce IS events and costs. This study shows that patient education and chronic disease co-management (two types of adherence improving intervention) can be cost-saving and cost-effective within a range of costs that appear reasonable to the Swedish healthcare system. The intervention costs identified to be cost-saving and cost-effective can be used to assist healthcare policy decision-makers in understanding the potential of adherence improving interventions when planning to reduce the significant clinical and economic burden of ischemic strokes associated with DOAC non-adherence.
It should be noted that certain nuances such as concomitant psychiatric conditions among patients would require a dedicated implementation approach for the intervention to be successful. Additional context may also be relevant to consider, such as socioeconomic and educational factors.
Limitations
This study was subject to several limitations. A key limitation of this analysis centred on the use of data from the Komen et al.Citation14 publication for key clinical parameters. First, the DOAC adherence definition used the MPR which assumes that a patient was in possession of a DOAC but does not consider whether it was prescribed or even consumed. Consequently, the association between different rates of poor adherence and IS may be over-estimated if there is a large proportion of patients who do not consume DOACs they have refilled. However, the sensitivity analyses explored the impact of any potential model uncertainties and found that the results remained consistent to that of the base case.
Second, 65% of the study population had hypertension, with a significant proportion of patients taking anti-hypertensive medications. Since non-adherence to antihypertensives and antiplatelet agents was not accounted for in the Komen et al.Citation14 analysis, this may have resulted in an over-estimation of the association between DOAC non-adherence and stroke risk, if DOAC non-adherence was also associated with anti-stroke mediation non-adherence. Third, there was no qualitative data available from the Komen et al.Citation14 publication to ascertain reasons for patient non-adherence. Therefore, the scenario analysis for putative adherence improving intervention did not specify actual strategies which targeted specific reasons for non-adherence. Furthermore, it is likely the ability to better target interventions to those patients most likely to be non-adherent to DOACs and amenable to behavior change would further improve the cost-effectiveness of an intervention. Finally, the assumption that both types of adherence improving interventions could increase the proportion of fully adherent patients by five percentage points may be an over-estimation. The target population of older adults is likely to have higher rates of physical and cognitive impairment than younger adults. Consequently, the effectiveness of a one-off patient education intervention is likely to be more limited than chronic disease co-management intervention composed of both educational and behavioral strategies. Furthermore, it was also assumed that the intervention would be equally effective for older adults, regardless of their socioeconomic position.
Lastly, although the relevance of adherence improving interventions should have broader applicability than to the Swedish healthcare system alone, the presented cost-effectiveness estimates indeed only apply to this setting.
Conclusions
This economic analysis shows that non-adherence to DOAC therapy has a substantial impact on outcomes in NVAF, both in terms of stroke-related events and costs. Consequently, we have been able to demonstrate that even small improvements in the proportion of those fully adherent to DOACs can deliver cost savings. This would be an important consideration for Swedish health policymakers planning quality improvement initiatives.
Transparency
Declaration of financial/other interests
CBL reports personal fees from Medtronic AB, Boston Scientific, Phillips, Bayer, BMS, MSD, Cathprint, and Aventis, during the conduct of the study. SS reports personal fees from Bayer AB during the conduct of the study. LGR reports grants from Bayer. OA, GJ, and AC are employed by Wickenstones Ltd, a company that received consultancy fees from Bayer. MH, KB, BS, HM, and LH are full-time employees of Bayer. LAL reports grants and personal fees from Bayer and Boehringer Ingelheim, and personal fees from Pfizer, outside the submitted work.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (112.8 KB)Acknowledgements
The authors would like to acknowledge James Harris and Peter Maguire (Wickenstones, Carlow, Ireland) for their medical writing assistance during the preparation of the manuscript.
Data availability statement
The data underlying this article are available at https://academic.oup.com/ehjcvp/article/7/FI1/f72/5824225?login=false.
References
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–847.
- Friberg L, Rosenqvist M, Lindgren A, et al. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;45(9):2599–2605.
- Lanitis T, Kongnakorn T, Jacobson L, et al. Cost-effectiveness of apixaban versus warfarin and aspirin in Sweden for stroke prevention in patients with atrial fibrillation. Thromb Res. 2014;134(2):278–287.
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151.
- Patel MR, Mahaffey KW, Garg J, the ROCKET AF Steering Committee, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2019;74(1):104–132.
- Jackevicius CA, Tsadok MA, Essebag V, et al. Early non-persistence with dabigatran and rivaroxaban in patients with atrial fibrillation. Heart. 2017;103(17):1331–1338.
- Ozaki AF, Choi AS, Le QT, et al. Real-World adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta-analysis. Circul Cardiovasc Qual Outcome. 2020;13(3):e005969.
- (TLV) TDaPBA. Ändring i Tandvårds- och läkemedelsförmånsverkets allmänna råd (TLVAR 2003:2) om ekonomiska utvärderingar 2017.
- Bowrin K, Briere JB, Levy P, et al. Real-world cost-effectiveness of rivaroxaban and apixaban vs VKA in stroke prevention in non-valvular atrial fibrillation in the UK. J Mark Access Health Policy. 2020;8(1):1782164.
- Bank EC. Swedish krona (SEK) Frankfurt: European Central Bank; 2021 [cited 2021 Aug 20]. Available from: https://www.ecb.europa.eu/stats/policy_and_exchange_rates/euro_reference_exchange_rates/html/eurofxref-graph-sek.en.html.
- Komen JJ, Heerdink ER, Klungel OH, et al. Long-term persistence and adherence with non-vitamin K oral anticoagulants in patients with atrial fibrillation and their associations with stroke risk. Eur Heart J Cardiovasc Pharmacother. 2021;7(Fi1):f72–f80.
- Komen JJ, Forslund T, Mantel-Teeuwisse AK, et al. Association of preceding antithrombotic therapy in atrial fibrillation patients with ischaemic stroke, intracranial haemorrhage, or gastrointestinal bleed and mortality. Eur Heart J Cardiovasc Pharmacother. 2021;7(1):3–10.
- Pennlert J, Overholser R, Asplund K, et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017;48(2):314–320.
- Aronsson M, Svennberg E, Rosenqvist M, et al. et al. Designing an optimal screening program for unknown atrial fibrillation: a cost-effectiveness analysis Europace 2017;19(10):1650–1656.
- Luengo-Fernandez R, Gray AM, Bull L, For the Oxford Vascular Study, et al. Quality of life after TIA and stroke: ten-year results of the oxford vascular study. Neurology. 2013;81(18):1588–1595.
- Lindgren P, Glader EL, Jönsson B. Utility loss and indirect costs after stroke in Sweden. Eur J Cardiovasc Prev Rehabil. 2008;15(2):230–233.
- World Health Organisation. Global Health Observatory data repository; 2020. Available from: https://apps.who.int/gho/data/?theme=main&vid=61600.
- Centralbyrån S. Konsumentprisindex (KPI). 2021. Available from: https://www.scb.se/hitta-statistik/statistik-efter-amne/priser-och-konsumtion/konsumentprisindex/konsumentprisindex-kpi.
