Abstract
Objective
Emerging SARS-COV-2 variants are spurring the development of adapted vaccines as public health authorities plan for fall vaccinations. This study estimated the number of infections and hospitalizations prevented by three potential booster strategies for adults (≥18 years) in the United States: boosting with either Moderna’s (1) licensed first generation monovalent vaccine mRNA-1273 (ancestral strain) or (2) candidate bivalent vaccine mRNA-1273.214 (ancestral + BA.1 variant of concern [VOC]) starting in September 2022, or (3) Moderna’s updated candidate bivalent vaccine mRNA-1273.222 (ancestral + BA.4/5 VOC) starting November 2022 due to longer development time.
Methods
An age-stratified, transmission dynamic, Susceptible-Exposed-Infection-Recovered (SEIR) model, adapted from previous literature, was used to estimate infections over time; the model contains compartments defined by SEIR and vaccination status. A decision tree was used to estimate clinical consequences of infections. Calibration was performed so the model tracks the actual course of the pandemic to present time.
Results
Vaccinating with mRNA-1273(Sept), mRNA-1273.214(Sept), and mRNA-1273.222(Nov) is predicted to reduce infections by 34%, 40%, and 18%, respectively, and hospitalizations by 42%, 48%, and 25%, respectively, over 6 months compared to no booster. Sensitivity analyses around transmissibility, vaccine coverage, masking, and waning illustrate that boosting with mRNA-1273.214 in September prevented more cases of infection and hospitalization than the other vaccines.
Limitations and Conclusions
With the emergence of new variants, key characteristics of the virus that affect estimates of spread and clinical impact also evolve, making parameter estimation difficult. Our analysis demonstrated that boosting with mRNA-1273.214 was more effective over 6 months in preventing infections and hospitalizations with a BA.4/5 subvariant than the tailored vaccine, simply because it could be deployed 2 months earlier. We conclude that there is no advantage to delay boosting until a more effective BA.4/5 vaccine is available; earlier boosting with mRNA-1273.214 will prevent the most infections and hospitalizations.
Introduction
The COVID-19 pandemic has been marked by the emergence of multiple variants of concern (VOC), including most recently Omicron, which arose in October 2021 and rapidly replaced all other circulating variants globallyCitation1. The World Health Organization (WHO) has stated that the available evidence suggests that Omicron VOC is more transmissible and shows evidence of immune escape, as there is a reduction in vaccine-induced neutralizing activity of antibody responses compared to previous VOCsCitation2. The original Omicron VOC, now termed BA.1, has already evolved into new sublineages. In the United States (US), the proportion of infections attributed to the BA.4 and BA.5 sublineages is increasing and, as of 6 July 2022, these are predicted to be the most common sublineages in the near futureCitation3,Citation4. While the characteristics of BA.4 and BA.5 are still being evaluated relative to BA.1, one concerning observation is that individuals produce lower neutralizing antibody titers compared to BA.1Citation2.
Since the successful launch of vaccines designed to protect against ancestral SARS-COV-2 infections, vaccine manufacturers have been developing new adapted vaccines targeting emerging VOCs. In April, Moderna reported superior neutralizing antibody responses generated by its candidate mRNA-1273.211 bivalent booster that encodes for the spike glycoproteins of the ancestral strain and Beta (B.1.351) variantCitation5. On 8 June 2022, Moderna reported superior antibody responses for its mRNA-1273.214 bivalent booster, that encodes for the ancestral strain and Omicron (BA.1) VOCCitation6,Citation7. Data from this latest clinical trial have now been submitted for review with regulators. In addition to superior neutralizing antibody responses to Omicron, next generation bivalent vaccines have also demonstrated a broader response to other VOCs, and this response was more durable for mRNA-1273.211 compared to the first-generation monovalent vaccine, mRNA-1273Citation4.
In anticipation of the peak winter season for respiratory viruses in the Northern Hemisphere and the registration of next generation COVID-19 vaccines, the WHO, European Medicines Agency (EMA), and US Food and Drug Administration (FDA) held several meetings where current epidemiological and vaccines data were reviewed. The WHO Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC) released a new interim statement in JuneCitation1. In this statement, TAG-CO-VAC reiterated that the primary objective of COVID-19 vaccination is to reduce hospitalization, severe disease and death, against which currently licensed vaccines continue to provide high levels of protection. However, in the context of uncertainty about the timing of the emergence, extent of global circulation, and antigenic characteristics of future variants, TAG-CO-VAC recommended that an additional vaccination objective be to broaden the immune response against circulating and emerging variants. As the most antigenically distinct SARS-CoV-2 VOC, they concluded that adding Omicron in an updated vaccine composition that includes the ancestral strain may be beneficial if administered as a booster dose. In their press briefing of 7 July 2022, EMA also communicated that the choice of vaccines will be based on the breadth of immunity against VOCs, given that no one can predict what variants will be circulating during the winterCitation8. Finally, following the Vaccines and Related Biological Products Advisory Committee (VRBPAC) vote on 28 June 2022, the FDA recommended for vaccine manufacturers to update booster formulations to include the ancestral strain and Omicron BA.4/5 given its current prevalence in the USCitation9. Such an update from the Omicron BA.1 currently included in candidate vaccines to BA.4/5 may have implications in terms of the timing of the fall vaccination strategy.
Our research objective was to estimate the number of infections and hospitalizations prevented by a booster strategy in those 18 years of age and older in the United States across a 6-month time horizon. We compared the following three scenarios: (1) Boosting with Moderna’s licensed first generation monovalent vaccine mRNA-1273 (ancestral) starting in September 2022, (2) Boosting with Moderna’s candidate bivalent vaccine mRNA-1273.214 (ancestral + BA.1 VOC) starting in September 2022, or (3) Boosting with Moderna’s updated candidate bivalent vaccine mRNA-1273.222 (ancestral + BA.4/5 VOC) starting in November 2022 due to longer development time.
Methods
For this analysis, we use a simple, age-stratified, Susceptible-Exposed-Infection-Recovered (SEIR) compartmental model, adapted from Shiri et al.Citation10, to estimate the number of infections over time and then use a decision tree to estimate the clinical consequences of those infections across a 6-month time horizon (September 2022 to February 2023), where healthcare systems are the most pressured. The model structure, inputs, and calibration process are described in more detail in the accompanying Technical Appendix; a brief overview of key features and inputs is presented here.
SEIR model structure
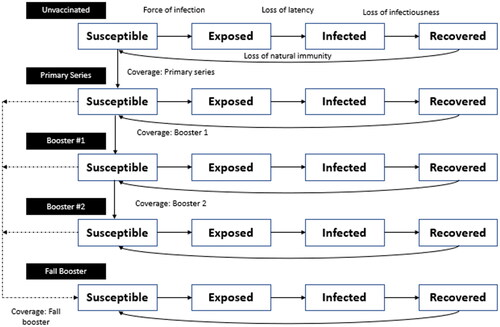
The SEIR model, which is sometimes referred to as a transmission model, contains compartments defined by both SEIR status and vaccination status. The time step for transitions in the model is one day. The model simulation begins on 31 January 2020, and, as described in more detail below, calibration methods are used to ensure that the model tracks as well as possible the actual course of the pandemic up to the present time, but prior to implementation of a new booster vaccine. As shown in , all individuals start in the unvaccinated compartments at the beginning of the pandemic. Individuals can move to the primary series compartments, then the booster 1 compartments, then the booster 2 compartments as more doses of vaccines are received. “Booster 1” and “booster 2” refer to doses of the currently available vaccine given after the primary series. For the primary series and each of these boosters, we assumed that a mix of the available vaccines (mRNA-1273; BTN162b2 [Pfizer-BioNTech]; AD26.COV2.S [Johnson & Johnson/Janssen]) were given based on US market shares at that time. We assume that no individuals receive primary series or booster 1 doses after 31 May 2022, and that no booster 2 doses are given after 15 June 2022. The model equations are presented in the Technical Appendix.
For this analysis, individuals may move into a final “Fall Booster” stratum when they receive a new booster starting in September or November 2022 (). Individuals may receive this fall booster if they have received the primary series, booster 1, or booster 2.
All individuals start in the susceptible (S) compartments and move through to the recovered (R) compartments as they develop an infection followed by natural immunity. The force of infection, which is indicated by arrows in the model figure, is a function of effective contact between the susceptible and infected people. This rate is dictated by an age-specific contact matrix, which is modified by reduction in social mobility and masking behavior, and transmissibility of the virus. Vaccination also acts to reduce the force of infection. The model was calibrated between 31 January 2020 to 31 May 2022 to match all infections as estimated by the Institute for Health Metrics Evaluation (IHME) by varying the transmissibility parameterCitation11. This process gave us an estimate of the number of people who had natural immunity and the average vaccine effectiveness (VE) for the proportions of the population in each of the vaccination strata, by age group in June 2022. All fixed model parameters and a more detailed description of the calibration process are described in the Technical Appendix.
Infection projections
For projections beyond May 2022, it was assumed that social distancing patterns would return to pre-pandemic levels and mask use would return to 0% by the end of July 2022. Both remain at these pre-pandemic levels for the remainder of the analysis time horizon. Projections of the potential impact of a new variant similar to the BA.4/5 sub-variant are highly variableCitation12. Initial indications from countries such as Portugal that have experienced an early BA.4/5 waveCitation13 are that BA.4/5 will not be as severe as the first BA.1 wave, but will be more severe than the BA.2 waves. We assumed that BA.4/5 would be the only sub-variants circulating by 15 August 2022. To create the projections, we therefore increased our transmissibility parameter until 1 September 2022, and held it constant thereafter. For the base case, we assumed that the peak incidence of infection without boosters would be approximately half of the Omicron BA.1 wave. These values are provided in the Technical Appendix.
Vaccine effectiveness
In order to model the impact of a changing mix of VOC on VE, we divided the model simulation timeframe into three periods: pre-Omicron (31 January, 2020–30 November 2021); Omicron BA.1/2 (1 December 2021–14 August 2022); and Omicron BA.4/5 (15 August 2022–28 February 2023).
VE was estimated for primary series, booster 1, and booster 2 using a weighted average of the types of vaccines received in the US. For the pre-Omicron period, initial VE (against infection and severe disease) and the monthly waning rates were obtained from an analysis of 20 studies conducted by the IHMECitation14. Data for boosters were obtained from a test-negative case-control study conducted in England, with assumptions applied for missing dataCitation15. For the Omicron BA.1 period, initial VE for primary series and booster and waning for the primary series were obtained from a meta-analysis by Pratama et al.Citation16; data collected in EnglandCitation17 were used where meta-analytic data were unavailable. The waning rates for the booster were assumed to be the same as the waning rates for the primary series. The values used in the model are displayed in , while further details are presented in the Technical Appendix.
Table 1. Vaccine effectiveness for primary series, booster 1 and booster 2 (pre-Omicron and Omicron BA.1 periods).
Real-world VE data against the BA.4/5 variant are not yet available. However, the method developed by Khoury et al.Citation18, and updated by Hogan et al.Citation19, which calculates VE over time based on relative neutralizing antibody levels (geometric mean titers, GMT), was used to estimate the impact of new vaccines and emerging variants. Moderna clinical data showed an 8.3 fold decrease between the neutralizing titers of mRNA-1273.214 against BA.4/5 and the ancestral SARS-CoV-2 at day 29, and an 11.5 fold decrease for mRNA-1273Citation20. Using the same method by Khoury et al.Citation21 and Hogan et al.Citation19, the initial infection VE against the ancestral strain for mRNA-1273.214 was estimated to be 95.24%. This was driven by the higher estimated GMT level produced against the ancestral strain by mRNA-1273.214 compared to mRNA-1273 (6,422 and 5,287, respectively)Citation20,Citation22. VE for the candidate mRNA-1273.222 BA.4/5-adapted bivalent vaccine was approximated using the GMTs of mRNA-1273.214 against BA.1, using the assumption that a BA.4/5-adapted bivalent vaccine would perform similarly against BA.4/5 as a BA.1 vaccine would perform against BA.1 (i.e. 2.6 fold decreased compared to ancestral GMT)Citation20,Citation22. The initial infection VE against the ancestral strain of 95.24% was also assumed for the mRNA-1273.222 vaccine. Waning for all three vaccine boosters was assumed to be the same as waning for primary series vaccination against BA.1/2 (7.3% for infection and 3.4% for severe disease; ). In order to adjust the VE of the primary series, booster 1, and booster 2 for the Omicron BA.4/5 period, we calculated that VE for the mRNA-1273 vaccine against infection drops to 72% of BA.1 effectiveness using the methods by Hogan et al.Citation19, while VE against severe disease drops to 88% of BA.1 effectiveness.
Table 2. Projected vaccine effectiveness against Omicron BA.4/5 for fall boosters.
Natural immunity
A review of the data during the pre-Omicron period concluded that the rate of waning of natural immunity is uncertain but likely equal to, or less than, that for vaccine-mediated immunityCitation23, and that the impact of Omicron on the duration of protection from natural immunity is also unclear. For our base case calibration, we assumed conservatively that this waning rate was equivalent to that of vaccine-mediated immunity in the pre-Omicron and Omicron BA.1 periods. We then held the rate constant during the BA.4/5 Omicron period to conduct projections.
Vaccine coverage
In this analysis, we attempt to determine the benefit of administering a new bivalent booster to individuals in the US who have received at least a primary series with a currently licensed vaccine. While not yet authorized by the FDA nor recommended by the Centers for Disease Control and Prevention (CDC), we assume this includes receipt of a third booster dose for those who have already received a primary series plus two booster doses, a second booster dose for those who have received only one booster dose, or a first booster dose for those who have received primary series only. The age-specific uptake of the primary series, booster 1, and booster 2 was based on data from the CDCCitation24. We assumed that no further primary series and first boosters were delivered after 15 May 2022 and that no further second boosters were delivered after 15 June 2022. The final coverage levels for each of these is therefore shown in and figures with daily coverage are provided in the Technical Appendix. For the fall boosters, we used the booster 1 uptake pattern and assumed that individuals are equally likely to receive the booster, regardless of their vaccination history. The age-specific uptake of the fall booster is shown in . For the November mRNA-1273.222 booster, the same uptake pattern was assumed except that vaccination started on 1 November 2022.
Figure 2. Cumulative vaccine uptake for a hypothetical fall booster strategy starting 1 September 2022.
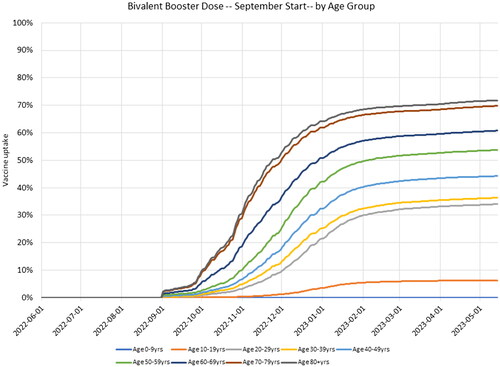
Table 3. Cumulative vaccine uptake by age group: primary series (31 May 2022); booster 1 (31 May 2022); and booster 2 (15 June 2022).
Consequences of infections
All patients who develop a symptomatic infection are assumed to move into an infection consequences decision tree (). The VE against severe infection is higher than protection against infection alone and this is modeled in the decision tree as a further reduction in hospitalization rates among those who are vaccinated. In sensitivity analysis, the impact of Paxlovid (Pfizer; nirmatrelvir/ritonavir) on the risk of hospitalization is also taken into account for a percentage of patients in each age stratum who are eligible and ultimately receive the treatment. The key model inputs are displayed in , with further details provided in the Technical Appendix.
Figure 3. Infection consequences decision tree.
*Risk is dependent on eligibility for and receipt of Paxlovid.
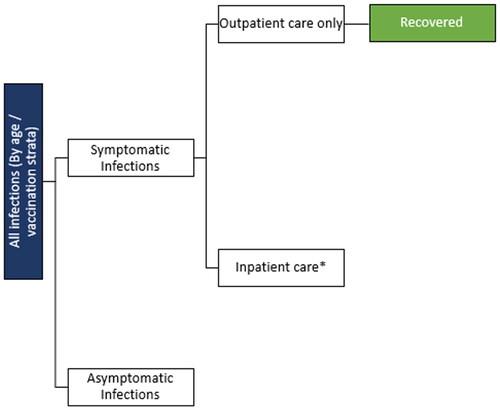
Table 4. Key consequences decision tree model inputs for the base case and sensitivity analyses.
Analyses conducted
We conducted a base case analysis to compare the clinical impact of the three potential strategies for fall 2022 to no additional boosters: 1) licensed mRNA-1273 (ancestral monovalent) booster starting in September; 2) candidate mRNA-1273.214 (ancestral + BA.1) booster starting in September; and 3) candidate mRNA-1273.222 (ancestral + BA.4/5) booster starting in November.
We conducted a series of sensitivity analyses with the SEIR model to determine how changes in our assumptions and input parameters alter prediction on the impact of a booster. First we recalibrated the model and varied the assumptions about waning of natural immunity and vaccine-mediated immunity to determine how waning would impact projections of infections for the fall. To do the recalibration, we altered the transmissibility of the virus from February 2020 to May 2022 but from June onwards, we did not change the transmissibility parameter input from the base case. We tested the impact of 1) reducing the waning of natural immunity by 50%, implying that it wanes at half the rate of vaccine-mediated immunity; 2) increasing the waning rate of vaccine immunity to 110% of the base values; and 3) decreasing the waning rate of vaccine immunity by 90% of the base values. We show results for the bivalent mRNA-1273.214 booster compared to no booster only. Using our original calibration, we also increased and decreased the virus transmissibility during the projection period by 10% and delayed the increase in transmissibility by one month. We present the impact of these changes on the projected patterns of infections.
We also did a series of sensitivity analyses on the characteristics of the new booster and the behaviour of people during the projection period. First, we altered the rate of waning effectiveness for the bivalent boosters. In the base case, we assumed that the monthly effectiveness waning rate was the same for the monovalent and bivalent boosters. However, there is emerging evidence suggesting that due to the bivalent composition of mRNA-1273.214, the duration of protection is extended (i.e. waning is reduced)Citation29. Therefore, we conducted three sensitivity analyses varying the waning rates of the bivalent boosters: a 25%, 50%, and 75% decrease in waning compared to the primary series. We did a separate analysis where we decreased the coverage rate associated with the fall boosters by 25%. We conducted one additional sensitivity analysis with the SEIR model where masking reduces to 9%Citation11 at end of July 2022 and remains at this level during the projection period from September to February 2023, and then is reduced to 0%.
Finally, we conducted a range of sensitivity analyses by varying our decision tree inputs as shown in . These changes impacted the number of hospitalizations only and did not impact the projections of infections with the transmission model.
Results
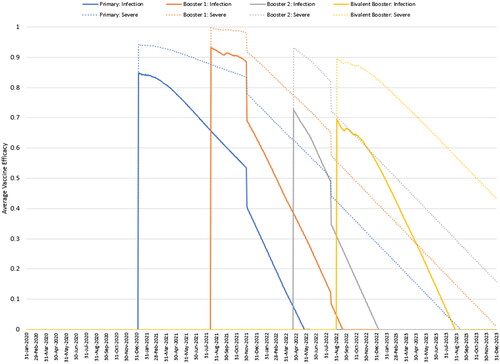
The average VE, by vaccination status, as projected by the model in the base case for the bivalent mRNA-1273.214, is shown in for those 80 years and above as an example. In the graph, solid lines represent VE against infection and dotted lines represent VE against severe disease. By September 2022, the model predicts that those who received only the primary series or booster 1 have no vaccine-mediated immunity against infection, while booster 2 VE is below 30%. VE against severe disease lasts longer, but it is projected to be below 50% for those receiving the primary series only. Among those who receive a mRNA-1273.214 booster, protection against infection and severe infection is boosted to 70% and 90%, respectively.
Figure 4. Model projected vaccine efficacy against infection and severe disease for primary series, booster 1, booster 2, and fall booster (mRNA-1273.214) groups for those 80 years and above.
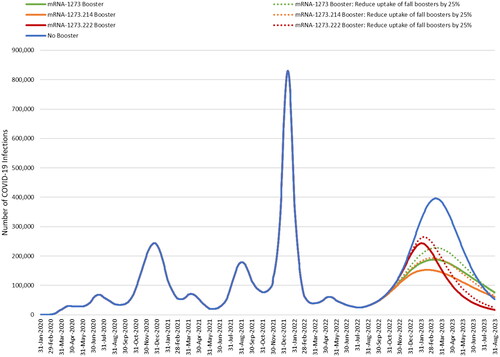
The base case model-projected numbers of infections with and without a fall booster are shown in . From September 2022 to February 2023, where healthcare systems are the most pressured, mRNA-1273 is predicted to prevent 34% of infections compared to no booster, mRNA-1273.214 prevents 40%, while mRNA-1273.222 prevents only 18% due to the delay in starting to boost. Given that the model predicts no protection against infection for many individuals, the administration of any fall booster reduces future infections.
Figure 5. Base case results: estimated COVID-19 infections with and without a fall booster strategy (mRNA-1273 or mRNA-1273.214 starting in September 2022; mRNA-1273.222 starting in November 2022).
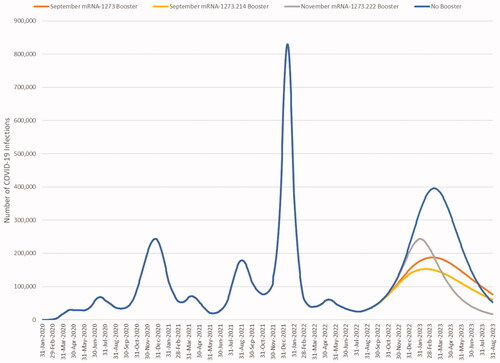
displays the cumulative number of infections predicted for each scenario by month and the percent change for each booster compared to no booster for the base case analysis. We have assumed that BA.4/5 will persist for the entire 6-month time horizon, however, if a new variant becomes dominant within the time period the relative effectiveness of the vaccines may change. If we further assume that BA.4/5 will persist over one year, the licensed mRNA-1273 booster is projected to reduce infections by 36%, the candidate bivalent mRNA-1273.214 by 47%, and the candidate mRNA-1273.222 by 45% compared to no boosters given in the fall.
Table 5. The cumulative number of infections predicted by the model by month for all booster scenarios.
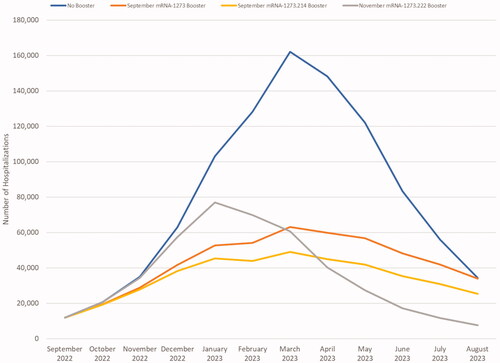
As with infections, starting to boost earlier (i.e. September) prevents more hospitalizations over the 6-month period than starting to boost two months later with a more effective vaccine: 42%, 48%, and 25% for mRNA-1273, mRNA-1273.214, and mRNA-1273.222, respectively in the base case analysis. The impact of different boosters on the expected number of hospitalizations is shown in . Overall, using a booster prevents hospitalizations mainly because it prevents infections in people in all strata, including those who do not receive the booster through the effect of herd immunity. There is some additional benefit from increasing protection against severe disease in those who received the fall booster as well. Assuming BA.4/5 will persist over one year, the licensed mRNA-1273 booster is projected to reduce hospitalizations by 47%, the candidate bivalent mRNA-1273.214 by 57%, and the candidate mRNA-1273.222 by 55% compared to no boosters given in the fall.
Figure 6. Estimated COVID-19 hospitalizations with and without a fall booster strategy (mRNA-1273 or mRNA-1274.214 starting in September; mRNA-1273.222 starting in November).
Our sensitivity analyses with the SEIR model demonstrate that assumptions on the waning of natural immunity, transmissibility and vaccine coverage impact the projected number of infections of mRNA-1273.214 compared to no booster as shown in .
Figure 7. Impact of changing calibration assumptions on the estimated number of infections projected with no fall booster strategy and the mRNA-1273.214 September strategy.
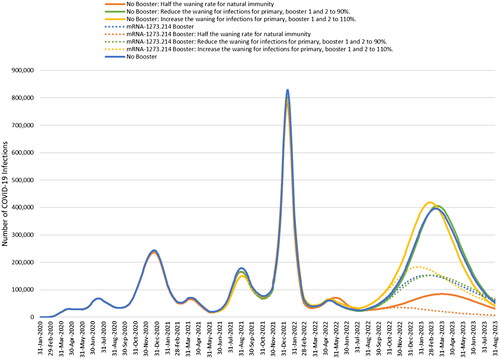
Figure 8. Impact of changing the transmissibility of the virus or delaying the increase in transmissibility caused by a new variant by 1 month.
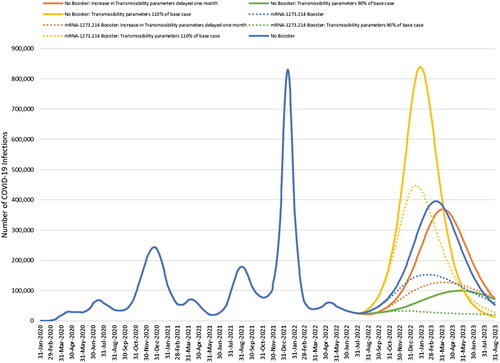
Figure 9. Impact of reducing the coverage of a fall booster strategy to 25% of base case on the number of infections.
The impact of varying the assumptions for the waning of immunity and then recalibrating the model is shown for no booster and mRNA-1273.214 in . When we varied the natural immunity and vaccine-induced immunity waning rates during our calibration period, natural immunity waning had the largest impact on the projected number of cases this fall. While the number of cases projected decreases with stronger natural immunity, mRNA-1273.214 is still effective when assuming half the waning rate of natural immunity and reduces the number of infections by 36% compared to no fall booster. However, even with this higher level of natural immunity, we can still achieve the same magnitude of number of infections starting September 2022 as predicted in the base case by increasing the transmissibility of the virus by 11%.
The impact of varying our assumptions about the transmissibility of the virus during our projection period is illustrated for no booster and mRNA-1273.214 in . When we used our base case calibration but increased or decreased the transmissibility of the future VOC, the absolute projected number of infections with no booster increased or decreased accordingly. The percent decreases in 6 months of cases when using the mRNA-1273.214 booster compared to no booster are 33% and 36%, respectively, which compares to 40% in the base case. If the increase in transmissibility of the virus is delayed from August 15 to 15 September 2022, but the fall boosters are still administered beginning in September, the decrease in number of infections with mRNA-1273.214 compared to no booster is 42%.
The impact of varying the assumptions about the projected vaccine uptake for all three booster scenarios is illustrated in . If uptake with the fall booster is 25% of what is predicted in base case, the decrease in the number of infections compared to no booster is lower: 27% with mRNA-1273, 32% with mRNA-1273.214 and 14% with mRNA-1273.222.
Decreasing the waning rate (i.e. increasing the duration of protection) of the bivalent boosters by 25%, 50%, and 75% had a small impact on the percent of infections averted compared to no booster: 41%, 42%, and 43% of infections, respectively, were prevented with mRNA-1273.214 while 18%, 18% and 19% respectively were prevented with mRNA-1273.222. Although increasing masking from the base case decreased the absolute number of infections by 41%, it had minimal impact on the proportion prevented compared to no booster: the percentage decrease remained at 34%, 40%, and 18% for mRNA-1273, mRNA-1273.214, and mRNA-1273.222, respectively.
Using an alternate source to inform the proportion of infections that are symptomatic or the age-specific hospitalization rates had no effect on total number of infections prevented by each of the boosters; likewise, including Paxlovid treatment only affected number of hospitalizations. Percentage decrease compared to no booster for each vaccine are presented in .
Table 6. Impact of varying inputs related to the consequences of infections on hospitalization results over 6 months.
Discussion
We conducted an analysis using mathematical modeling to compare three different booster strategies that involved providing an additional booster dose in Fall 2022 to adults who had previously been vaccinated with at least a primary series. The options were: (1) the licensed monovalent mRNA-1273; (2) the candidate bivalent mRNA-1273.214; or (3) the candidate bivalent mRNA-1273.222 updated for BA.4/5. We compared all of these to the counter-factual of no additional boosting. As additional development time would be required for the mRNA-1273.222 bivalent booster, we assumed that boosting would start 2 months later than boosting with the other two vaccines.
Our analysis demonstrated that vaccinating with the bivalent mRNA-1273.214 was more effective over a 6-month period in preventing infections with a BA.4/5 subvariant than the tailored vaccine, simply because it could be deployed earlier. By September 2022, our model predicts that vaccine-induced protection against infection is quite low, especially in those who have only received a primary series or one booster. The earlier deployment allows reduction in circulating virus in the population sooner and prevents an exponential rise in the number of infections. This result was consistent with one of our sensitivity analyses in which the mRNA-1273.214 booster was even more effective in reducing infections if the BA.4/5 wave is delayed by 1 month. Focusing on the initial 6-month period when healthcare systems are the most pressured, vaccinating with either the monovalent mRNA-1273 or the bivalent-1273.214 is more effective than delaying to boost with a BA.4/5-specific vaccine (mRNA-1273.222). Reduction in infections is important because it leads to a decrease in the number of hospitalizations across the population, even amongst those who are not vaccinated or up to date on the recommended boosters.
Our analysis also demonstrates that the candidate bivalent mRNA-1273.214 has substantial incremental benefits in terms of infections and hospitalizations compared to the licensed monovalent mRNA-1273. Based on antibody titers, we estimated a 11.8% difference in initial VE against infection, and 6.4% difference against severe disease against BA.4/5, using the same waning rate over time. Although it is not known how these vaccines will protect against future VOCs, current immunogenicity data suggests that bivalent vaccines offer a broader protection across current VOCs and are potentially more durable, compared to first generation monovalent vaccines. Using neutralizing antibody titer levels, mRNA-1273.214 was shown to elicit higher GMTs at day 29 compared to mRNA-1273 against ancestral and all variants tested (Alpha, Beta, Delta, Gamma, BA.1, and BA.4/5)Citation30.
Data on VE for mRNA-1273, BNT162b2, and AD26.COV2.S administered prior to 15 June 2022, were obtained from published meta-analyses, adding strength to our data. However, there are a lack of VE data for BA.4/5 since a new VOC is often dominating by the time the study data are disseminated. Extensive work has been done on algorithms to predict, with some degree of certainty, VE against current strains based on GMT levelsCitation18. The VEs against BA.4/5 for the fall booster mRNA-1273, candidate mRNA-1273.214 and mRNA-1273.222 bivalent vaccines are not known, and relative GMT levels were used instead. Therefore, all fall booster VEs were derived using the same method. Real world effectiveness studies will quantify the impact of these vaccines against BA.4/5 infection, however, this modelling exercise allows us to understand the relative impact of the different boosting policies. Waning rates for the bivalent vaccines were assumed to be the same as monovalent primary series rates, which is a conservative estimate, as there is emerging evidence suggesting a bivalent vaccine maintains the duration of protection longerCitation29. Sensitivity analyses decreasing the waning rate of mRNA-1273.214 by 25–75% (i.e. assuming more durable protection) of the base case value increased the number of prevented infections compared to no booster from 40% to 41–43%. Decreasing the coverage rate by 25% had a larger impact, with the percent of infections prevented with mRNA-1273.214 compared to no booster falling to 32%.
The remaining sensitivity analyses did not greatly alter the impact of the vaccines, suggesting they were not key drivers of the model results. Increasing the proportion masking decreased cases of infection compared to the base case but did not change the percentage reduction in infections compared to no booster with masking. Varying the inputs to the infection consequences model, including the estimates for proportion of infections that are symptomatic, age-specific hospitalization rates, or including Paxlovid had minimal impact on the percentage decreases in hospitalization.
With the emergence of new variants, key characteristics of the virus that affect estimates of spread and clinical impact, such as transmissibility, disease severity, risk of reinfection, and vaccine effectiveness also evolve, making estimation of these parameters difficult, especially in heterogeneous populations. While mathematical models have had some success at predicting the course of infections across a 1-month time horizon, longer-term predictions have been challengingCitation12. Longer-term projections also necessitate assumptions about the emergence of future VOCs and their characteristics, as well as the population’s behavior to protect themselves in reaction to their emergence, which are inherently difficult to predict over the long-term. As we demonstrated in our sensitivity analyses, higher proportions of natural immunity in the population will blunt the number of infections caused by future variants. However, it has been observed that natural immunity obtained from past variants such as Delta may be significantly reduced with newer variants such as Omicron BA.1Citation31. It is not possible to know how well infections with past variants protect against future variants. Even with different levels of natural immunity, the magnitude of infection incidence also depends on the transmissibility of future VOCs. Given the unknowns, we acknowledge that our projections will not represent the incidence that will be seen in a heterogeneous and geographically diverse country like the US. However, our projections of the impact of the vaccine have shown that a fall booster is useful under multiple scenarios to prevent both illness and hospitalizations.
Future work will include updating the model and estimates with data as they emerge, conducting a cost-effectiveness analysis on the use of different boosters, as well as examining the impact of adverse events such as vaccine-associated compared to COVID-19-associated myocarditis.
Conclusions
We conclude that providing a fall booster prevents infections and hospitalizations compared to no further vaccination. A more effective bivalent vaccine will prevent more infections than a monovalent vaccine. Furthermore, the use of any booster prior to the rise in incidence of a new VOC, whether it be a monovalent (mRNA-1273) or next generation bivalent vaccine (mRNA-1273.214) in September 2022 is predicted to be more effective than boosting with a BA.4/5 vaccine (mRNA-1273.222) in November 2022. We conclude that there is no advantage to delay boosting until a BA.4/5 vaccine is available; earlier boosting with mRNA-1273.214 will prevent the most infections and the most hospitalizations.
Transparency
Declaration of financial/other interests
MK is a shareholder in Quadrant Health Economics Inc, which was contracted by Moderna, Inc. to conduct this study. KF, AL, MM and MW are consultants at Quadrant Health Economics Inc. PB and NV are employees of Moderna, Inc. and hold stock/stock options in the company.
Author contributions
AL, KF, MK, MM, PB, NV and MW were involved in study design and interpretation of the analysis. MM programmed the model with quality assurance by MK, AL, and KF. All authors were involved in model estimation. MM, MK and KF conducted the analysis. AL, KF and MK wrote the initial draft of the manuscript, and all remaining co-authors critically revised the manuscript and approved the final version.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (356.9 KB)Acknowledgements
None.
Data availability statement
There is no data set associated with this publication.
References
- World Health Organization (WHO). Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC). Interim statement on the composition of current COVID-19 vaccines. 2022. https://www.who.int/news/item/17-06-2022-interim-statement-on–the-composition-of-current-COVID-19-vaccines
- COVID-19 Weekly Epidemiological Update. Edition 95. 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- Centers for Disease Control and Prevention. Variant proportions 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- Hadfield J, Megill C, Bell SM, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34(23):4121–4123. PubMed PMID: 29790939; PubMed Central PMCID: PMCPMC6247931.
- MODERNA. Moderna announces clinical update on bivalent COVID-19 booster platform 2022. https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-Clinical-Update-on-Bivalent-COVID-19-Booster-Platform/default.aspx
- MODERNA. New Release: Moderna announces Omicron-containing bivalent booster candidate mRNA-1273.214 demonstrates superior antibody response against omicron. 2022. https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-Omicron-Containing-Bivalent-Booster-Candidate-mRNA-1273.214-Demonstrates-Superior-Antibody-Response-Against-Omicron/default.aspx
- Hoge S. mRNA-1273.214 Moderna COVID-19 Invetigational bivalent vaccine C(original + Omicron). Presentation to the Vaccines and Related Biological Products Advisory Committee. 2022. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-june-28-2022-meeting-announcement
- European Medicines Agency. EMA regular press briefing on COVID-19. 2022. https://www.ema.europa.eu/en/events/ema-regular-press-briefing-covid-19-19
- FDA U.S. Food & Drug Administration. Vaccines and related biological products advisory committee June 28, 2022 Meeting Announcement. 2022. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-june-28-2022-meeting-announcement
- Shiri T, Evans M, Talarico CA, et al. Vaccinating adolescents and children significantly reduces COVID-19 morbidity and mortality across all ages: a Population-Based modeling study using the UK as an example. Vaccines. 2021;9(10):1180.
- Institute for Health Metrics Evaluation (IHME). COVID-19 Projections. United States of America. Used with permission. 2022. https://covid19.healthdata.org/united-states-of-america
- Lessler J. COVID-19 Scenario Modeling Hub. Round 13: Planning scenarios projecting COVID-19 burden March 2022-March 2023 under current vaccination policy. Presentation to the Vaccines and Related Biological Products Advisory Committee meeting. 2022. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-june-28-2022-meeting-announcement
- Centers for Disease Control and Prevention. Interim updated planning guidance on allocating and targeting pandemic influenza vaccine during an influenza pandemic. 2018. https://www.cdc.gov/flu/pandemic-resources/pdf/2018-Influenza-Guidance.pdf
- Institute for Health Metrics and Evaluation (IHME). COVID-19 model update: Omicron and waning immunity. 2021. www.healthdata.org
- Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 vaccines against the omicron (B.1.1.529) variant of concern. medRxiv. 2021.
- Pratama NR, Wafa IA, Budi DS, et al. Effectiveness of covid-19 vaccines against SARS-CoV-2 omicron variant (B.1.1.529): a systematic review with Meta-analysis and Meta-regression. medRxiv. 2022.
- UK Health Security Agency. COVID-19 vaccine surveillance report. Week 24. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1083443/Vaccine-surveillance-report-week-24.pdf
- Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211.
- Hogan AB, Wu SL, Dooha P. Imperial College COVID-19 response team. Report 48: the value of vaccine booster doses to mitigate the global impact of the Omicron SARS-CoV-2 variant. 2021. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-48-global-omicron/
- Chalkias S, Harper C, Vrbicky K, et al. A bivalent Omicron-containing booster vaccine against Covid-19. N Engl J Med. 2022. https://doi.org/10.1056/NEJMoa2208343
- Khoury DS, Steain M, Triccas JA, et al. A Meta-analysis of early results to predict vaccine efficacy against omicron. medRxiv. 2021.
- Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against covid-19. medRxiv. 2022.
- Pilz S, Theiler-Schwetz V, Trummer C, et al. SARS-CoV-2 reinfections: overview of efficacy and duration of natural and hybrid immunity. Environ Res. 2022;209:112911.
- Centers for Disease Control and Prevention. COVID data tracker. 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographic
- Reese H, Iuliano AD, Patel NN, et al. Estimated incidence of coronavirus disease 2019 (COVID-19) illness and Hospitalization-United States, February-September 2020. Clin Infect Dis. 2021;72(12):e1010–e1017.
- Ma Q, Liu J, Liu Q, et al. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and Meta-analysis. JAMA Netw Open. 2021;4(12):e2137257.
- Wang L, Berger NA, Kaelber DC, et al. COVID infection rates, clinical outcomes, and racial/ethnic and gender disparities before and after omicron emerged in the US. medRxiv. 2022.
- Qasmieh SA, Robertson MM, Teasdale CA, et al. The prevalence of SARS-CoV-2 infection and uptake of COVID-19 antiviral treatments during the BA.2/BA.2.12.1 surge, New York city, April-May 2022. medRxiv. 2022.
- Chalkias D, Eder F, Khetan S, et al. Safety, immunogenicity and antibody persistence of a bivalent beta-containing booster vaccine. 2022. https://www.researchsquare.com/article/rs-1555201/v1
- Barbut F, Galperine T, Vanhems P, et al. Quality of life and utility decrement associated with Clostridium difficile infection in a French hospital setting. Health Qual Life Outcomes. 2019;17(1):6.
- Head E, van Elsland S. Omicron largely evades immunity from past infection or two vaccine doses [updated 17 December 2021; cited 2022 June 30]. https://www.imperial.ac.uk/news/232698/omicron-largely-evades-immunity-from-past/