Abstract
Aims
The goal of this study was to review the economic evaluations of health technologies in multiple myeloma (MM) and provide guidance and recommendations for future health economic analyses.
Materials and methods
A systemic literature review (SLR) was conducted on original economic assessment studies and structured review papers focusing on the studies in MM. The search was limited to English language papers published from 1 January 2000 onwards. Publications not applying any type of modelling methodology to describe disease progression and patient pathways over a specific time horizon were excluded.
Results
A total of 2,643 publications were initially identified, of which 148 were eligible to be included in the full-text review phase. From these, 49 publications were included in the final analysis. Most published health economic analyses supported by models came from high-income countries. Evaluations from middle-income countries were rarely published. Diagnostic technologies were rarely modelled and integrated care had not been modelled. Very few models investigated MM treatments from a societal perspective and there was a relative lack of evaluations regarding minimal residual disease (MRD).
Limitations
Limitations of the publications included differences between trial populations and modelled populations, justification of methods, lack of confounder analyses, and small trial populations. Limitations of our study included the infeasibility of comparing MM economic evaluations due to the significant variance in modelled therapeutic lines and indications, and the relative scarcity of published economic evaluations from non-high-income countries.
Conclusions
As published economic models lacked many of the elements of the complex and heterogeneous patient pathways in MM and they focused on single decision problems, a thorough, open-source economic whole disease modelling framework is needed to assess the economic value of a wide range of technologies across countries with various income levels with a more detailed view on MM, by including patient-centric and societal aspects.
Introduction
Multiple myeloma (MM) is a type of plasma cell neoplasm that represents about 10–15% of all hematological malignancies, ranking 2nd in prevalence after non-Hodgkin lymphoma within this subgroup. The annual age-adjusted incidence rate is 5.6–7 cases per 100,000 person-years resulting in an estimated 32,000 yearly new cases in the United States (US) and 160,000 worldwide, leading to 13,000 yearly deaths in the US and 106,000 worldwide attributed to MM. The disease primarily affects the elderly with a median age at diagnosis of 65–74 yearsCitation1–3. MM is an incurable, progressive condition; however, with recent therapeutic advancements, survival rates have significantly increased over the past 30 yearsCitation4–6. The outcomes of MM patients are heterogeneous, depending on the age, overall fitness, affected organs and other important prognostic factors. On the other hand, a wide range of therapeutic options are available, including autologous stem cell transplantation (SCT) and various anti-tumour drug and cellular therapiesCitation7,Citation8. Due to these factors, the patient pathways in MM can be extremely complex. Capturing this heterogeneity in an economic model is a substantial challenge. Generally, most health economic models only focus on the economic analysis of a single decision and only vaguely consider the broader contextCitation9. Very few models exist across all disease areas that investigate treatments from a societal perspective instead of the payer perspective. Economic modelling should strive to be designed in a comprehensive way to assess the added value of specific disease biomarkers to fully capture the real-world value of those biomarkers and the disease in generalCitation10.
Whole disease modelling (WDM) is a way to look at the “broader picture” by simulating entire disease and treatment pathways in detail within a single modelCitation9. For example, WDM may include preclinical and post-diagnostic elements in an attempt to capture different service pathways for specific subgroups of patients. It not only represents events, costs and outcomes, but also attempts to present the structural relationships between these, even allowing for the use of multiple economic decision rules. Recent appearance of several new treatment options, expected uptake of technologies for response assessment (such as MRD) and prognostic parameters (like iFISH, gene expression-based risk assessment or MRD), potential uptake of integrated care solutions provides basis for improving the comparability of value judgement of various technologies by the development of an WDM in MM.
This systemic literature review (SLR) was developed under the umbrella of the Beyond Medicines’ Barriers (BMB) Consortium, which was established in January 2020.
Rationale and research objectives
The objective of this research was to review existing economic evaluations of health technologies in MM and provide guidance and recommendations for future health economic analyses. The ultimate objective was to support the development of a whole disease model by synthesizing the knowledge from published developed economic models. The study aimed to answer the following questions: What type of economic evaluation methodology was used by previous economic studies in MM? What are the main assumptions and characteristics of economic assessments that are developed in MM? What are the differences between the economic assessments used in various jurisdictions?
Methods
To support the aim of developing a whole disease model for MM, an SLR was conducted on original economic assessment studies and structured review focusing on the studies in MM using the following databases: Medline, Scopus, Cochrane Database of Systematic Reviews, the National Institute for Health Research (NIHR) PROSPERO and Centre for Reviews and Dissemination (CRD) databases, the Center for Evaluation of Value and Risk in Health (CEVR) Cost-Effectiveness Analysis (CEA) Registry. Websites of Health Technology Assessment (HTA) Agencies, namely Canadian Agency for Drugs and Technologies in Health (CADTH) and the National Institute of Health and Care Excellence (NICE) from England was also searched. The search was limited to English language papers published from 1 January 2000 onwards. International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Conference abstracts were also included from the years 2017 to 2021, and the International ISPOR Conference from May 2022. Details of the search in ISPOR conference abstracts are described in Supplementary Table 1.
Eligibility criteria
Publications were eligible to be included in the study if they were either economic assessment studies or structured review economic assessment studies in MM. Systematic, targeted and scoping reviews were considered structured review papers. Publications not applying any type of modelling methodology to describe disease progression and patient pathways over a specific time horizon were excluded (e.g. descriptions of a single case).
Search strategy
The search strategy was built up as a combination of search strings, allowing the capture of all relevant keywords and synonyms that appeared in the publications. In order to cover the time period between the date the first search was conducted (13 January 2020) and the actual date the project started (fall of 2020), the same search strings were used once again to update the search in the same databases, only for the year 2020. The search strings are presented in Supplementary Tables 2–4. Due to the significant changes in the NICE database during the year this research was conducted, the decision was made to re-run the entire search first in the fall of 2020 on that particular platform. This was repeated on 17 June 2022.
Screening process
After the removal of duplicates, the title and abstract screening took place using the software Endnote. The title and abstract screening of all identified and de-duplicated papers was conducted by two independent researchers. Disagreements between reviewers were resolved by the independent principal researcher. The following exclusion criteria were hierarchically applied during title-abstract screening:
No English abstract;
Not related to myeloma;
Not full economic evaluation or disease model (e.g. cost-comparison without assessment of health benefits);
Not relevant for economic modelling purposes.
Articles not fitting any of these exclusion criteria were eligible for full text screening. Only articles that were selected for full text screening were downloaded and stored in the cloud storage.
The full text screening phase was also conducted by two independent researchers, and disagreements between reviewers were once again resolved by the principal researcher. During full text screening, articles were excluded if they fit in any of the following exclusion criteria:
Duplication;
No English full text;
Not related to myeloma;
Not economic evaluation or disease model;
Not relevant for economic modelling purposes including abstract or commentary;
Not original paper (e.g. model framework already involved in review without major changes in criteria) or
No detailed model description
Other specified reasons (e.g. budget impact model)
Articles which did not fit any of the previous exclusion criteria were included in the data extraction phase.
Data collection process
A pilot, annotated, data extraction sheet was circulated to all reviewers, together with an example extraction of a study. Reviewers carried out pilot extraction of a study that had been selected at random. Following the pilot extraction, the data extraction grid was finalized according to the comments of the reviewers. Data from included studies were extracted by two independent reviewers followed by quality assessment of the included studies. Data extraction was limited to data detailed in section on data items. Extracted data from all included studies were double-checked by a second reviewer. If more than one publication was based on the exact same model, the research group aimed to find and keep the initial country adaptation only. If the same “core” model underwent significant changes over the years, multiple publications, one per each of the different versions were kept in the analysis. It is important to note that only assumed structural changes were taken into account, not the necessary level of changes required for various local adaptations (e.g. changing the cost inputs).
The following information was extracted from each included study, in order to support our efforts in developing a whole disease model for MM:
the first author and year of publication;
country/region of (planned) implementation;
Perspective of the analysis
Health care perspective
Societal perspective (includes cost elements that occur at stakeholders other than the health care payer organizations);
Modell type/design;
Modell structure;
Health states;
Therapeutic line at which patients enter the model;
Number of therapeutic lines are included in the model;
Type of technologies being investigated in the model;
Utility estimates linked to different health states or parameters;
Time horizon;
Key clinical parameters modelled.
Quality assessment of included studies
Quality assessment increases the robustness of research findings in SLRs. Methodological quality of included economic modelling studies were evaluated using the Quality of Health Economic Studies (QHES) instrumentCitation11. Disease progression models were evaluated using the CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS)Citation12, as applicable. The answers for each criterion included in the checklists were summarized in a Microsoft Excel spreadsheet. The results of the quality assessment were not considered as basis for exclusion of studies, it was rather used to explore potential connections between the quality of reporting and other investigated aspects (as listed above).
Results
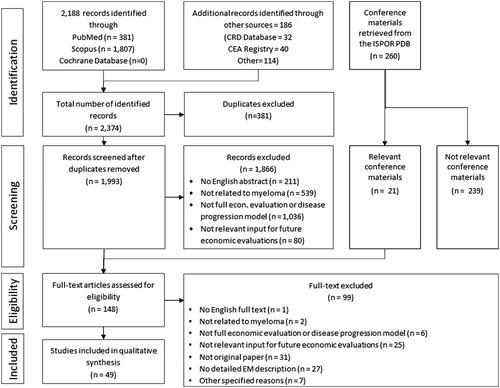
A total of 2,643 publications were initially identified, of which 148 were eligible to be included in the full-text review (FTR) phase. Within this step, 79 studies were excluded at this phase for various reasons (not being related to myeloma, not being full economic evaluations or disease progression models, not containing relevant input for future economic evaluations, etc.). A total of 49 studies were selected for data extraction, with eight from HTA agencies (i.e. NICE and CADTH) and four scientific posters from the ISPOR conferences. Based on the QHES quality assessment tool, 77% (38/of 49) of the included studies were rated high quality and 11 studies were assessed as not high quality. The list and key details of the included studies are available in Supplementary Table 5Citation13–Citation61 ( and ).
Modelling approaches
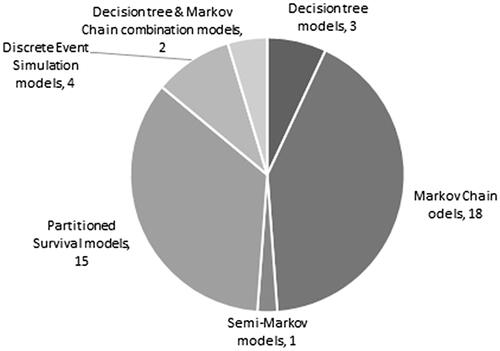
The most common modelling methods applied were Markov chain and partitioned survival, in 18 and 15 studies, respectively. Other models used discrete event simulation, decision tree and various combinations of modelling methods. The three-health state approach of pre-progression, post-progression and death was used in 58% of the Markov and partitioned survival models. Regarding the time horizon of the models, 90% of the studies used time horizons of at least 10 years or lifetime length. A significant number of papers (18 models) did not specify the cycle lengths of their model, 16 studies used 4-week long cycles, three studies worked with 3-week long cycles and eight studies used 1-week long cycles ().
Therapeutic lines
There was great variance observed in the therapeutic line at which patients entered the model and across the total number of lines incorporated in the model. In a substantial number of the studies (in 17 cases), it was not explicit or clear which therapeutic line the patient entered the model at or how many therapeutic lines were included in the model (20 studies). Patients entered the first line in 23 studies, the 2nd line in six studies, in the 3rd line in three studies.
Treatment types and used compounds
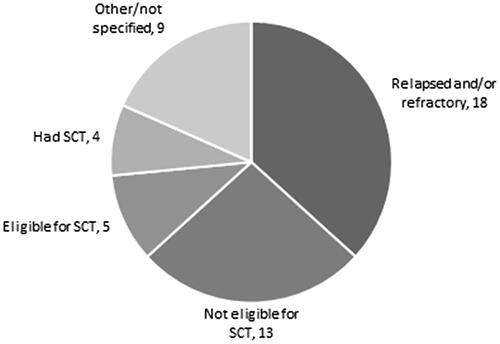
The included models assessed a number of different indications. Eighteen studies examined patients with relapsed or refractory MM, five papers concentrated on patients eligible for SCT, four studies modelled patients who had SCT before, 13 models examined patients who were not eligible for SCT and nine studies used other or not-specified indications. Due to the complex nature of certain indications, we grouped them together into basic categories in the following figure ().
Most analyses compared conventional health technologies, provided to a patient with the main intent of fully or partially resolving an illness; therefore, not as a diagnostic procedure, best supportive care (BSC), or integrated care intervention. Diagnostic technologies were only modelled in three cases. The use of combination therapies, and multiple therapeutic arms in the same models resulted in inclusion of dexamethasone on 104 occasions. Bortezomib was included in 53 modelled therapeutic arms, while lenalidomide was included in 42. Further included compounds were daratumumab (24), prednisone (23), melphalan (14), and thalidomide (16).
Clinical parameters
Only two models incorporated the clinical features. Blommestein et al.Citation13 modelled World Health Organization (WHO) status, International Staging System (ISS) status, serum-β2 microglobulin, albumin level, hemoglobin, creatinine, platelets, lactate dehydrogenase (LDH), serum calcium, Salmon Durie stage, comorbidity and other malignancies. Möller et al.Citation14 modelled Eastern Cooperative Oncology Group (ECOG) performance status, M-protein, β2 microglobulin, albumin, plasmacytomas, creatinine level, the ratio of platelet count plasma cells and the number of prior relapses. A novel topic, minimal residual disease (MRD) was incorporated into only four models.
Perspectives used
Most studies took healthcare/payer perspectives accounting for costs and benefits over a lifetime horizon. Only four studies were identified that took a societal perspective for the analysis. Borg et al.Citation15 included patient and caregiver productivity loss, Prinja et al.Citation16 looked at patient and caregiver direct costs, and Raje et al.Citation17 focused on quality-adjusted life years (QALY) monetization and direct non-medical costs (driving and parking) and indirect costs such as short-term disability and productivity loss. Lazzaro et al.Citation18 calculated with out-of-pocket and productivity loss, in addition to caregiver burden.
Observations by region, income status, and sponsorship
The vast majority of the studies were applied to Western countries. Twenty-three models were developed for North America, 26 studies examined Western Europe, two studies concentrated on Central-Eastern-Europe and two on Latin America and the Caribbean, four studies were based in Asia, while two studies took a multi-country approach ().
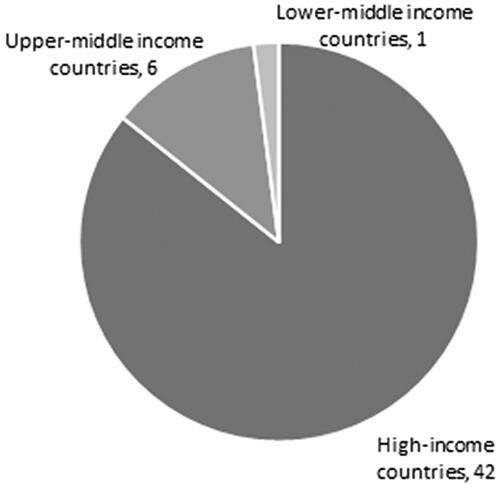
More than 85% of the models concentrated on high-income countries according to the latest (2019–2020) World Bank ClassificationCitation62. One model came from a lower-middle income country (India), and six models came from upper-middle income countries (one each from Brazil, North Macedonia, Serbia, and three from China). These seven non-high income country models examined different MM treatments throughout the MM disease pathway. One model incorporated a decision tree, one combined a decision tree and a Markov Chain, and the remaining five used Markov Chains. Six (5) of these seven studies took healthcare/payer perspectives, and one used a societal perspective. Based on the QHES quality assessment tool, reporting quality among these seven studies was on par with the average of higher income countries, with two cases not being of high quality.
In three out of seven cases (43%), the studies developed outside the high-income countries came from research team without any reported sponsorship from industry. Overall, we found 17 non-sponsored publications among the 49 included studies, therefore, the rate of industry sponsored models was higher in high-income countries. Overall, two pharmaceutical companies were sponsors of nearly a third of included studies. Of the four studies that utilized a societal perspective, only one was not sponsored by industry.
Utilities of health states
Van Agthoven et al.Citation63 was the most common source cited for health state utilities. If available and applicable, utilities were extrapolated from the relevant clinical trials. It was assumed that these utility values would be valid for the study jurisdiction’s population. Utility adjustments were made for factors such as on/off treatments, adverse events, and specific line of treatment. Due to the variability in utility sources used and adjustments, cross-model comparison was not feasible.
Discussion
In order to support efforts in building a whole disease model for MM, a substantial number of health economic models investigating MM interventions were identified in the scientific literature. The number of published models increased in the last decade. Significant heterogeneity was observed across modelling methods in terms of methodology, though we noted several common elements in the approaches. Partitioned survival modelling was the most frequently applied approach, which was also observed by a previous SLR on MM economic evaluations by Asrar et al.Citation64
Most models focused on the value assessment of new treatments. Technologies for response assessment (such as MRD) were only included in one model, and complex solutionsCitation65 (such as integrated care with personalized pathway system to manage patients from first symptoms of MM to more advanced disease stages by a multidisciplinary team of health and social care professionals) and prognostic parameters (like iFISH, gene expression-based risk assessment or MRD) were not modelled.
Economic evaluations in MM have primarily taken a healthcare/payer perspective, in adherence to minimal requirements of country-specific HTA regulatory body guidelines. Individual level decision-making aspects were not modelled. This traditional health economic scope can potentially underestimate the full value of MM technologies to patients and the society.
Overall, we found that the capacity of published models to investigate the complex and heterogeneous patient pathways in MM throughout the entire disease was limited.
Limitations of economic evaluations
There were several main limitations identified in the economic evaluations that could have potentially affected the results. These include differences between trial populations and modelled populations, lack of utility data specific to the modelled population, extrapolating outcomes beyond trial data, absence of justification for the chosen modelling approach, lack of head-to-head clinical trial data on modelled interventions and small trial populations. In most cases, assumptions and limitations of the studies were stated, but potential biases were rarely discussed and there was a deficiency of confounder analyses. None of the identified models used complex methodology, such as WDM, which is capable of assessing various interventions throughout the entire patient pathway.
Limitations of our study
There were some limitations associated with our own study. Drawing general conclusions regarding the cost-effectiveness of certain health technologies was not feasible to compare between studies due to the use of different indications, different comparators, and adhering to the settings of different jurisdictions. Cost data are always country-specific; therefore, the results of cost-effectiveness analyses are country-specific as wellCitation66. Thus, we are not aiming to make general conclusions on e.g. the cost-effectiveness of certain therapies, as it is highly country-specific.
Comparability between MM economic evaluations was also not feasible because of the significant variance seen in therapeutic lines and indications modelled. Publication bias was an issue, as authors were more likely to publish results favouring a particular health technology, especially in the settings of high-income countries. Published economic evaluations were relatively scarce for non-high-income countries, which aligned with previous research illustrating a gap in HTA capacity in low- and middle-income countriesCitation67. As we strive to provide global access to novel therapies to the myeloma community, the lack of geographical scope in MM HTAs is definitely among our key concerns.
Our study was restricted to English language papers and the search included only selected HTA agencies and ISPOR conferences from 2017 to 2021 and early 2022; thus, economic evaluations not published in English or published earlier were not included. The QHES tool utilized for methodological quality assessment was considered an appropriate instrument, as it was designed and validated to support prompt, accurate initial assessments of health economic study quality; however, several points on the checklist may be open to interpretation by different reviewers. This was partially mitigated by having two reviewers for each study. Alternative instruments, such as the checklist of Drummond and Jefferson for assessing economic evaluationsCitation68, could also lead to different results. However, despite the aforementioned limitations and heterogeneity, we were able to make several key conclusions regarding the modelling methods used.
Similar studies
To our knowledge, this is the most comprehensive systematic review on models developed for MM that were employed in various jurisdiction across the world. Compared to previous SLRs on MM, our study put more emphasis on the geographical distribution of conducted MM economic evaluations to assess the prevalence of disparities across nations with different income levels. In addition to the generally investigated characteristics of applied modelling methods (i.e. model structure, utilities applied, time horizon, clinical parameters modelled), we also strived to assess novel aspects, for example, the use of MRD or molecular prognostication.
Our study made several observations similar to Asrar et al. For example, the majority of MM economic evaluations took a healthcare/payer perspective and incorporated a time horizon of at least 10 yearsCitation64. Gaultney et al. evaluated MM economic evaluations through 2009Citation19, noting it was difficult to assess quality with the Drummond checklistCitation69 due to inadequate descriptions of the analyses. It was unclear whether this was attributed to poor methodology or documentation. Aguiar et al. cited poor compliance of MM economic evaluationsCitation70 to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklistCitation71. Similarly, our study also noted a consistent lack of clarity in studies regarding the number of therapeutics lines modelled and where patients entered models; however, this deficiency may not have been fully reflected by the QHES tool. Rochau et al. noted the numerous possible sequencing of MM medications could significantly hinder cost-effective treatment allocation as only a small subset of therapeutic sequences can be tested in classic randomized trialsCitation72. Cooper et al. compared three MM models, demonstrating that such comparisons were indeed feasible, and hinted at the possibility of reference models aiding decision-makers in certain disease areasCitation73.
Gonzalez-McQuire et al. conducted an SLR to guide the development of an MM conceptual model that could be applied in MM economic modellingCitation74. Their conceptual model focused on the relevance of disease and patient characteristics on MM progression and clinical outcomes; a Delphi panel of hematologists emphasized the significance of disease activity (i.e. M-protein, serum-free light chain levels, bone marrow plasma cell count). As noted in our SLR, these disease activity markers were rarely tracked in the previously developed MM health economic models. To our knowledge between our literature search date and the submission of this manuscript, no papers on whole disease models have been published, that are capable of covering the entirety of the disease spectrum.
Implications
We identified certain data gaps that can be the subject of future research. These include the need to investigate utilities of MM subgroups, real-world cost analysis (including laboratory tests, imaging, other procedures and cost of human resource), concomitant medication costs, and subsequent therapy patterns (e.g. the length, the therapy choices, and the treatment-free periods between further lines). Van Agthoven et al.Citation63 the most frequently cited study for utilities, was published in 2004. After nearly two decades the question can be raised regarding the current applicability of these utility values, identifying quality of life in MM research as an area of need. There is a demand for individual-based modelling incorporating different patient characteristics and a need for full indirect comparison of competing health technologies. Unfortunately, clinical parameters were hardly incorporated. For example, only one economic evaluation study was identified that assessed MRD and none of the publications addressed molecular prognostication using e.g. iFISH or gene expression based assessment. Very few models investigated treatments and disease from a societal perspective. Currently, published literature lacks high-quality models concentrating on lower-income countries. This relatively low number is most likely a result of the lack of funding available for health economic research in most of these countries.
The future development of an MM whole disease model can contribute to bridging the gap between high-income countries and the rest of the world by introducing a more detailed approach to the field of MM modelling and can expand the scope of research at a societal level. It is anticipated that more complex modelling structures building on the traditional three-stage approach (pre-progression; post-progression; death) will be required. Harmonization of modelling efforts can be suggested for the economic modelling community with the involvement of representatives from HTA agencies from countries with different economic status and health care system archetypes. The harmonization process may cover several other aspects beyond the WDM design, such as recommendation on national adaptation of the core WDM, guidance on which input data have limited transferability, use of default input data (e.g. health utilities) or comprehensive reporting of studies based on the WDM.
Conclusions
Scientific publications were more likely to come from high-income countries and lacked many of the elements of the complex and heterogeneous patient pathways in MM. In general, most of the published modelling studies focused on single decision problems. A thorough, open-source economic evaluation framework is needed that is capable of assessing the cost-effectiveness of a wide range of interventions in various jurisdictions, in the spirit of a WDM.
Transparency
Declaration of financial/other relationships
BN, MT, TZ, and ZK are employees of Syreon Research Institute, Budapest, Hungary, and XYJ conducted his internship there. Syreon received funding from the International Myeloma Foundation for performing the SLR and conducting the analysis. MCQ is an employee of the International Myeloma Foundation. JLH and DH declare no conflict of interest. The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions
BN, MCQ, TZ, MT, XYJ and ZK made substantial contributions to the conception and design of this study. Data acquisition and analysis was conducted by BN, MT, XYJ, TZ, and ZK. All authors contributed to the interpretation of data, took part in the drafting and revising of the manuscript, and gave final approval of the manuscript to be published. All authors agree to be accountable for all aspects of this work.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Previous presentations
A scientific abstract on this research was presented to ISPOR 2021 Annual Conference (May 17-20, 2021).
Supplemental Material
Download MS Word (15.1 KB)Supplemental Material
Download MS Word (17.4 KB)Supplemental Material
Download MS Word (17.5 KB)Supplemental Material
Download MS Word (17.9 KB)Supplemental Material
Download MS Word (27.7 KB)Acknowledgements
Editorial assistance in the preparation of the article was provided by Laconic Medical Writing Inc.
Additional information
Funding
References
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060.
- Bray F, Ferlay J, Soerjomataram I, et al. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
- Andres M, Feller A, Arndt V, et al. Trends of incidence, mortality, and survival of multiple myeloma in Switzerland between 1994 and 2013. Cancer Epidemiol. 2018;53:105–110.
- Braunlin M, Belani R, Buchanan J, et al. Trends in the multiple myeloma treatment landscape and survival: a U.S. analysis using 2011–2019 oncology clinic electronic health record data. Leuk Lymphoma. 2021;62(2):377–386.
- Chang-Chan DYL, Ríos-Tamayo R, Rodríguez BM, et al. Trends of incidence, mortality and survival of multiple myeloma in Spain. A twenty-three-year population-based study. Clin Transl Oncol. 2021;23(7):1429–1439.
- Kumar SK, Callander NS, Hillengass J, et al. NCCN guidelines insights: multiple myeloma, version 1.2020. J Natl Compr Canc Netw. 2019;17(10):1154–1165.
- Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl. 4):iv52–iv61.
- Tappenden P, Chilcott J, Brennan A, et al. Whole disease modeling to inform resource allocation decisions in cancer: a methodological framework. Value Health. 2012;15(8):1127–1136.
- Oosterhoff M, van der Maas ME, Steuten LMG. A systematic review of health economic evaluations of diagnostic biomarkers. Appl Health Econ Health Policy. 2016;14(1):51–65.
- Ofman JJ, Sullivan SD, Neumann PJ, et al. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9(1):53–61.
- Moons KGM, Groot J, Bouwmeester W, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10):e1001744.
- Blommestein HM, Verelst SGR, de Groot S, et al. A cost-effectiveness analysis of real-world treatment for elderly patients with multiple myeloma using a full disease model. Eur J Haematol. 2016;96(2):198–208.
- Möller J, Nicklasson L, Murthy A. Cost-effectiveness of novel relapsed-refractory multiple myeloma therapies in Norway: lenalidomide plus dexamethasone vs bortezomib. J Med Econ. 2011;14(6):690–697.
- Borg S, Nahi H, Hansson M, et al. Cost effectiveness of pomalidomide in patients with relapsed and refractory multiple myeloma in Sweden. Acta Oncol. 2016;55(5):554–560.
- Prinja S, Kaur G, Malhotra P, et al. Cost-effectiveness of autologous stem cell treatment as compared to conventional chemotherapy for treatment of multiple myeloma in India. Indian J Hematol Blood Transfus. 2017;33(1):31–40.
- Raje N, Roodman GD, Willenbacher W, et al. A cost-effectiveness analysis of denosumab for the prevention of skeletal-related events in patients with multiple myeloma in the United States of America. J Med Econ. 2018;21(5):525–536.
- Lazzaro C, Castagna L, Lanza F, et al. Chemotherapy-based versus chemotherapy-free stem cell mobilization (±plerixafor) in multiple myeloma patients: an Italian cost-effectiveness analysis. Bone Marrow Transplant. 2021;56(8):1876–1887.
- Gaultney JG, Redekop WK, Sonneveld P, et al. Critical review of economic evaluations in multiple myeloma: an overview of the economic evidence and quality of the methodology. Eur J Cancer. 2011;47(10):1458–1467.
- Aceituno S, Gozalbo I, Appierto M, et al. Cost-effectiveness of lenalidomide in combination with dexamethasone compared to bortezomib in combination with dexamethasone for the second-line treatment of multiple myeloma in Chile. Medwave. 2018;18(3):e7220.
- Asano E, Maiolino A, Martins E. Treatment sequencing for patients with multiple myeloma with at least one prior line: comparing progression-free survival and costs under a private payer perspective. Value Health. 2017;20(9):A876.
- Brown RE, Stern S, Dhanasiri S, et al. Lenalidomide for multiple myeloma: cost-effectiveness in patients with one prior therapy in England and Wales. Eur J Health Econ. 2013;14(3):507–514.
- Büyükkaramikli NC, de Groot S, Fayter D, et al. Pomalidomide with dexamethasone for treating relapsed and refractory multiple myeloma previously treated with lenalidomide and bortezomib: an evidence review group perspective of an NICE single technology appraisal. Pharmacoeconomics. 2018;36(2):145–159.
- Cai H, Zhang L, Li N, et al. Cost-effectiveness analysis on binary/triple therapy on the basis of ixazomib or bortezomib for refractory or relapsed multiple myeloma. Leuk Lymphoma. 2019;60(12):2951–2959.
- Cao Y, Zhao L, Zhang T, et al. Cost-effectiveness analysis of adding daratumumab to bortezomib, melphalan, and prednisone for untreated multiple myeloma. Front Pharmacol. 2021;12:608685.
- Delea TE, El Ouagari K, Rotter J, et al. Cost-effectiveness of zoledronic acid compared with clodronate in multiple myeloma. Curr Oncol. 2012;19(6):392–403.
- Dolph M, Tremblay G, Leong H. Cost effectiveness of triplet selinexor–bortezomib–dexamethasone (XVd) in previously treated multiple myeloma (MM) based on results from the phase III BOSTON trial. Pharmacoeconomics. 2021;39(11):1309–1325.
- Garrison LP Jr., Wang ST, Huang H, et al. The cost‐effectiveness of initial treatment of multiple myeloma in the US with bortezomib plus melphalan and prednisone versus thalidomide plus melphalan and prednisone or lenalidomide plus melphalan and prednisone with continuous lenalidomide maintenance treatment. Oncologist. 2013;18(1):27–36.
- Grima DT, Airia P, Attard C, et al. Modelled cost-effectiveness of high cut-off haemodialysis compared to standard haemodialysis in the management of myeloma kidney. Curr Med Res Opin. 2011;27(2):383–391.
- Hornberger J, Rickert J, Dhawan R, et al. The cost‐effectiveness of bortezomib in relapsed/refractory multiple myeloma: Swedish perspective. Eur J Haematol. 2010;85(6):484–491.
- Jakubowiak AJ, Campioni M, Benedict Á, et al. Cost-effectiveness of adding carfilzomib to lenalidomide and dexamethasone in relapsed multiple myeloma from a US perspective. J Med Econ. 2016;19(11):1061–1074.
- Kaló Z, Vályi-Nagy I, Székely A, et al. PPM6 early phase economic evaluation of new generation sequence diagnostics in multiple myeloma. Value Health. 2020;23:S326–S327.
- Li S, Li J, Peng L, et al. First-line daratumumab in addition to chemotherapy for newly diagnosed multiple myeloma patients who are transplant ineligible: a cost-effectiveness analysis. Clin Ther. 2021;43(7):1253–1264.e5.
- Lu J, Chen W. Cost-effectiveness of lenalidomide plus low-dose dexamethasone for newly diagnosed multiple myeloma patients ineligible for stem cell transplantation in China. J Comp Eff Res. 2019;8(12):979–992.
- Marchetti M, Gale RP, Barosi G. Cost-effectiveness of post-autotransplant lenalidomide in persons with multiple myeloma. Mediterr J Hematol Infect Dis. 2021;13(1):e2021034.
- Muto RL, Lenzi M, Franzini JM, et al. Cost-effectiveness analysis of daratumumab in heavily pre-treated multiple myeloma patients for the Italian Healthcare System. Value Health. 2017;20(9):A442–A443.
- Narsipur N, Bulla S, Yoo C, et al. PCN69 cost-effectiveness of daratumumab added to lenalidomide and dexamethasone for transplant ineligible multiple myeloma. Value Health. 2020;23:S35.
- Narsipur N, Bulla S, Yoo C, et al. Cost-effectiveness of adding daratumumab or bortezomib to lenalidomide plus dexamethasone for newly diagnosed multiple myeloma. J Manag Care Spec Pharm. 2021;27(12):1691–1702.
- Nikolaou A, Ambavane A, Shah A, et al. Belantamab mafodotin for the treatment of relapsed/refractory multiple myeloma in heavily pretreated patients: a US cost-effectiveness analysis. Expert Rev Hematol. 2021;14(12):1137–1145.
- Ollendorf D, Chapman R, Khan S. Treatment options for relapsed or refractory multiple myeloma: effectiveness, value, and value-based price benchmarks. Boston: Institute for Clinical and Economic Review; 2016.
- Olry de Labry Lima A, Gimeno-Ballester V, Ríos Tamayo R, et al. Cost-effectiveness of lenalidomide maintenance in patients with multiple myeloma who have undergone autologous transplant of hematopoietic progenitor cells. Bone Marrow Transplant. 2019;54(11):1908–1919.
- Pandya C, Hashmi S, Khera N, et al. Cost‐effectiveness analysis of early vs. late autologous stem cell transplantation in multiple myeloma. Clin Transplant. 2014;28(10):1084–1091.
- Patel KK, Giri S, Parker TL, et al. Cost-effectiveness of first-line versus second-line use of daratumumab in older, transplant-ineligible patients with multiple myeloma. J Clin Oncol. 2021;39(10):1119–1128.
- Pelligra CG, Parikh K, Guo S, et al. Cost-effectiveness of pomalidomide, carfilzomib, and daratumumab for the treatment of patients with heavily pretreated relapsed–refractory multiple myeloma in the United States. Clin Ther. 2017;39(10):1986–2005.e5.
- Qerimi V, Nestorovska AK, Sterjev Z, et al. Cost-effectiveness analysis of treating transplant-eligible multiple myeloma patients in Macedonia. Clinicoecon Outcomes Res. 2018;10:327–338.
- Usmani SZ, Cavenagh JD, Belch AR, et al. Cost-effectiveness of lenalidomide plus dexamethasone vs bortezomib plus melphalan and prednisone in transplant-ineligible US patients with newly-diagnosed multiple myeloma. J Med Econ. 2016;19(3):243–258.
- Uyl D, Groot CA, Ramsden R, et al. Lenalidomide as maintenance treatment for patients with multiple myeloma after autologous stem cell transplantation: a pharmaco‐economic assessment. Eur J Haematol. 2020;105(5):635–645.
- Vukićević Đ, Rochau U, Savić A, et al. Long-term effectiveness and cost effectiveness of multiple myeloma treatment strategies for elderly transplant-ineligible patients in Serbia. Zdr Varst. 2020;59(2):83–91.
- Walzer S, Krenberger S, Vollmer L, et al. A cost impact analysis of clonoSEQ® as a valid and CE-Certified minimal residual disease (MRD) diagnostic compared to no MRD testing in multiple myeloma in Germany. Oncol Ther. 2021;9(2):607–619.
- Yamamoto C, Minakata D, Koyama S, et al. Daratumumab in first-line therapy is cost-effective in transplant-eligible patients with newly diagnosed myeloma. Blood. 2022;140(6):594–607.
- Zeng X, Peng L, Peng Y, et al. Economic evaluation of adding daratumumab to a regimen of bortezomib + dexamethasone in relapsed or refractory multiple myeloma: based on the latest updated analysis of CASTOR. Clin Ther. 2020;42(2):251–262.e5.
- Zeng X, Liu Q, Peng L, et al. Cost-effectiveness analysis of adding daratumumab to a regimen of bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma. Adv Ther. 2021;38(5):2379–2390.
- Zhang TT, Wang S, Wan N, et al. Cost-effectiveness of daratumumab-based triplet therapies in patients with relapsed or refractory multiple myeloma. Clin Ther. 2018;40(7):1122–1139.
- National Institute of Health and Care Excellence (NICE). Bortezomib and thalidomide for the first line treatment of multiple myeloma TA228 27; 2011. Available from: https://www.nice.org.uk/guidance/ta228/resources/bortezomib-and-thalidomide-for-the-firstline-treatment-of-multiple-myeloma-pdf-82600316845765
- National Institute of Health and Care Excellence (NICE). Bortezomib for induction therapy in multiple myeloma before high-dose chemotherapy and autologous stem cell transplantation TA311 23; 2014. Available from: https://www.nice.org.uk/guidance/ta311/resources/bortezomib-for-induction-therapy-in-multiple-myeloma-before-highdose-chemotherapy-and-autologous-stem-cell-transplantation-pdf-82602421404613
- National Institute of Health and Care Excellence (NICE). Panobinostat for treating multiple myeloma after at least 2 previous treatments TA380; 2016 [cited 2016 Jan 27]. Available from: https://www.nice.org.uk/guidance/ta380/resources/panobinostat-for-treating-multiple-myeloma-after-at-least-2-previous-treatments-pdf-82602842988229
- National Institute of Health and Care Excellence (NICE). Isatuximab with pomalidomide and dexamethasone for treating relapsed and refractory multiple myeloma TA658; 2020 [cited 2020 Nov 18]. Available from: https://www.nice.org.uk/guidance/ta658/resources/isatuximab-with-pomalidomide-and-dexamethasone-for-treating-relapsed-and-refractory-multiple-myeloma-pdf-82609205373637
- Canadian Agency for Drugs and Technologies in Health (CADTH). Darzalex in combo with bortezomib, melphalan and prednisone for multiple myeloma (newly diagnosed) – final economic guidance report; 2019 [updated 2019 Sep 16]. Available from: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10148Daratumumab%2BVMPforMM_fnEGR_NOREDACT-ABBREV_Post_29Aug2019_final.pdf
- Canadian Agency for Drugs and Technologies in Health (CADTH). Revlimid (in combo) bortezomib + dexamethasone for newly diagnosed multiple myeloma – final economic guidance report; 2019 [updated 2019 Jul 5]. Available from: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10141LenalidomideBorDexMM_fnEGR_NOREDACT-ABBREV_EC-Post_19Jun2019_final.pdf
- Canadian Agency for Drugs and Technologies in Health (CADTH). Ninlaro for multiple myeloma (2nd-beyond) – final economic guidance report; 2019 [updated 2019 Jul 22]. Available from: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10164IxazomibMM_fnEGR_NOREDACT-ABBREV_Post_05Jul2019_final.pdf
- Canadian Agency for Drugs and Technologies in Health (CADTH). Pomalyst in combination with dexamethasone and bortezomib for multiple myeloma (second-line or beyond) – final economic guidance report; 2019 [updated 2019 Oct 3]. Available from: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10165PomalidomideBortezomibMM_fnEGR_NOREDACT-ABBREV_EarlyConv_Post_18Sep2019_final.pdf
- World Bank Country and Lending Groups; 2022. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- Van Agthoven M, Segeren CM, Buijt I, et al. A cost–utility analysis comparing intensive chemotherapy alone to intensive chemotherapy followed by myeloablative chemotherapy with autologous stem-cell rescue in newly diagnosed patients with stage II/III multiple myeloma. Eur J Cancer. 2004;40(8):1159–1169.
- Asrar MM, Lad DP, Prinja S, et al. A systematic review of economic evaluations of treatment regimens in multiple myeloma. Expert Rev Pharmacoecon Outcomes Res. 2020;21:1–11.
- Kostopoulos IV, Ntanasis-Stathopoulos I, Gavriatopoulou M, et al. Minimal residual disease in multiple myeloma: current landscape and future applications with immunotherapeutic approaches. Front Oncol. 2020;10(10):860.
- Drummond M, Barbieri M, Cook J, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force Report. Value Health. 2009;12(4):409–418.
- Tantivess S, Chalkidou K, Tritasavit N, et al. Health technology assessment capacity development in low- and middle-income countries: experiences from the international units of HITAP and NICE. F1000Res. 2017;6:2119.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313(7052):275–283.
- Drummond MF, Sculpher MJ, Stoddart GL, et al. Methods for the economic evaluation of health care programme. 3rd ed. Oxford: Oxford University Press; 2005.
- Aguiar PM, Lima TM, Storpirtis S. Systematic review of the economic evaluations of novel therapeutic agents in multiple myeloma: what is the reporting quality? J Clin Pharm Ther. 2016;41(2):189–197.
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) — explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250.
- Rochau U, Jahn B, Qerimi V, et al. Decision-analytic modeling studies: an overview for clinicians using multiple myeloma as an example. Crit Rev Oncol Hematol. 2015;94(2):164–178.
- Cooper K, Picot J, Bryant J, et al. Comparative cost-effectiveness models for the treatment of multiple myeloma. Int J Technol Assess Health Care. 2014;30(1):90–97.
- Gonzalez-McQuire S, Campioni M, Bennison C, et al. Development and validation of a conceptual model of multiple myeloma. Value Health. 2015;18(7):A698.