Abstract
Objective
To estimate clinical events and evaluate the financial implications of introducing ferric carboxymaltose (FCM) to treat iron deficiency (ID) at discharge in patients hospitalized for acute heart failure (AHF) with left ventricular ejection fraction (LVEF) <50% in the UK, Switzerland and Italy.
Methods
A decision analytic cost-offset model was developed to evaluate the costs associated with introducing FCM for all eligible patients in three countries compared to a world without FCM, over a five-year time horizon. Data from AFFIRM-AHF clinical trial were used to model clinical outcomes, using an established cohort state-transition Markov model. Country-specific prevalence estimates were derived using data from real-world studies to extrapolate number of events and consequent cost totals to the population at risk on a national scale.
Results
The cost-offset modeling demonstrated that FCM is projected to be a cost-saving intervention in all three country settings over a five-year time horizon. Savings were driven primarily by reduced hospitalizations and avoided cardiovascular deaths, with net cost savings of −£14,008,238, −CHF25,456,455 and −€105,295,146 incurred to the UK, Switzerland and Italy, respectively.
Limitations
Although AFFIRM-AHF was a multinational trial, efficacy data per country was not sufficiently large to enable country-specific analysis, therefore overall clinical parameters have been assumed to apply to all countries.
Conclusions
This study provides further evidence of the potential cost savings achievable by treating ID with FCM at discharge in patients hospitalized for AHF with LVEF <50%. The value of FCM treatment within the healthcare systems of the UK, Switzerland and Italy was demonstrated even within a limited time frame of one year, with consistent cost savings indicated over a longer term.
Introduction
Heart failure (HF) is a life-threatening chronic disease, currently affecting approximately 1–2% of the adult population in developed countriesCitation1. Patients with HF have impaired cardiac function, are at increased risk of cardiovascular (CV) events and CV-related mortalityCitation2, and the symptom burden associated with HF, notably edema, fatigue and dyspnea, is substantial and debilitatingCitation3–7. HF is most common in elderly patients, affecting ≥10% of patients aged over 70 years. Consequently, with an aging population, HF prevalence is set to riseCitation1,Citation2,Citation8.
An estimated 40–50% of patients with HF with left ventricular ejection fraction (LVEF) <50% have concomitant iron deficiency (ID)Citation9–13. In patients with HF, ID has a significant impact on morbidity and mortality and is associated with reduced quality of life (QoL)Citation14, New York Heart Association (NYHA) class severity and increased risk of hospitalizationsCitation15, independent of anemia. ID is now recognized as a comorbidity of HF and routine screening for ID in this population is recommended in the 2021 European Society of Cardiology (ESC) guidelines for acute and chronic HF, in order to improve disease managementCitation16.
Over the last decade the economic burden of HF has risen sharply in Europe, with the highest proportion of healthcare costs driven by hospitalizations. In the United Kingdom (UK), Italy and Switzerland, HF hospitalizations account for approximately 5% of all emergency medical hospital admissions and readmission rates are high, ranging from 24% at 3 months to 44% at 12 months post-hospital dischargeCitation17,Citation18. Overall, HF treatment costs account for approximately 1–2% of total healthcare budget and is projected to riseCitation19.
Oral iron therapy is inexpensive and a frequently-used therapy for ID, however most HF patients with ID fail to respondCitation20–22 and it often has suboptimal resultsCitation21. In addition, oral iron therapy can be poorly tolerated, with significant gastrointestinal perturbation reported in the majority of hospitalized patientsCitation23. Hence, the ESC guidelines recommend not using oral iron to treat ID in patients with HFCitation16.
Intravenous (IV) iron therapy is now gaining traction for the treatment of iron deficiency in HFCitation24,Citation25. Despite reports on various AE risks, associated with different formulations of IV iron, findings from these studies yield contradictory results for the relative frequencies of certain AEsCitation26–32. Furthermore, a Cochrane review of iron deficiency anemia treatments concluded no significant differences in the rates of AEs arising from different IV iron therapiesCitation33.
Ferric carboxymaltose (FCM) is a high dose IV therapy for ID with a robust, established benefit-risk profile based on numerous clinical trial programs, including in patients with HFCitation16,Citation34–40. In AFFIRM-AHF, a multi-center randomized controlled trial, FCM treatment reduced risk of subsequent hospitalizations with no apparent effect on the risk of CV death in patients admitted for acute HF (AHF) with LVEF <50% and concomitant ID, compared to placeboCitation38. Patients receiving FCM reported significant improvements in their health status from week 4 to week 24Citation36. Accordingly, the ESC guidelines recommend that treatment with FCM should be considered in symptomatic HF patients recently hospitalized for HF and with LVEF <50%Citation16. Based on data from AFFIRM-AHF, cost-effectiveness analysis demonstrated that FCM dominates standard care (quality-adjusted life year [QALY] gains combined with cost savings) in the UK and Switzerland, and is highly cost effective in Italy, with an incremental cost-effectiveness ratio of €1,269 per QALYCitation41.
Given the population-level burden of hospitalizations in HF patients, treatment of ID with FCM has the potential to have a significant beneficial impact on healthcare budgets as well as improving patient outcomes. Currently, no published literature exists for the UK, Swiss or Italian public healthcare settings regarding the budget impact of FCM from the payer’s perspective. Hence, the objectives of this study were to estimate the clinical events (hospitalizations, adverse events [AEs] and mortality) and assess the financial implications of introducing FCM to treat ID in eligible patients with LVEF <50% who had stabilized after hospitalization for an acute HF episode, in three European countries; the UK, Switzerland and Italy.
Methods
A decision analytic cost-offset model was developed to assess the potential cost savings of implementing IV FCM for the treatment of concomitant ID in patients at discharge after AHF with LVEF <50% in three European countries; the UK, Switzerland and Italy. The model evaluated the costs associated with treating all eligible patients with FCM, compared to those associated with a world without IV iron therapy (referred to hereafter as “standard care”), over a time horizon of five years. Clinical outcomes were modeled using data from AFFIRM-AHF, using a previously published cohort state-transition Markov modelCitation41. The estimated cumulative number of clinical events and cumulative monthly costs were then used as inputs in the cost-offset model. Real-world epidemiology data were used to derive country-specific prevalence estimates in order to extrapolate the number of events and consequent overall cost to the population at risk on a national scale. Clinical data underlying the model remained consistent across country adaptations, assuming patients in AFFIRM-AHF were representative of the country-specific patient populations in the UK, Italy and Switzerland. Cost parameters and epidemiological inputs were varied to more accurately model economic outcomes within the specific country settings.
Costs were considered primarily from a healthcare’s perspective and summarized as i) the incremental healthcare costs of implementing FCM versus standard care, with standard care represented by the placebo arm of AFFIRM-AHF, and ii) costs saved due to avoided events associated with FCM treatment. All costs were discounted at 3.5% for the UK and 3% for Switzerland and Italy.
Epidemiology and eligible population
The target population was aligned with the inclusion and exclusion criteria of the AFFIRM-AHF (NCT02937454) trialCitation38. As in the AFFIRM-AHF trial, the study population was defined as patients hospitalized for AHF, with LVEF <50% within a 12-month period prior to randomization, and established ID; the latter defined as serum ferritin <100 ng/mL, or between 100 and 299 ng/mL with transferrin saturation <20%.
To derive the eligible population in each country, a targeted literature review was carried out to identify estimates of the population hospitalized for AHF, the prevalence of ID within AHF patients and the population with comorbid ID and LVEF <50% in UK, Italy and Switzerland (). No Italy-specific data for the prevalence of ID within hospitalized AHF patients could be identified, therefore the prevalence of ID within AHF observed from France and Spain were used as a proxyCitation42.
Table 1. Eligible population based on country-specific epidemiology
Clinical outcomes
The modeled outcomes integrated into the cost-offset analysis were hospitalization for HF (HHF), hospitalization for non-HF causes (HnHF), CV death, all-cause mortality (ACM), and serious AEs that occurred in at least 1% of patients in each treatment arm in AFFIRM-AHF: atrial fibrillation (AF), acute kidney injury (AKI), pneumonia and sepsisCitation41. The incidence of HHF and HnHF events were modeled using generalized estimating equations in order to capture recurrent events (Table S1).
Patient mortality outcomes were estimated using parametric survival models fitted to the AFFIRM-AHF patient-level data. The survival model fitting and selection process were consistent with published guidelinesCitation43–45 and explored standard parametric survival functions: Weibull, log-logistic, log-normal, Gompertz, generalized gamma and gamma. Goodness-of-fit was assessed using the Akaike Information Criteria. A Weibull distribution was selected on the basis of clinical plausibility of long-term survival estimates in addition to providing the best fit to the trial data. Modeled mortality outcomes were adjusted for age, sex and other clinical characteristics.
Input values to the cost-offset model are presented in Table S2–S4. Incidence of AEs was derived from the AFFIRM-AHF trial data (Table S2). The base case analyses assume a market share of 100% over the five-year horizon period.
Cost parameters
All costs were based on 2020 prices. Direct medical costs accrued in the cost-offset model consisted of drug acquisition and administration, hospitalization, treatment-emergent AEs, and CV-related mortality.
Drug acquisition costs presented in Table S3 were aligned with AFFIRM-AHF treatment protocol. Participants received IV FCM or placebo prior to their initial discharge for AHF, and again at further study visits if the patient continued to present with ID. Acquisition costs for FCM were supplied by CSL Vifor and were calculated according to the mean dose recorded in the AFFIRM-AHF trial data. Acquisition and administration costs for placebo were assumed to have no charge.
Hospitalization and AE costs were applied as discrete events. Costs of the incident AHF hospitalization were not included. Mortality costs were applied only for CV mortality; all other cause mortality was assumed to incur no cost to the healthcare system. CV mortality costs compromised end of life care costs for people with worsened CV conditions. All event costs and sources are summarized in Table S3.
Scenario and sensitivity analyses
The base case for the UK and Switzerland used country-specific studies to inform the proportion of patients with ID, which provided conservative estimates compared to alternative published sources from other country settings. A scenario where the proportion of patients with ID in the UK and Switzerland was increased to reflect that reported in prospective studies from other countries was undertaken. For the Italian case, ID prevalence within the population was assumed to be 77.5%, a midpoint of the range 72–83% observed in Spain and FranceCitation42. Subsequent scenario analysis was conducted applying an average of the UK and Swiss ID figures in order to present a more optimistic scenario of FCM impact in Italy.
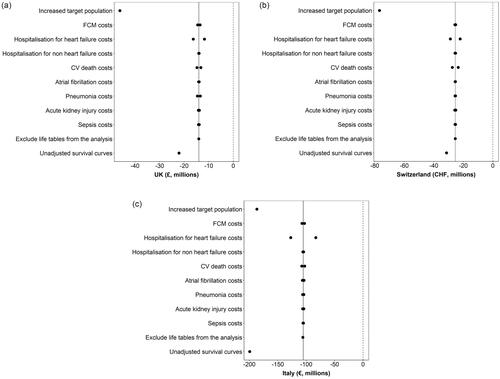
A deterministic sensitivity analysis (DSA) was performed to assess the impact of changes in parameters on model results. The parameters varied in the DSA included the target population in each country, exclusion of life tables from analyses, the use of unadjusted survival equations, the cost of FCM, hospitalization costs, CV death and AE costs. Standard errors were used to vary parameters included in the DSA.
Results
Derivation of the population eligible for treatment in the UK, Switzerland and Italy
The base case population size eligible for treatment with FCM in this indication derived for the UK, Switzerland and Italy was 17,528, 5,330 and 69,003, respectively (). The UK eligible population stemmed from a retrospective cohort study of 78,805 incident cases of AHF in England requiring hospitalization, of which 33.7% patients had concomitant IDCitation46. The proportion of those patients with LVEF <50% was derived from a survey of patients based in England and Wales, which reported 66% of AHF patients had LVEF <50%Citation47.
In Switzerland, an analysis of hospital records identified 65,807 incident cases of AHF requiring hospitalization between 2012 and 2015, which averages to 21,936 per yearCitation48. In another Swiss study, 45% of admitted AHF patients presented with LVEF <50%. The study found no statistical difference in ID prevalence across HF phenotypes, so the proportion of 54% of LVEF <40% patients presenting with comorbid ID was assumed to apply to the full LVEF <50% populationCitation49.
In Italy, 167,047 incident cases of AHF requiring hospitalization were reported in 2018Citation50. In patients admitted for AHF, the proportion of persons presenting with LVEF <50% was 53.3%Citation51, while a recent review estimated the prevalence range of ID in acute decompensated HF to be 72%–83%; in the absence of Italy-specific data, the average of these bounds were taken as a proxyCitation42.
Predicted impact of FCM on hospitalizations
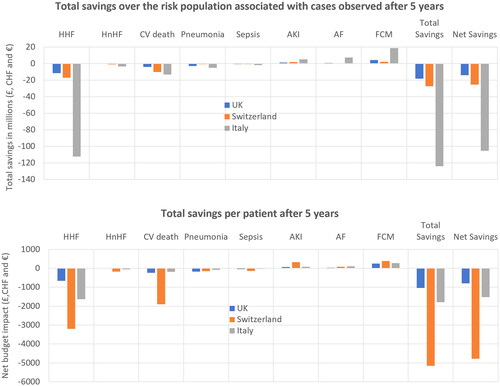
Without FCM intervention, the predicted number of HF-related hospital bed days were 20,942, 6,370 and 82,363 within the UK, Swiss and Italian patient populations, respectively (). Treatment with FCM was associated with a reduction of 4,100, 1,248 and 16,097 HF-hospitalizations in the UK (with a target at risk population of 17,528), Switzerland (population of 5,330) and Italy (population of 69,003), respectively, over five years, versus standard care ( and ), leading to total HF-hospitalization cost savings of £11,609,915, CHF17,028,407, and €112,418,925, respectively. This corresponded to a saving of £662, CHF3,195 and €1,629 in hospital costs per patient with FCM treatment over five years in the UK, Switzerland and Italy, respectively ().
Table 2. Modeled outcomes, associated costs and net budget impact over five years.
Figure 1. Events averted in five years after introducing FCM in the UK, Switzerland and Italy. The number of hospitalizations, CV deaths and AEs averted over five years in the UK, Switzerland and Italy after introducing FCM. Abbreviations. AE, adverse events; AF, atrial fibrillation; AKI, acute kidney injury; CV, cardiovascular; HHF, heart failure hospitalizations; HnHF, hospitalizations due to non-heart failure.
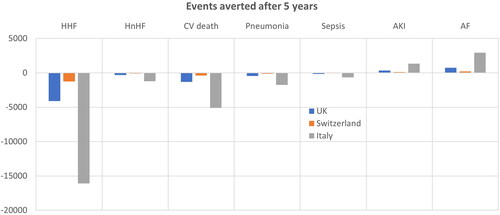
Figure 2. Budget impact of introducing FCM in the UK, Switzerland and Italy. Budget impact over five years for the UK, Switzerland and Italy. Abbreviations. AF, atrial fibrillation; AKI, acute kidney injury; CV, cardiovascular; HHF, heart failure hospitalizations; HnHF, hospitalizations due to non-heart failure.
In addition, FCM treatment was predicted to result in reductions in non-HF related hospitalizations: 309 in the UK, 94 in Switzerland and 1,202 in Italy across the five-year time horizon (). The reduction in non-HF hospitalization was associated with cost savings of £23, CHF172 and €52 per patient in the UK, Switzerland and Italy, respectively.
Predicted impact of FCM on CV deaths and adverse events
The model predicted 8,986, 2,734 and 35,339 CV-related deaths in the world without FCM treatment, compared to 7,683, 2,337 and 30,229 CV-related deaths in the world with FCM treatment, for the UK, Switzerland and Italy, respectively (). This was associated with cost savings of £4,072,771, CHF 10,117,478 and €13,126,285 in the UK, Switzerland and Italy, respectively.
In the UK, the net AE-related costs associated with the use of FCM in the UK resulted in cost savings of £2,174,152 over a five-year time horizon compared to standard care ( and Figures S1–S6). In contrast, the net cost of AE management in Switzerland and Italy increased with FCM compared to standard care, by CHF582,485 and €5,130,994, respectively ( and Figures S1–S6).
Budget impact of FCM
The total combined cost associated with hospitalizations, AEs and CV mortality was lower when the predicted eligible population was treated with FCM compared to standard care (), therefore FCM is projected to be cost saving. The differences in these outcomes between FCM and standard care was modeled to result in total cost-savings of £18,266,627, CHF27,479,443 and €123,978,272 were estimated for the UK, Switzerland and Italy, respectively. When FCM acquisition costs were accounted for, the net budget impact was −£14,008,238, −CHF25,456,456 and −€105,295,147 for the UK, Switzerland and Italy, respectively. This corresponds to net cost savings of £799, CHF4,776 and €1,526 per patient over five years for the UK, Switzerland and Italy, respectively.
Scenario and sensitivity analysis
The base case in the UK and Switzerland used country-specific estimates of the proportion of eligible patients with ID, which are potentially conservative, since several prospective studies from other European countries have reported higher estimatesCitation42. In order to explore the budget impact in the UK and Switzerland if a higher proportion of patients are eligible, a scenario was constructed where at least 70% of the patients admitted for AHF with LVEF <50% had ID, based on a range 72-83% reported in France and SpainCitation42. If all eligible patients in this scenario were treated with FCM, cost savings of £46 million and CHF77 million were estimated for the UK and Switzerland, respectively, after considering drug acquisition costs.
For Italy, to explore the eventuality of a smaller eligible population, an ID prevalence aligned with the other country base cases was applied. Averaging the proportion of patients with diagnosed ID within the UK and Switzerland returned a lower estimate of 43.9%, in contrast to the 77.5% applied in the Italian base case. This scenario returned five-year potential cost savings of over €59 million when accounting for FCM administration within the population.
The results of the DSA indicated that the analyses were robust to parameter changes ( and Table S5). The three most influential parameters were increased target populations in the countries, which led to increased net budget impact, hospitalization for heart failure costs and use of unadjusted survival curves.
Discussion
This study is the first to capture the impact of FCM treatment for the treatment of concomitant ID in patients hospitalized for AHF with LVEF <50% on the healthcare budgets of the UK, Italy and Switzerland. Additionally, this analysis was novel in the contextualization of preventable hospital bed days under an economic lens. The cost-offset modeling demonstrates that FCM is a cost-saving intervention in all three country settings over a five-year time horizon. Although FCM acquisition costs may appear high (£4,258,391, CHF2,022,987 and €18,683,125 for the UK, Switzerland and Italy, respectively), when offset against the savings associated with adverse clinical outcomes avoided, the overall impact on total healthcare expenditure is small, and is fully offset within one year. Total cost savings of £14,008,238, CHF25,456,456 and €105,295,147 were incurred to the respective healthcare systems after accounting for FCM acquisition costs. These savings were driven primarily by reduced hospitalization events for worsening HF associated with FCM use, which accounted for economic savings of £662, €1,629 and CHF3,195 per patient within the UK, Italian and Swiss healthcare budgets.
The model also predicted cost savings in each country setting attributable to fewer CV mortality events associated with FCM use. The AFFIRM-AHF trial demonstrated no statistically significant effect of FCM on CV mortality, however, there was a small numerical difference in CV mortality between the trial armsCitation38. In the cost-effectiveness model, patient-level data from AFFIRM-AHF were used to extrapolate CV mortality using parametric multivariable survival analysisCitation41. When this extrapolation was performed, in order to predict outcomes over a longer time than the trial period, and applied on a population level, this numerical difference in CV mortality events resulted in the cost savings presented in the current analysis.
When considered at the population level, modeled AEs were also predicted to impact costs. Although the overall incidence in AFFIRM-AHF of each of the modeled AEs was low, as illustrated in Figures S1–S3, small numerical differences between the arms applied on the scale of the eligible population in each country, combined with country-specific AE management costs, translated to a small cost increase associated with FCM in Switzerland and Italy, while in the UK model, the net AE costs resulted in a cost saving. However, despite higher total AE costs in Switzerland and Italy, there is little uncertainty around the overall cost-offsetting capacity of FCM; FCM treatment remained a cost saving option in these countries as the AE costs were outweighed by the savings accrued from other avoided events, including HHF, HnHF and CV-related mortality.
A discrepancy in the prevalence of ID reported in the eligible patient population was identified, particularly when comparing retrospective cohort studies versus prospective cohort studies, which is presumed to reflect underdiagnosis in real-world populations. The base case in the UK and Switzerland used prevalence figures from country-specific studies to estimate the eligible population, as a conservative assumption reflecting likely current prescribing patterns. However, the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic HFCitation16 recommend that all patients with HF are regularly screened for ID, and that IV iron supplementation with FCM should be considered in symptomatic HF patients recently hospitalized for HF and with LVEF <50%. Implementation of these guidelines may lead to increased diagnosis and treatment rates and therefore the potential for greater budget impact, therefore data from a prospective study reflecting the potential eligible population were used in a scenario analysis. As expected, the cost savings associated with this larger population were greater, highlighting that guideline-recommended treatment should provide economic benefit to healthcare systems as well as clinical benefit to patients.
In Europe, HF prevalence is rising due to increasing life expectancy and advances in treatment, particularly for patients with reduced LVEF. In addition, increased use of revascularization procedures and improved acute treatment has resulted in increased post-myocardial infarction survival rates, leading to a larger pool of patients at risk of developing HFCitation52–59. The number of hospital admissions for HF is projected to double over the next 25 yearsCitation60. Given the high prevalence of ID in this populationCitation12,Citation61, particularly if patients are screened and treated for ID with FCM in accordance with the ESC 2021 HF guidelines, the impact of FCM therapy worldwide on hospital bed availability, which has potential implications for cost savings as well as freeing up capacity that could be directed elsewhere, would be significant. Increasing hospital capacity within highly saturated healthcare systems, particularly during the Covid-19 pandemic, would have a great impact on both emergency and elective procedures, with significant health economic and resourcing advantagesCitation62. Furthermore, HHF is associated with worsening functional status for the patient, leading to vicious cycle of reduced QoL and recurrent hospitalizations. FCM treatment would reduce time spent in hospital and potentially break this cycle, positively impacting on the patient’s QoL and re-hospitalizations.
Health economic studies have consistently demonstrated the benefits of FCM treatment in HF patients with ID in the USCitation41 and across Europe, including AustriaCitation63,Citation64, DenmarkCitation65, FinlandCitation65, ItalyCitation41,Citation66, NorwayCitation65, SpainCitation67,Citation68, SwedenCitation65, UKCitation41 and SwitzerlandCitation41. This budget impact analysis was based on data for patients with a recent AHF episode, representing a very high-risk subgroup; however, the findings are relevant to all patients with HF and align with those from prior budget impact studies in patients with chronic HF and ID. Cost savings are driven mainly by reduced hospitalizations in AustriaCitation63, FranceCitation69, ItalyCitation66, SpainCitation68 and RomaniaCitation70, respectively. In Austria three years after introducing FCM, savings reached €684,443Citation63. A French budget analysis showed that FCM treatment in HF patients with ID led to savings of €0.9M over five yearsCitation69. While a Romanian budget impact analysis demonstrated overall healthcare savings, with an annual saving of €8,800 per 1000 patients treated with FCMCitation70. In a Spanish healthcare system, FCM reduced annual costs by €534.8 per patientCitation68. Furthermore, with the adoption of increased screening for ID in HF patients, FCM treatment was associated with cost saving of up to €69.9 million in an Italian settingCitation66,Citation71. In a German budget impact analysis, despite beneficial impact with FCM treatment in HF patients with ID, including improved symptoms, lowered NYHA class and reduced rates of hospitalization, significant savings from reduced hospitalizations were not fully offset by additional treatment costs, resulting in a small incremental costCitation72. These data support our findings and demonstrate cost savings or small incremental cost with FCM treatment in a broad spectrum of patients with HF and ID.
There were some limitations to the analysis presented. While AFFIRM-AHF was a multinational trial across Europe, South America and Singapore, efficacy data per country was not sufficiently large to enable reliable country-specific analysis. Consequently, the results from each country adaptation of the cost-offset model, where clinical parameters were unchanged, may not fully represent the treatment guidelines and diversity of clinical outcomes for each local setting. Tailoring of model inputs within the country adaptations was made wherever possible; although these inputs were subject to the availability and quality of the data that could be sourced from relevant literature. The application of country-specific prevalence data was successful in the UK and Swiss models, but the use of a European proxy for ID prevalence in the Italian base case was required. Further, the population at risk of AHF in Italy was reported as patients hospitalized for an episode of either HF or stroke, with no disaggregated figure reported. The combined impact of these input assumptions results in an undetermined effect on the population at risk and associated cost savings generated of FCM in the Italian setting; nevertheless, the consistency of the DSA results with the base case give confidence to the robustness of the central model.
A further limitation is that incidence of hospitalization events did not take into account the potential impact of length of stay (LOS), which varies between countries. Given the improved prognosis of the FCM treatment cohort, cost savings could be greater than currently presented if the analysis had accounted for uncertainty around LOS. Additionally, the analysis does not account for any potential disparity in background healthcare utilization between patients at different stages of the HF disease pathway or between treatment groups i.e. any differences in patient experience of HF with and without FCM, which may have cost implications that were consequently not captured; related to this, the model did not include costs of standard pharmacotherapy in HF patients with LVEF <50% in either cohort. Furthermore, despite clinical and physiological differences between newly-diagnosed acute heart failure (de novo HF) and acute decompensation of pre-existing chronic HF, it was not possible to distinguish between them in our eligible population since there is a paucity of available country specific epidemiology data in HF population with ID. A final limitation is that trial follow-up was limited to one year, requiring the extrapolation of outcomes data over a five-year time horizon. Nevertheless, model results indicated FCM to be cost saving within the first year, demonstrating its value over a short time horizon.
The results of this study are intended to be informative specifically for the UK, Switzerland and Italy, as the specification of the country adaptations limits the transferability of results to alternative settings. Further studies from other country perspectives would contribute more accurate results to the discourse on the cost-saving capacity of FCM treatment for patients with ID at discharge after acute HF.
Conclusion
ID has a substantial impact on both clinical and economic outcomes in HF, contributing to increased hospitalizations, rehospitalization, QoL and worsening of symptoms. Treatment with FCM has potential to save on costs and improve outcomes in HF patients with ID. This study provides further evidence of the potential cost savings associated with using FCM to treat ID at patients discharge after acute HF with LVEF <50%. In this multinational study, savings were driven primarily by reduced hospitalizations and avoided CV deaths, with total net cost savings of £14,008,238, CHF25,456,455 and €105,295,146 incurred in the UK, Switzerland and Italy, respectively. Overall, the value of FCM treatment within these healthcare systems is demonstrated even within a limited time frame of one year, with consistent cost savings indicated in the longer term. This evaluation of the budgetary impacts of FCM therapy provides healthcare decision makers with a range of potential cost benefits that may be realized through the utilization of FCM treatment, in alignment with the ESC 2021 HF guideline recommendations, which focus on the provision of improved diagnosis and treatment rates for ID.
Transparency
Declaration of funding
This work was supported by CSL Vifor who provided support for data analysis, model development and medical writing for this study, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
GMCR was supported by the Italian Ministry of Health (Ricerca Corrente) 20/1819.
Declaration of financial/other relationships
AJSC reports honoraria from Astra Zeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, CSL Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Gore, Impulse Dynamics, Respicardia and Viatris. EAJ is a honorary lecturer and a consultant and has participated in advisory boards for: CSL Vifor, Novartis, AstraZeneca, Boehringer Ingelheim, Servier, Pfizer, Berlin Chemie, Respicardia, Bayer, Gedeon Richter, Abbott, Cardiac Dimensions, Takeda. Co-principal investigator of the AFFIRM-AHF trial sponsored by CSL Vifor. PP reports participation in clinical trials for and grants and personal fees from CSL Vifor during the conduct of the study; participation in clinical trials for and personal fees from Amgen, Bayer, Novartis, Abbott Vascular, Boehringer Ingelheim, Pfizer, Servier, Astra Zeneca, Cibiem, BMS, and Impulse Dynamics, outside the submitted work; participation in clinical trials for Cardiac Dimensions, outside the submitted work; and personal fees from Berlin Chemie, outside the submitted work. ARdA and FD are employees of CSL Vifor. PM and TS are employees of HEOR Ltd. HEOR Ltd received fees from CSL Vifor in relation to this study. Reviewer disclosures: A reviewer on this manuscript has disclosed that they have received research funding for data management from Covis Pharma and have provided an educational non-promotional program for Pharmacosmos. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
PM and ARdA conceptualized and designed the study. TS was responsible for data analysis. All authors contributed to interpretation of the results, preparation and review of the manuscript and approval of the final manuscript for publication.
Previous presentations
The manuscript, or part of it, has neither been published nor is currently under consideration for publication by any other journal. Preliminary analyses have previously been published in abstract form.
Supplemental Material
Download MS Word (1.6 MB)Acknowledgements
The authors thank Ruth Lewis, Kerrie Ford, Rhona Binnie, Melodi Kosaner. Kliess and George Mann of Health Economics and Outcomes Research Ltd. for providing medical writing support/editorial support, which was funded by CSL Vifor in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
References
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200.
- Your guide to heart failure. British Heart Foundation. 2018. Available from: www.bhf.org.uk/informationsupport/publications/heart-conditions/your-guide-to-heart-failure
- Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21(5):365–371.
- Nieminen MS, Dickstein K, Fonseca C, et al. The patient perspective: quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol. 2015;191:256–264.
- Peters-Klimm F, Kunz CU, Laux G, et al. Patient-and provider-related determinants of generic and specific health-related quality of life of patients with chronic systolic heart failure in primary care: a cross-sectional study. Health Qual life Outcomes. 2010;8(1):1–11.
- Pisa G, Eichmann F, Hupfer S. Assessing patient preferences in heart failure using conjoint methodology. Patient Prefer Adherence. 2015;9:1233.
- Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1(1):4–25.
- Lippi G, Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Med J. 2020;5:15.
- Ezekowitz JA, McAlister FA, Armstrong PW. The interaction among sex, hemoglobin and outcomes in a specialty heart failure clinic. Can J Cardiol. 2005;21(2):165–171.
- Beverborg NG, Klip IT, Meijers WC, et al. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail. 2018;11(2):e004519.
- Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J. 2012;34(11):827–834.
- Jankowska EA, von Haehling S, Anker SD, et al. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34(11):816–829.
- Shah R, Agarwal AK. Anemia associated with chronic heart failure: current concepts. Clin Interv Aging. 2013;8:111–122.
- Enjuanes C, Klip IT, Bruguera J, et al. Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol. 2014;174(2):268–275.
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165(4):575.e3–582.e3.
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726.
- Cleland JG, Swedberg K, Follath F, et al. The EuroHeart Failure survey programme – a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24(5):442–463.
- Maggioni AP, Dahlström U, Filippatos G, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15(7):808–817.
- Lesyuk W, Kriza C and Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord. 2018;18(1):1–11.
- Ambrosy AP, Lewis GD, Malhotra R, et al. Identifying responders to oral iron supplementation in heart failure with a reduced ejection fraction: a post-hoc analysis of the IRONOUT-HF trial. J Cardiovasc Med. 2019;20(4):223–225.
- Drozd M, Jankowska EA, Banasiak W, et al. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs. 2017;17(3):183–201.
- Niehaus ED, Malhotra R, Cocca-Spofford D, et al. Repletion of iron stores with the use of oral iron supplementation in patients with systolic heart failure. J Card Fail. 2015;21(8):694–697.
- Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS one. 2015;10(2):e0117383.
- Kalra PR, Cleland JG, Petrie MC, et al.; IRONMAN Study Group. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): an investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial. Lancet 2022;4:S0140-6736(22)02083-9.
- Ponikowski P, Kirwan BA, Anker SD, et al. Rationale and design of the AFFIRM-AHF trial: a randomised, double-blind, placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalisations and mortality in iron-deficient patients admitted for acute heart failure. Eur J Heart Fail. 2019;21(12):1651–1658.
- Blumenstein I, Shanbhag S, Langguth P, et al. Newer formulations of intravenous iron: a review of their chemistry and key safety aspects–hypersensitivity, hypophosphatemia, and cardiovascular safety. Exp Opin Drug Saf. 2021;20(7):757–769.
- Ehlken B, Nathell L, Gohlke A, et al. Evaluation of the reported rates of severe hypersensitivity reactions associated with ferric carboxymaltose and iron (III) isomaltoside 1000 in Europe based on data from EudraVigilance and VigiBase™ between 2014 and 2017. Drug Saf. 2019;42(3):463–471.
- Glaspy JA, Lim-Watson MZ, Libre MA, et al. Hypophosphatemia associated with intravenous iron therapies for iron deficiency anemia: a systematic literature review. Therapeut Clin Risk Manag. 2020;16:245.
- Kumar A, Brookes MJ. Iron therapy in inflammatory bowel disease. Nutrients. 2020;12(11):3478.
- Mulder MB, van den Hoek HL, Birnie E, et al. Comparison of hypersensitivity reactions of intravenous iron: iron isomaltoside‐1000 (Monofer®) versus ferric carboxy‐maltose (Ferinject®). A single center, cohort study. Br J Clin Pharmacol. 2019;85(2):385–392.
- Pollock RF, Biggar P. Indirect methods of comparison of the safety of ferric derisomaltose, iron sucrose and ferric carboxymaltose in the treatment of iron deficiency anemia. Exp Rev Hematol. 2020;13(2):187–195.
- Schaefer B, Tobiasch M, Viveiros A, et al. Hypophosphataemia after treatment of iron deficiency with intravenous ferric carboxymaltose or iron isomaltoside—a systematic review and meta‐analysis. Br J Clin Pharmacol. 2021;87(5):2256–2273.
- Gordon M, Sinopoulou V, Iheozor-Ejiofor Z, et al. Interventions for treating iron deficiency anaemia in inflammatory bowel disease. Cochrane Database Syst Rev. 2021;1(1):CD013529.
- Vifor Pharma Limited. Ferinject (ferric carboxymaltose). 2021. Available from: https://www.medicines.org.uk/emc/product/5910/smpc.
- Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436–2448.
- Jankowska EA, Kirwan BA, Kosiborod M, et al. The effect of intravenous ferric carboxymaltose on health-related quality of life in iron-deficient patients with acute heart failure: the results of the AFFIRM-AHF study. Eur Heart J. 2021;42(31):3011–3020.
- Khan MS, Usman MS, von Haehling S, et al. Ferric carboxymaltose for the treatment of iron-deficient heart failure patients: a systematic review and meta-analysis. ESC Heart Fail. 2020;7(6):3392–3400.
- Ponikowski P, Kirwan B-A, Anker SD, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. 2020;396(10266):1895–1904.
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657–668.
- van Veldhuisen DJ, Ponikowski P, van der Meer P, et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136(15):1374–1383.
- McEwan P, Ponikowski P, Davis JA, et al. Ferric carboxymaltose for the treatment of iron deficiency in heart failure: a multinational cost-effectiveness analysis utilising AFFIRM-AHF. Eur J Heart Fail. 2021;23(10):1687–1697.
- Rocha BML, Cunha GJL, Menezes Falcao LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol. 2018;71(7):782–793.
- Bagust A, Beale S. Survival analysis and extrapolation modeling of time-to-event clinical trial data for economic evaluation: an alternative approach. Med Decis Making. 2014;34(3):343–351.
- Latimer NR. NICE decision support unit technical support documents, in survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. London: National Institute for Health and Care Excellence (NICE); 2013.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657.
- Beattie JM, Khatib R, Phillips CJ, et al. Iron deficiency in 78 805 people admitted with heart failure across England: a retrospective cohort study. Open Heart. 2020;7(1): e001153.
- Cleland JG, McDonagh T, Rigby AS, et al. The national heart failure audit for England and Wales 2008-2009. Heart. 2011;97(11):876–886.
- Kutz A, Gut L, Ebrahimi F, et al. Association of the Swiss diagnosis-related group reimbursement system with length of stay, mortality, and readmission rates in hospitalized adult patients. JAMA Netw Open. 2019;2(2):e188332.
- Beale A, Carballo D, Stirnemann J, et al. Iron deficiency in acute decompensated heart failure. J Clin Med. 2019;8(10):1569.
- Annual report on hospitalization activity (SDO data 2018). Minister of Health Italy. 2019. Available from: www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=2898
- Orso F, Pratesi A, Herbst A, et al. Acute heart failure in the elderly: setting related differences in clinical features and management. J Geriatr Cardiol. 2021;18(6):407–415.
- Capewell S, Livingston B, MacIntyre K, et al. Trends in case-fatality in 117718 patients admitted with acute myocardial infarction in Scotland. Eur Heart J. 2000;21(22):1833–1840.
- Hardoon SL, Whincup PH, Petersen I, et al. Trends in longer-term survival following an acute myocardial infarction and prescribing of evidenced-based medications in primary care in the UK from 1991: a longitudinal population-based study. J Epidemiol Commun Health. 2011;65(9):770–774.
- Stewart A, Beaglehole R, Jackson R, et al. Trends in three-year survival following acute myocardial infarction, 1983–1992. Eur Heart J. 1999;20(11):803–807.
- Bata IR, Gregor RD, Wolf HK, et al. Trends in five-year survival of patients discharged after acute myocardial infarction. Can J Cardiol. 2006;22(5):399–404.
- Briffa T, Hickling S, Knuiman M, et al. Long term survival after evidence based treatment of acute myocardial infarction and revascularisation: follow-up of population based Perth MONICA cohort, 1984-2005. BMJ. 2009;338:b36.
- Botkin NF, Spencer FA, Goldberg RJ, et al. Changing trends in the long-term prognosis of patients with acute myocardial infarction: a population-based perspective. Am Heart J. 2006;151(1):199–205.
- Shotan A, Gottlieb S, Goldbourt U, et al. Prognosis of patients with a recurrent acute myocardial infarction before and in the reperfusion era—a national study. Am Heart J. 2001;141(3):478–484.
- Buch P, Rasmussen S, Gislason GH, et al. Temporal decline in the prognostic impact of a recurrent acute myocardial infarction 1985 to 2002. Heart. 2007;93(2):210–215.
- UK NGC. Chronic heart failure in adults: diagnosis and management. National Institute for Health and Care Excellence (NICE). 2018. Available from: https://www.nice.org.uk/guidance/ng106
- Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138(1):80–98.
- McCabe R, Schmit N, Christen P, et al. Adapting hospital capacity to meet changing demands during the COVID-19 pandemic. BMC Med. 2020;18(1):1–12.
- Ressl S, Walter E, Bauer M. Budget-impact-analysis of iron treatment using intravenous ferric carboxymaltose in patients with chronic heart failure and iron deficiency in Austria. Value Health. 2015;18(7):A384.
- Walter E, Bauer M, Ressl S. Cost-effectiveness of ferric carboxymaltose in patients with iron deficiency and chronic heart failure in Austria. Value Health. 2015;18(7):A392.
- Hofmarcher T, Cabrales Alin D, Linde C. Cost effectiveness of implementing ESC guidelines for treatment of iron deficiency in heart failure in the Nordic countries. Scand Cardiovasc J. 2018;52(6):348–355.
- Rognoni C, Gerzeli S. Ferric carboxymaltose for patients with heart failure and iron deficiency in Italy: cost-effectiveness and budget impact. J Comp Eff Res. 2019;8(13):1099–1110.
- Comín-Colet J, Rubio-Rodríguez D, Rubio-Terrés C, et al. A cost-effectiveness analysis of ferric carboxymaltose in patients with iron deficiency and chronic heart failure in Spain. Rev Esp Cardiol. 2015;68(10):846–851.
- Delgado JF, Oliva J, González-Franco Á, et al. Budget impact of ferric carboxymaltose treatment in patients with chronic heart failure and iron deficiency in Spain. J Med Econ. 2020;23(12):1418–1424.
- Bourguignon S, Faller M, Champs FO, et al. Budget impact of intravenous ferric carboxymaltose in patients with chronic heart failure and iron deficiency in France. ESC Heart Fail. 2019;6(3):559–569.
- Lorenzovici L, Székely A, Farkas-Ráduly S, et al. Budget impact of intravenous iron therapy with ferric carboxymaltose in patients with chronic heart failure and iron deficiency in Romania. J Cardiovasc Emerg. 2019;5(4):131–139.
- Rognoni C, Gerzeli S. Ferric carboxymaltose for patients with heart failure and iron deficiency in Italy: cost–effectiveness and budget impact. J Comp Eff Res. 2019;8(11):1099–1110.
- Theidel U, Väätäinen S, Martikainen J, et al. Budget impact of intravenous iron therapy with ferric carboxymaltose in patients with chronic heart failure and iron deficiency in Germany. ESC Heart Fail. 2017;4(3):274–281.
- National heart failure audit. 2019 summary report (2017/18 data). NICOR. 2019. Available from: https://www.nicor.org.uk/wp-content/uploads/2019/09/Heart-Failure-2019-Report-final.pdf