Abstract
Aims
This study aimed to estimate the utility values of the factors associated with intravenous (IV) iron infusion treatment in Japanese patients with iron-deficiency anemia (IDA) from the patient’s perspective.
Methods
A conjoint analysis based on online survey data was conducted in May 2022 (registration number: UMIN000047756). Respondents in the main group were selected from the general population (20–69 years). Seven attributes were included in this analysis: waiting time before receiving an IV infusion, pain due to IV infusion, time required for IV infusion, number of IV infusions required to achieve treatment effect, frequency of hypophosphatemia as a side effect of IV infusion, frequency of skin discoloration by the drug solution, and out-of-pocket cost for one IV infusion visit. The utility of each level for each attribute was estimated using a logistic regression model as the difference from non-treatment.
Results
The responses were collected from 1,026 people. The utilities decreased with higher pain (−0.189 for pain level of 3.05), longer time for the IV infusion (−0.145 or −0.212 for 5 or 15 min), greater number of required IV infusions (−0.773 or −1.899 for 3 or 25 times), and higher frequency of adverse events (−0.373 or −0.385 for 13.0% or 14.2% of hypophosphatemia incidences; −0.502 for 2.3% of skin discoloration per one infusion).
Limitations
Since this study was based on an online survey, the reliability of the results depends on whether the respondents understood the questions accurately. Further, the respondents were selected from an online panel, potentially affecting finding generalizability.
Conclusions
The results indicate that utilities differ depending on the factors associated with IV iron infusion treatment. The findings of this study may be useful for informing future treatments or improving current treatment regimes, supporting the achievement of complete iron repletion for Japanese patients with IDA.
Introduction
Iron-deficiency anemia (IDA) is the most common type of anemia, which can cause symptoms such as fatigue, headache, paleness, dyspnea on exertionCitation1, pagophagiaCitation2,Citation3, and restless legs syndromeCitation2,Citation4. According to a systematic analysis of the global anemia burden, anemia affects 27% of the world’s population and iron deficiency is the main cause (≥60%) of anemiaCitation5. Among Japanese women, the frequency of IDA is estimated to be 8.5%Citation6. According to the National Health and Nutrition Survey conducted in Japan in 2019, among women, 13.5% of the total respondents aged ≥20 years reported hemoglobin (Hb) concentrations of less than 12.0 g/dL, as did 18.3% of those in their 30 s, and 14.9% of those over 60 years; among men, 10.7% of the total respondents aged ≥20 years reported concentrations of Hb less than 13.0 g/dL, as did 0.4% of those in their 20–40 s, and 15.9% of those over 60 yearsCitation7. IDA is known to be associated with poorer health outcomes, such as higher mortality in patients with chronic renal failure or undergoing cardiac surgery, and a higher risk of cardiovascular malfunction after percutaneous coronary interventionCitation8–10.
Two types of treatment for IDA are available in clinical practice: oral and intravenous (IV) iron treatment. IV iron treatment is prescribed for patients with IDA in need of rapid delivery of iron or when oral iron treatment is considered inappropriate due to lack of efficacy or poor tolerability. Various types of IV iron formulations are available, including those that require only one or two infusions to fully replenish iron in patients with IDA, and the types of formulations approved for use differ between countries. In Japan, two types of IV iron formulations—saccharated ferric oxide (SFO) and ferric carboxymaltose (FCM)—are currently commercially available for patients with IDA. The non-inferiority of FCM to SFO in improving Hb levels was verified in a randomized open-label study of Japanese patients with IDACitation11. The iron dose per infusion differs by IV iron formulation type: 40 − 120 mg for SFO and 500 mg for FCM for an adult patient, and therefore, the total number of administrations needed to provide full correction of IDA also differs by IV iron formulation type. Notably, the maximum iron dose per infusion for FCM differs between countries. It is 750 mg or 1,000 mg in the USCitation12,Citation13 and 1,000 mg in the EUCitation12. In addition, there are differences in time required for infusion between the formulations.
In clinical settings in Japan, data show that the actual amount of infused IV iron may not be sufficient to fully replenish patients with IDA. Based on an analysis of patients with IDA using the claims database provided by DeSC Healthcare, Inc.Citation14, 98.6% of SFO and 36.4% of FCM infusions had actual cumulative infused amounts per episode that accounted for less than the estimated iron need, suggesting insufficient compliance with IV iron treatment standards. Further, the proportion of patients who received just one infusion of SFO and FCM was 11.5% and 47%, respectively. There may be various reasons for the suggested insufficient compliance with relevant treatment, such as concern for adverse events and factors associated with treatment process, including frequency of visits and required time for infusionCitation15,Citation16. These concerns and factors may differ by IV iron formulation type and by treatment strategy or skills at the individual level (i.e. depending on the physicians or nurses performing the treatment).
Although these factors, together with effectiveness, may influence patients’ preference for IV iron treatment, possibly affecting compliance and full iron repletion, they have not been comprehensively evaluated from a patient’s perspective. A systematic review reported that convenience-related process utility exists independent of the health outcome, but the magnitude differed depending on various factors including study settings and disease typesCitation17. Regarding IV iron treatment of patients with IDA, a vignette-based time trade-off (TTO) study found significant differences in health state utilities due to differences in process attributes such as required time for infusion and the risk of hypophosphatemic osteomalaciaCitation18.
In this study, the utility values (values representing respondents’ preference) associated with IV iron treatment for patients with IDA were estimated using a conjoint analysis based on an online survey conducted in Japan. By estimating the utility values of each factor, we compared which and to what extent factors influence treatment preferences. This study aims to provide information to assist physicians in the continuous treatment of IDA through understanding patients’ preferences, which in turn should support the improvement of the treatment process in Japan and elsewhere. It may contribute to improving compliance and the achievement of full iron repletion in patients with IDA. Although the availability of IV iron formulations and valuation of process attributes differs between countries, we believe the results also have wider relevance because the importance of treatment process attributes has been reported globallyCitation17–23.
Methods
Study design
We conducted a conjoint analysis based on an online survey. A conjoint analysis is a stated preference method that can quantitatively calculate the impact of each attribute level on preferences based on survey data. It is widely used to elicit patient preferences in healthcareCitation17,Citation19,Citation24,Citation25. The actual health situation of patients with IDA and IV iron treatment was simultaneously investigated.
Data source and participants
An online survey was conducted from 24–26 May 2022, via an online panel managed by INTAGE Inc. Data from the following populations were included in the analysis: a primary respondents group comprising respondents between 20 and 69 years who were randomly selected from a general population panel according to the distribution of the Japanese population (Group 1), patients who have been diagnosed with IDA for which they had previously received IV iron treatment (Group 2), and patients who have been diagnosed with IDA and had not previously received IV iron treatment (Group 3). The target sample size for each group was 1,000. The sample size was determined to obtain robust results and is larger than that reported in the checklist by ISPORCitation25. It additionally considered the availability of respondents, particularly those in Group 3, and budget. Invitations to the survey were sent to a certain number of potential respondents, and the answers were accepted until the planned number of respondents for each group was received. Individuals who began a response were able to withdraw at any time; they were excluded from the respondents. Some patients with IDA may have been included in Group 1 because their data were extracted without screening for disease history. The respondents were limited to those aged 20–69 years in the general population panel (Group 1) to ensure that respondents could respond appropriately to the online questionnaire. However, no age limit was set for patients with IDA because it could have led to the number of applicable respondents not reaching the target number.
Survey methods
Patients with IDA (Groups 2 and 3) were asked questions regarding their health situation, including underlying causes of IDA, the experience of oral drug treatment for IDA, and symptoms of IDA (see Table S1 for survey questions). In addition, participants in Group 2 were asked about their IV iron treatment experiences, including the degree of pain due to IV infusion and the presence or absence of IV iron induced hypophosphatemia and skin discoloration as side effects of the treatment (see Table S1 for survey questions). Responses to these questions were analyzed to interpret the results of the conjoint analysis and estimate the relative impact of the various levels of attributes on patient preferences in relation to the available IV iron treatments.
Table 1. Attributes and attribute levels for the conjoint analysis in the survey.
The conjoint analysis survey was conducted to estimate the utility values for factors associated with IV iron infusion. To ensure that the attributes follow current standard of care, we selected attributes and levels of each attribute used in the conjoint cards utilized in the survey () mainly based on information from clinicians with expert knowledge of IV iron in Japan. We also referred to information about treatment status with IV iron infusion used in Japan from literature. The seven attributes included waiting time before receiving an IV infusion, pain due to IV infusion, the time required for IV infusion, the number of IV infusions required to achieve treatment effect, frequency of hypophosphatemia as a side effect after the IV infusion, frequency of skin discoloration by the drug solution, and out-of-pocket cost for one IV infusion. The six-level Wong-Baker FACES Pain Rating Scale (0–10) was used for pain evaluation. While there are 17,280 level combinations, the types of combinations were limited in this study according to orthogonal programming using “conjoint” package in R to reduce the survey burden for respondents. Each respondent was provided with question sentences and two different conjoint cards, and then asked to select the preferable one (an example of the options is shown in ).
Figure 1. Example of choice task questions for the conjoint analysis in the survey. Each respondent was presented with two different cards with illustrations and asked to select the preferable card. Twenty sets of conjoint cards were presented to each respondent.
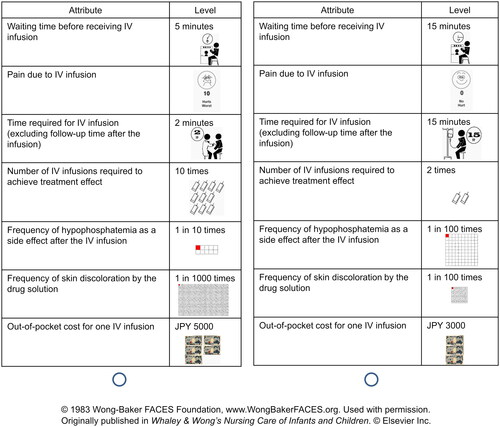
In total, 20 sets of conjoint cards were presented to each respondent. The actual question sentences were as follows: “You are a patient with anemia (iron-deficiency anemia) and have symptoms such as tiredness, fatigue, and shortness of breath when climbing stairs, which interfere with your daily life. You consulted a family doctor in a clinic and took the oral iron treatment; however, it was not effective, so you decided to receive IV iron treatment. Which of the following treatments would you choose? Treatments are conducted while sitting in a chair. A 15-min follow-up is also required after the treatment. The effect of the treatment is to eliminate symptoms such as tiredness, fatigue, and shortness of breath when climbing stairs. Side effects of the treatment such as IV iron induced hypophosphatemia and skin discoloration may occur. If you experience hypophosphatemia, it can cause lethargy, loss of appetite, muscle weakness, and bone and joint pain, which may require further treatment. If the drug is deposited on the skin, a dark color resembling a bruise or tattoo will remain on the skin for approximately 2 years.” Notably, we consider the symptoms, “tiredness” and “fatigue” to be different—tiredness (tsukare-yasusa in Japanese) means getting tired easily and requiring rest or sleep, and fatigue (darusa in Japanese) means extreme tiredness and not easily cured even after rest or sleep.
Statistical analysis
The utility value of each level for each attribute was estimated in the conjoint analysis based on the preferences of the respondents in each group using a logistic regression model. The utility of no treatment was considered to be 0. For example, suppose the utility of pain 5 on the Pain Rating Scale (0–10) is −0.565, which is the mean of the utilities of pain 4 (−0.243) and pain 6 (−0.887). The utility of pain 5 (−0.565) is 4.31 times larger than that of pain 2 (−0.131), not 2.5 times, which implies that the strength of response to pain increases beyond the scale of pain. When the utility of pain 2 (−0.131) is close to that of time required for the IV infusion of 5 min (−0.145), it implies that people dislike pain 2 almost the same as a required time for the IV infusion of 5 min.
In addition, the utilities were estimated assuming the available IV iron formulations. The conditions applied in this estimation were assumed or calculated as follows. The waiting time before receiving an IV infusion was assumed to be the typical related waiting time of 15 min. Pain due to infusion applied the average pain level as reported by respondents in Group 2 in this study. The time required for the IV infusion of SFO and FCM was assumed by referring to their package insertsCitation26,Citation27, which were 5 and 15 min, respectively. The number of IV infusions required to achieve treatment effect was calculated for FCM and SFO as three and 25 times, respectively. To calculate these numbers, we checked the required iron amount based on their package inserts, assumed the delivery of treatment for a patient with a body weight of 50 kg and Hb of 8.0 g/dlCitation26,Citation27, and assumed that the single iron doses for FCM and SFO were 500 and 80 mg, respectively. We considered a body weight of 50 kg to be a reasonable number because the average weight of Japanese women is within the 50 kg range in all age groups. In addition, 50 kg is the median of the weight ranges listed in the package inserts of SFO and FCM. We applied 80 mg, not the maximum dose, 120 mg, for a single dose of SFO considering the current clinical practice in Japan. Our previous analysis using the DeSC Healthcare database (unpublished data) showed that 40 mg was the most common single dose of SFO, and 120 mg was infused in approximately 2% of all prescriptions.
For the frequency of hypophosphatemia for SFO, the percentage of respondents in Group 2 who reported having experienced hypophosphatemia was applied because most patients in Japan with IDA seem to use SFO as IV iron treatment. The frequency for FCM was estimated by multiplying this percentage (considered as the frequency for SFO) by the following ratio of blood phosphorus decreased as an adverse event reported in a randomized controlled trial (18.5% for FCM to 20.2% for SFO)Citation28. The frequency of skin discoloration by the drug solution was calculated using the frequency per infusion, considering the percentage of respondents in Group 2 who described having experienced IV iron induced skin discoloration and the average number of infusions for SFO by a prior claims database analysis (9.6 times). As SFO was the mostly used IV iron treatment in Japan, the number for SFO was applied in this estimation. Each utility was calculated by linear interpolation of the results of the conjoint analysis.
Additionally, the utility value for each attribute per episode was estimated by assuming the available treatment in Japan; SFO and FCM were assumed as Drug A and Drug B, respectively, to support an interpretation of the results. The intangible costs were further estimated by comparing the utility value of each attribute and the monetary attribute (out-of-pocket cost for one IV infusion). The utilities of each factor associated with one infusion (waiting time, pain, time required for infusion, and skin discoloration by the drug solution) were multiplied by the required number of infusions to achieve treatment effect. These utility values per episode and their intangible costs were also estimated for Drug B′, an IV iron formulation not yet commercially available in Japan, by referring to the results of an open-label trial of ferric derisomaltose (FDI)Citation29. In Drug B′, the number of infusions required and frequency of hypophosphatemia were reduced compared with Drug B. The number of infusions required was assumed to be twice, and the frequency of hypophosphatemia was estimated by multiplying the frequency estimated for SFO by the following ratio of hypophosphatemia in an open-label trial (5.9% for FDI to 55.5% for SFO)Citation29.
SAS version 9.4 and Microsoft Excel 2016 were used for data analyses.
Ethics statement
This study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki, the Ethical Guidelines for Life Science and Medical Research Involving Human Subjects by the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labour and Welfare, and the Ministry of EconomyCitation30, and the checklist of good research practices for conjoint analysis formulated by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR)Citation25. Before conducting the survey, the respondents were informed of its purpose, the nature of the information to be obtained, and the right to withdraw from the survey via the website before they provided their consent to participate. Only data from those who answered all questions were included in the analysis. This study was approved by the Ethics Committee of the Research Institute of Healthcare Data Science (No. RI2022001) before the online survey was conducted. In addition, this study is registered under the University Hospital Medical Information Network Clinical Trial Registry (UMIN000047756).
Results
Characteristics of the respondents
The number of respondents was 1,026, 1,052, and 1,049 for Groups 1, 2, and 3, respectively, with an average age of 46.2, 48.0, and 48.2 years, respectively ().
Table 2. Number of respondents by gender and age group for men (A) and women (B).
Actual situation of IDA and IV iron treatment
The underlying cause of IDA by gender, the experience of oral iron treatment, and symptoms of anemia in patients with IDA in Groups 2 and 3 are shown in Table S2. The most common response for the underlying cause of IDA was “unknown” for both genders (men: 62.3% and women: 48.4%) in Group 3. For Group 2, renal disease/renal failure was the most common response for men (30.0%) and menstrual bleeding for women (35.6%). Respondents who reported having received oral iron treatment were 84.8% and 54.0% in Groups 2 and 3, respectively. The top three symptoms of IDA were fatigue (20.1% and 20.0% for those in Groups 2 and 3, respectively), tiredness (21.6% and 23.0%, respectively), and dizziness (19.1% and 22.0%, respectively).
shows the responses of Group 2 regarding their IV treatment in terms of pain, hypophosphatemia, and skin discoloration by the drug solution. The most frequent response to the degree of pain was 4 (hurts a little more; 41.5%) with an average response level of 3.05. Further, 14.2% and 20.3% of participants in Group 2 reported having experienced IV iron induced hypophosphatemia and skin discoloration, respectively.
Table 3. Degree of pain (A), experiences of hypophosphatemia (B), and skin discoloration by the drug solution (C) due to intravenous iron infusion treatment.
Utility value for each factor based on the conjoint analysis
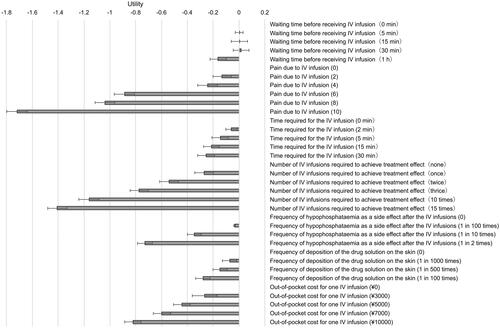
The utilities of each level for each attribute based on the conjoint analysis of Group 1 are shown in and Table S3. Other than the increase in waiting time before receiving an IV infusion from 5 to 15 min and 30 min, the utilities decreased with increases in time, number, and frequency of the factors. The utilities for each attribute assuming two IV iron treatment types are as follows. For 15 min of waiting time before receiving an IV iron infusion, the utility was estimated to be 0.004 (95% confidence interval [CI]: −0.061, 0.068). For the pain level of 3.05, the utility was estimated to be −0.189 (95% CI: −0.263, −0.116). For 5 and 15 min of time required for the IV infusion, the utilities were estimated to be −0.145 (95% CI: −0.209, −0.081) and −0.212 (95% CI: −0.275, −0.150), respectively. For 3 and 25 times of the infusion required to achieve treatment effect, the utilities were estimated to be −0.773 (95% CI: −0.845, −0.702) and −1.899 (95% CI: −1.968, −1.829), respectively. The frequencies of hypophosphatemia were estimated to be 13.0% for FCM and 14.2% for SFO, and the utilities for these frequencies were estimated to be −0.373 (95% CI: −0.427, −0.319), and −0.385 (95% CI: −0.439, −0.331), respectively. The frequency of skin discoloration by the drug solution was estimated to be 2.3% per IV infusion, and the utility for this frequency was estimated to be −0.502 (95% CI: −0.558, −0.445).
The utilities of each level for each attribute for Groups 2 and 3 are shown in . In most attributes, the utilities tended to be lower in Groups 2 and 3 than in Group 1. Particularly, the utility of out-of-pocket costs for both Groups 2 and 3 was much lower than that for Group 1. When comparing Groups 2 and 3, the utilities tended to be lower for Group 3 regarding most attributes.
Table 4. Utility of each level for each attribute for patients with iron-deficiency anemia undergoing intravenous iron treatment (Group 2; A) and those not receiving intravenous iron treatment (Group 3; B).
Estimated intangible costs of IV iron treatment per episode
Drug A (with shorter treatment time and a greater number of required infusions to achieve treatment effect) had larger intangible costs per episode for pain, time required for infusion, required number of infusions to achieve treatment effect, and skin discoloration by the drug solution than Drug B (). Among these factors, the difference in intangible costs between these drugs was the greatest for skin discoloration, followed by pain, time required for infusion, and required number of infusions. Comparing the intangible costs per episode between Drug B and Drug B′, the costs of Drug B were larger for discoloration by the drug solution, hypophosphatemia, and required number of infusions to achieve treatment effect in descending order of the difference ().
Table 5. Estimation of utilities and intangible costs for one intravenous iron treatment episode assuming commercially available intravenous iron treatments (A) and when changing the number of required infusions and the incidence of hypophosphatemia of drug B (B).
Discussion
This study estimated the utility values for factors associated with IV iron treatment among patients with IDA in Japan. To the best of our knowledge, this is the first study of its kind in Japan. A conjoint analysis was performed based on an online survey comprising members of the general population of Japan. The results indicated that the utilities decreased with higher pain, longer time required for the IV infusion, a greater number of required infusions to achieve treatment effect, and a higher frequency of adverse events. When considering available treatments for each attribute, the utility for the required number of infusions had a higher negative value than that for side effect-related attributes. Although the magnitude of the decrease varied by factor, differences in factors among treatments seemed to affect the patients’ preferences.
The number of required infusions to achieve treatment effect affected the total utilities per episode, and the number itself seems to have a significant effect. A physician (acknowledged in the Declarations section) mentioned that, based on his clinical experience, it was difficult to continue IV iron treatment frequently and for a long time. In fact, a database analysis indicated that even for SFO, whose maximum single dose is just 120 mg per infusion, more than 10% of patients received only one infusion (unpublished data). The physician also suggested that a treatment strategy sometimes must consider the possible frequency and episode length in addition to the required number of infusions for full iron repletion. Many physicians including himself have desired IV iron formulations with fewer required number of infusions to achieve treatment effect.
The utility values did not decrease when the waiting time before receiving an IV infusion was increased from 5 to 15 min and 30 min. Given the relatively large range of 95% CIs of the utility values, it does imply that the impact of an increase in waiting time (within 30 min) varied among the respondents, with some demonstrating not being bothered by the increase in waiting time.
It is noted that this study did not incorporate an opt-out alternative, which allows the respondents not to choose either treatment option, in the conjoint survey. This is because we considered the situation described in the scenario to be not so serious and that the respondents would be able to choose the preferable one of two different treatment options each time. In addition, it was clearly stated in the introduction of the survey that respondents were able to withdraw at any time, so we considered that an opt-out alternative was not necessary in order to protect them.
This study quantitatively examined the influence of factors associated with IV iron treatment aside from treatment effectiveness. The importance of treatment process attributes, such as administration route and dose frequency, has been recently observed in related literature. A review study reported that treatment process attributes have a quantifiable impact on patients’ preference and willingness to pay for treatment, although safety and efficacy were the primary patient concerns in many situationsCitation19. A systematic review suggested preferences for convenience-related process attributes exist, independent of health outcomesCitation17. Notably, the last cited study mentioned the difficulties related to assessing the magnitude of the preferences owing to the differences in countries or regions and disease types, diverse methodologies, and the attributes being assessed. Nevertheless, prior research has suggested the influence of the treatment process on treatment adherence, which can in turn affect health outcomesCitation20–22. Some health technology assessment agencies including the National Health and Care Excellence in the United Kingdom have recently recognized the importance of treatment process characteristics and that they could be considered in the assessment if the value could be fully explained and additional benefits could be quantifiedCitation17,Citation23. Regarding the treatment process attributes for IV iron treatment among patients with IDA, a Chinese study assessed the disutility of the process attributes using the TTO method among respondents from the general population. The study reported that shorter required time for infusion and treatment not associated with a risk of hypophosphatemic osteomalacia were associated with higher utility value for patients with IDACitation18; these results are consistent with our current findings.
The impact in the differences in factors associated with IV iron treatment by drug types was indicated as the difference in intangible costs per episode when assuming available treatment in Japan. In addition, when comparing Drug B, which assumed available treatment (with a smaller number of required infusions to achieve treatment effect among available treatment), to Drug B′, which assumed the treatment not yet commercially available in Japan, the intangible costs related to skin discoloration showed the largest decrease, followed by hypophosphatemia, and required number of infusions. Considering the results, if Drug B′ becomes available, patients may be less concerned about the side effects and frequency of infusions, which would in turn increase their treatment satisfaction, support treatment compliance, and thereby support the overall treatment goal of achieving full iron repletion.
Approximately 20% of study respondents reported having experienced skin discoloration from the drug solution, which was calculated to be 2.3% per infusion. In Japan, the package inserts of the IV iron formulations state that extravascular leakage of the drug solution may cause long-term skin staining and care should be taken to avoid leakage when infusing the drug. However, the frequency of this outcome is unknown. In clinical trials performed outside Japan, skin discoloration occurred in 0.7Citation31 to 1.3%Citation32 of the patients, which is lower than the frequency in our study. An Australian study indicated that iron staining is an unwanted adverse effect of IV iron treatmentCitation33, and that though the incidence of iron staining appears to be relatively low, it may be under-reported. Factors suggested to minimize the risk of skin discoloration in previous literatureCitation33 include ensuring correct injection site, administration technique, and closely monitoring for signs and symptoms of extravasation, which may have contributed to the difference in the incidences among the studies. Differences in characteristics of populations between countries may also be associated with the difference in incidence rates of skin discoloration. A physician mentioned that one of the reasons for the difference in this incidence could be that more information may be provided before the treatment in other countries than in Japan to prevent the risk of lawsuits, which may make patients less sensitive to skin discoloration. The physician also suggested that patients with IDA tend to have thinner blood vessels than regular patients, so skin discoloration may happen more often in such patients; Japanese patients may have thinner blood vessels than their Western counterparts. These characteristics may have contributed to the higher incidence rates in the current study.
Limitations
Although the present study reveals important findings, it has several limitations. Since this study is based on an online survey of internet panels, the reliability of the results depends on whether the respondents accurately understood the questions and the extent to which they answered the questions truthfully. Particularly, although IV iron treatment and symptoms of IDA were explained to participants at the beginning of the survey, respondents may understand the symptoms and treatments differently depending on their experiences, thereby affecting their responses. Moreover, for the conjoint analysis, we intended for the attribute “frequency of hypophosphatemia” to be a side effect after the IV infusion for one episode. However, respondents may have gotten confused regarding whether it was intended for one episode or one infusion session in the attribute options written in Japanese. Nevertheless, we decided to examine the utility as per episode as originally intended, which yields a more conservative data set for comparing the results between drugs. Additionally, since most cases of hypophosphatemia are asymptomatic or similar to anemia symptoms, it often cannot be discovered unless blood tests are performedCitation34. In this study, we assumed that many respondents who experienced hypophosphatemia had been diagnosed based on the symptoms, so that we estimated the utility value of hypophosphatemia by applying its percentage, rather than the incidence in the RCTs, upon the explanation of its symptoms to respondents of the conjoint analysis survey. In RCTs, as hypophosphatemia might be determined based on serum phosphorus level with or without symptoms, the incidence was higher than the percentage based on this survey. We also cannot ignore the possibility of recall bias due to the data having been collected using an online survey.
Next, the respondents of this study were obtained from an online panel, which may affect the generalizability of the results due to selection bias. Still, respondents in Group 1 were recruited nationwide and their proportions of gender and age corresponded to the Japanese population. However, the demographics and clinical situations of patients with IDA, such as IDA severity and comorbidities, were not considered in this study. Therefore, they may not represent Japanese patients with IDA and IV iron treatment in Japan. Consequently, although we estimated the impact of utilities on available IV iron treatments in which the patients’ answers were considered, the results may still be biased due to sample characteristics. Additionally, this study allowed the respondents to withdraw from the survey at any time. For these respondents all previous responses were not reflected in the results and the opportunity to answer subsequent questions was lost, which may affect the generalizability. Nevertheless, they were consistently excluded because the reasons for leaving from the survey may vary and could not be identified.
Despite these limitations, this study evaluated the utility values of factors associated with IV iron treatment aside from effectiveness-related factors, and examined actual treatment situations of IV iron treatment, both topics which have been scarcely investigated in Japan. Considering that the database analysis showed potential insufficiency related to the provision of IV iron treatment in Japan for meeting the patients’ needs for iron, this study provides significant data for the improvement of IV iron treatment process, potentially helping Japanese patients with IDA to achieve full iron repletion.
Conclusions
This study suggests that the factors associated with IV iron treatment contribute to patients’ preferences. Among the factors investigated, skin discoloration by the drug solution showed a more negative impact for the drug with a greater number of required infusions but a shorter treatment time when assuming actual IV iron formulations available in Japan. The treatment that required a lower number of infusions (from thrice to twice) and had lower incidence rates for hypophosphatemia (from 13.0 to 1.5%) showed a significant improvement in the utilities, particularly for IV iron induced hypophosphatemia and skin discoloration. Consequently, new treatments and/or improvements in available treatments may improve the treatment process for patients and thereby support treatment compliance and the achievement of full iron repletion. The information provided by this study regarding the relative impact of different treatment factors on patient utility may be useful for the assessment of future treatments and treatment selection for patients with IDA.
Transparency
Author contributions
All authors contributed to the study conception, design, and interpretation of findings. Data analysis was performed by KI and CH. The first draft of the manuscript was written by TT, and KI and CH revised it critically for important intellectual content. All authors approved the final version of the manuscript for submission.
Acknowledgements
The authors are grateful to Dr. Seiji Eto, ETO Clinic, for the valuable discussions on the interpretation of the results. We would like to thank Dr. Shoichi Irie, Milliman Inc., for his support in drafting the manuscript. We also thank INTAGE Healthcare Inc. for the execution of the online survey and Editage for English language editing.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Previous presentations
Not applicable.
Supplemental Material
Download PDF (221.6 KB)Declaration of funding
This study was supported by Nippon Shinyaku Co., Ltd. and Pharmacosmos A/S.
Declaration of financial/other relationships
TT, CH, and KI are employees of Milliman Inc., which has received research funding from Nippon Shinyaku Co., Ltd. and Pharmacosmos A/S. The supporting companies provided information regarding IV iron treatment and advice on this study, but was not involved in the study design, the design and implementation of the survey, data analysis, interpretation of data, writing of the report, and decision on submission of the report for publication.
References
- United Nations, World Health Organization. Iron deficiency anaemia: assessment, prevention, and control: a guide for programme managers. Geneva: world Health Organization; 2001.
- Achebe MO, Mandell E, Jolley K, et al. Pagophagia and restless legs syndrome are highly associated with iron deficiency and should be included in histories evaluating anemia. Am J Hematol. 2022 [cited 2022 Dec 12]; [3 p.]. DOI: 10.1002/ajh.26775.
- Borgna-Pignatti C, Zanella S. Pica as a manifestation of iron deficiency. Expert Rev Hematol. 2016;9(11):1075–1080.
- Allen RP, Auerbach S, Bahrain H, et al. The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol. 2013;88(4):261–264.
- Kassebaum NJ, GBD 2013 Anemia Collaborators. The global burden of anemia. Hematol Oncol Clin North Am. 2016;30(2):247–308.
- Uchida T, Kawachi Y, Sakamoto Y[, et al. Prevalence and pathogenesis of iron deficiency in Japanese women (1981–1991). Rinsho Ketsueki. 1992;33(11):1661–1665.
- Ministry of Health, Labour and Welfare. The national health and nutrition survey in Japan. Tokyo: Ministry of Health, Labour and Welfare; 2019.
- Iimori S, Naito S, Noda Y, et al. Anaemia management and mortality risk in newly visiting patients with chronic kidney disease in Japan: the CKD-ROUTE study. Nephrology. 2015;20(9):601–608.
- Mansurova JA, Karazhanova LK. Independent predictors of adverse cardiovascular events in patients with acute coronary syndrome after percutaneous coronary intervention during hospitalization. Kardiologiia. 2018;58(12):22–29.
- Rössler J, Schoenrath F, Seifert B, et al. Iron deficiency is associated with higher mortality in patients undergoing cardiac surgery: a prospective study. Br J Anaesth. 2020;124(1):25–34.
- Ikuta K, Ito H, Takahashi K, et al. Safety and efficacy of intravenous ferric carboxymaltose in Japanese patients with iron-deficiency anemia caused by digestive diseases: an open-label, single-arm study. Int J Hematol. 2019;109(1):50–58.
- Keating GM. Ferric carboxymaltose: a review of its use in iron deficiency. Drugs. 2015;75(1):101–127.
- Injectafer [package insert]. American Regent, Inc.; 2022 [cited 2022 Dec 12]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/203565Orig1s019lbl.pdf.
- Iwasaki K, Takeshima T, Tateyama M, et al. Utilization of intravenous iron preparations for iron deficiency anemia: a longitudinal study using a Japanese health insurance claims database with annual health check-ups in 2018–2021. Forthcoming ISPOR Europe 2022; 2022 Nov 6 − 9; Vienna, Austria and Virtual.
- Toblli J, Angerosa M. Optimizing iron delivery in the management of anemia: patient considerations and the role of ferric carboxymaltose. Drug Des Devel Ther. 2014;8:2475–2491.
- Rund D. Intravenous iron: do we adequately understand the short- and long-term risks in clinical practice? Br J Haematol. 2021;193(3):466–480.
- Higgins A, Barnett J, Meads C, et al. Does convenience matter in health care delivery? A systematic review of convenience-based aspects of process utility. Value Health. 2014;17(8):877–887.
- Wu D, Zhang Y, Boegelund M, et al. Development of a diminishing marginal disutility model for intravenous iron infusions based on data from a time trade-off study in China. Virtual ISPOR Europe 2021; 2021 Nov 30 − Dec 3; Virtual.
- Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385–1399.
- Hixson-Wallace JA, Dotson JB, Blakey SA. Effect of regimen complexity on patient satisfaction and compliance with warfarin therapy. Clin Appl Thromb Hemost. 2001;7(1):33–37.
- Shikiar R, Rentz AM. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health. 2004;7(2):204–215.
- Matza LS, Stewart KD, Lloyd AJ, et al. Vignette-based utilities: usefulness, limitations, and methodological recommendations. Value Health. 2021;24(6):812–821.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. London: National Institute for Health and Care Excellence; 2013 (Process and methods (PMG9).
- Bien DR, Danner M, Vennedey V, et al. Patients’ preferences for outcome, process and cost attributes in cancer treatment: a systematic review of discrete choice experiments. Patient. 2017;10(5):553–565.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413.
- Fesin injection 40mg. Package insert. Nichi-iko Pharmaceutical Co., Ltd.; 2014 [cited 2022 Dec 12]. Available from: https://www.info.pmda.go.jp/go/pack/3222400A1058_1_03 (Japanese).
- Ferinject solution for injection/infusion 500mg. Package insert. Zeria Pharmaceutical Co., Ltd.; 2021 [cited 2022 Dec 12]. Available from: https://www.info.pmda.go.jp/go/pack/3222404A1021_1_03/ (Japanese).
- Ikuta K, Hanashi H, Hirai K, et al. Comparison of efficacy and safety between intravenous ferric carboxymaltose and saccharated ferric oxide in Japanese patients with iron-deficiency anemia due to hypermenorrhea: a multi-center, randomized, open-label noninferiority study. Int J Hematol. 2019;109(1):41–49.
- Kawabata H, Tamura T, Tamai S, Study Group, et al. Intravenous ferric derisomaltose versus saccharated ferric oxide for iron deficiency anemia associated with menorrhagia: a randomized, open-label, active-controlled, noninferiority study. Int J Hematol. 2022;116(5):647–658.
- Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labour and Welfare, and Ministry of Economy, Trade and Industry. Ethical guidelines for life science and medical research involving human subjects. Tokyo: ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labour and Welfare, and Ministry of Economy, Trade and Industry; 2021 (partial revision in 2022, Japanese).
- Qunibi WY, Martinez C, Smith M, et al. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant. 2011;26(5):1599–1607.
- Anker SD, Comin Colet J, Filippatos G, FAIR-HF Trial Investigators, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436–2448.
- Canning M, Grannell L. A stain on iron therapy. Aust Prescr. 2020;43(5):160–163.
- Tebben PJ. Hypophosphatemia: a practical guide to evaluation and management. Endocr Pract. 2022;28(10):1091–1099.