Abstract
Aims
To compare the efficacy and safety of abobotulinumtoxinA (aboBoNT-A) and onabotulinumtoxinA (onaBoNT-A) for the treatment of refractory neurogenic detrusor overactivity (NDO), using an indirect treatment comparison (ITC).
Materials and methods
A systematic literature review was used to identify randomized controlled trials (RCTs) that evaluated botulinum toxin type A for the treatment of refractory NDO. Treatments were compared using a Bucher ITC approach. Efficacy outcomes were reduction in number of weekly urinary incontinence (UI) episodes at 6, 12, and 24 weeks of follow-up. The safety outcome was the proportion of patients with treatment-emergent urinary tract infections (TE-UTIs) during follow-up. Subgroup/sensitivity analyses were performed to investigate the impact of heterogeneity.
Results
Fifteen studies of botulinum toxin type A were identified. Among these, onaBoNT-A 200 U was the only botulinum toxin type A considered an appropriate comparator for aboBoNT-A 600 U and 800 U. As such, six RCTs that evaluated onaBoNT-A or aboBoNT-A were included in the ITC. In base-case analyses, there were no statistically significant differences between aboBoNT-A and onaBoNT-A in terms of UI episodes or TE-UTIs. Numerically, the trend favored aboBoNT-A (either dose) for all endpoints and time points. At 12 and 24 weeks, the difference in reduction of UI episodes per week was considered clinically relevant when comparing aboBoNT-A 800 U with onaBoNT-A 200 U, but not when comparing the lower dose of aboBoNT-A (600 U) with onaBoNT-A 200 U. Results from subgroup/sensitivity analyses were consistent with the base case.
Limitations
Heterogeneity across studies was observed; however, strong consistency of trends across analyses suggests the impact of heterogeneity is low.
Conclusions
There may be potential advantages of aboBoNT-A over onaBoNT-A, in terms of UI reduction, in patients with refractory NDO. More confirmatory studies are needed owing to the sparsity of current evidence.
PLAIN LANGUAGE SUMMARY
Neurogenic detrusor overactivity (NDO) is a condition in which the bladder muscle wall is overactive and does not function normally. This can lead to urinary incontinence (i.e. accidental leakage of urine). NDO may also cause urinary tract infections and upper urinary tract damage if it is left untreated or if treatment does not work (i.e. refractory NDO).
Botulinum toxin is a treatment that relaxes muscles in patients with refractory NDO, so they have less chance of experiencing urinary incontinence. This study used results from clinical trials to compare two types of botulinum toxin – abobotulinumtoxinA (aboBoNT-A) and onabotulinumtoxinA (onaBoNT-A) – to see if one works better than the other.
Clinical trials are experiments to assess how well treatments work by giving different treatments to different patients and observing the results. When there is no clinical trial that compares the two treatments you are interested in, it is possible to combine results from a number of different clinical trials instead. This is known as an indirect treatment comparison.
We used an indirect treatment comparison to compare aboBoNT-A and onaBoNT-A for the treatment of refractory NDO. Results showed that aboBoNT-A may be more effective than onaBoNT-A in reducing the frequency of urinary incontinence episodes. On average, patients treated with aboBoNT-A had at least three fewer episodes of urinary incontinence per week than those treated with onaBoNT-A. These results suggest that patients treated with aboBoNT-A could have a better quality of life than those treated with onaBoNT-A.
Introduction
Neurogenic detrusor overactivity (NDO) is a urodynamic bladder dysfunction that may lead to urinary incontinence (UI), increased urgency to urinate, and nocturia. Untreated NDO is associated with increased risk of urinary tract infections (UTIs) and upper urinary tract deteriorationCitation1. Anticholinergic (antimuscarinic) agents are typically the first-line treatment for NDO and are used to reduce UI and to increase bladder capacityCitation2,3. However, long-term management with these oral therapies is limited owing to undesirable adverse events (AEs), including blurred vision, cognitive impairment, and dry mouth, or contraindications, including glaucoma, myasthenia gravis, and ulcerative colitisCitation2–4. For patients unable to urinate spontaneously or to empty their bladder, clean intermittent catheterization (CIC) is considered the standard of care, requiring patients to self-catheterize multiple times per dayCitation2,5,6. In addition, anticholinergics may still be prescribed to prevent incontinence episodes between CIC proceduresCitation7,8.
Alternative treatments to anticholinergics are botulinum toxins type A, such as abobotulinumtoxinAFootnotei (aboBoNT-A) and onabotulinumtoxinAFootnoteii (onaBoNT-A). Botulinum toxin type A is among the seven neurotoxin subtypes (named A to G) produced by Clostridium botulinum. Of the subtypes, botulinum toxin type A has the longest duration of action, making it most relevant, clinicallyCitation9. Botulinum toxin type A is available in different brands according to the proteins that wrap the toxin. In the case of onaBoNT-A and aboBoNT-A, the cover proteins cause a substantial difference in the molecular weight (900 kDa and 400 kDa, respectively)Citation9. These neurotoxins can be delivered to an affected muscle via intramuscular injection, therein blocking the release of acetylcholine at the neuromuscular junction to prevent muscle contractionCitation10. For urological conditions, botulinum toxin type A can be delivered to the bladder wall to prevent involuntary detrusor contractions that lead to incontinenceCitation11. OnaBoNT-A is licensed in the USA and the EU for the treatment of NDO associated with UI (NDOi)Citation12, based on evidence from randomized controlled trials (RCTs)Citation2,6. AboBoNT-A is approved in the EU for the treatment of NDOiCitation13. This approval is supported by evidence from two RCTs in which aboBoNT-A intradetrusor injections led to a reduction in UI episodes in patients with NDOi in whom anticholinergic therapy failed to induce an adequate response (i.e. refractory NDOi) or was unsuitable owing to intoleranceCitation14–16.
Direct comparative evidence of onaBoNT-A versus aboBoNT-A is not yet available to inform NDOi treatment decisions. However, in the absence of head-to-head clinical trial data, indirect treatment comparisons (ITCs) are well-established methods that can be used to compare the efficacy of treatmentsCitation17,18.
The objective of this study is to compare the efficacy and safety of aboBoNT-A and onaBoNT-A in refractory NDOi using an ITC.
Methods
Systematic literature review
A systematic literature review (SLR) was conducted in accordance with the standards required by health technology assessment organizations and other established guidelines (i.e. Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] and the Cochrane Handbook for Systematic Reviews of Interventions)Citation19,20. Note, the protocol for this review is not registered or otherwise saved online, but is described in full in this manuscript. Searches of global electronic databases (MEDLINE, Embase, the Cochrane Library, and the Database of Abstracts of Reviews of Effectiveness) were performed in August/September 2020, to identify RCTs that assessed aboBoNT-A and onaBoNT-A for the treatment of patients with NDOi in whom prior oral medical therapy induced an inadequate response or was unsuitable owing to intolerance. The search terms used to search the electronic databases can be found in Supplementary Table S1. These include key terms related to disease, intervention, and study designs of interest. For disease, we restricted search terms to NDO because this aligns with the indication of aboBoNT-A. As such, we did not search for related definitions such as neurogenic lower urinary tract dysfunction, because these are not of direct relevance to aboBoNT-A.
Table 1. Baseline characteristics of studies included in Bucher indirect treatment comparisons.
No date restriction was applied to the electronic database search. A supplemental search of ClinicalTrials.gov was conducted to identify clinical trials registered between 2016 and 2020 that had not been detected in other searches. Relevant conference proceedings that took place during 2018–2020 were also searched. In September 2021, targeted searches were performed to identify any new RCTs for which data were published.
Identified abstracts were screened, double blind, by two systematic reviewers against Population, Intervention, Comparator, Outcome, and Study design (PICOS) eligibility criteria, with uncertainties resolved by a third reviewer. Full texts of relevant articles were then screened, using the same double-blind approach. Full details of the PICOS inclusion and exclusion criteria can be found in Supplementary Table S2.
Data were extracted from eligible studies by one reviewer and validated by a second reviewer. Study quality was assessed using the Cochrane Risk of Bias tool for RCTs v2.0Citation20.
Feasibility assessment of indirect treatment comparison
A feasibility assessment was conducted to determine which of the RCTs identified in the SLR should be included in the ITCs, as well as which endpoints could be analyzed. Efficacy endpoints reported in all trials, and therefore suitable for analysis, were change from baseline in number of weekly UI episodes and the proportion of patients with 100% reduction in number of weekly UI episodes. Several studies reported daily UI episodes; conversion from daily to weekly number of UI episodes was performed by multiplying by seven. Assessment time points for all endpoints were consistent across studies, and included weeks 6, 12, and 24 after treatment.
Suitability of the RCTs included for each endpoint analysis was partly based on the likelihood of clinical heterogeneity among the studies, determined by observing potential differences in patient characteristics across study populations. The characteristics used were based on a list of potential treatment effect modifiers for patients with NDO identified via literature review and discussion with clinical experts; the list included patient demographics, disease characteristics, and prior and concomitant treatments. Following assessment, studies were not excluded based on differences in potential treatment effect modifiers. Instead, several approaches were planned, a priori, to address the potential impact of heterogeneity. In one approach, we planned to investigate the potential impact of CIC experience at baseline through subgroup analysis of CIC-experienced patients. In addition, although clinical experts did not expect age, sex, and ethnicity to be treatment effect modifiers, sensitivity analyses were used to investigate the impact of removing study populations with demographic characteristics that were notably different from other studies.
Suitability of the RCTs was also based on comparability of interventions. OnaBoNT-A 200 units (U) was considered the only relevant comparator for aboBoNT-A, based on treatments licensed for NDO in the USA and EU at the time searches were conducted. Therefore, the ITC was restricted to evaluation of onaBoNT-A at the 200 U dose.
The feasibility assessment was also used to confirm that a network of evidence could be linked for the RCTs of interest.
Study endpoints
Efficacy outcomes of interest for the analyses were change from baseline in number of weekly UI episodes and the proportion of patients with 100% reduction in number of weekly UI episodes. Efficacy findings were interpreted as clinically meaningful if a mean reduction of three UI episodes per week was observed. This is in accordance with the minimal clinically important change threshold reported in the literature, which was based on UI and health-related quality of life data in patients with overactive bladder syndrome (OAB) with incontinenceCitation21.
The safety outcome of interest was the proportion of patients with treatment-emergent UTIs (TE-UTIs).
Outcomes were assessed at common time points across the studies: 6, 12, and 24 weeks for number of weekly UI episodes; 6 weeks for proportion of patients with 100% reduction in number of weekly UI episodes; and the end of the study for TE-UTIs.
Statistical analyses
ITCs were conducted based on the Bucher approach, using random-effects metamodels to synthesize aggregate data when feasibleCitation22. Fixed-effects models were fitted when a single study generated the clinical data for each comparison or when between-study heterogeneity could not be measured; in these cases, no meta-analyses were feasibleCitation22. Arm-level data (i.e. outcomes measured within individual treatment arms) were used in all calculations because they were more widely available than contrast-level data (i.e. relative effects between two treatment arms). When an analysis involved data with zero events, continuity correction was used. Given that a division by zero is not possible, 0.5 was added to all events and nonevents in the analysis.
Bucher ITCs generated estimates of relative effects, along with 95% confidence intervals (CIs) and two-tailed p-values. For odds ratios (ORs), all comparisons were calculated based on the log-estimates, after which the point estimates and 95% CIs were exponentiated. For mean differences, all calculations were done in the original metric. See Supplementary Table S3 for a list of outcomes and corresponding analytical approaches. All analyses were carried out using the metafor packageCitation23 in R 3.5.1.
Base-case and subgroup analyses were performed to analyze efficacy endpoints. In addition, sensitivity analyses were conducted that excluded one studyCitation24 because its population was younger and included more male patients than the other studiesCitation23. Analyses included all patients with NDOi in whom prior oral medical therapy induced an inadequate response or was unsuitable owing to intolerance. Where available, data from the intention-to-treat (ITT) analysis sets of each trial were used. Otherwise, analysis included all randomized patients who received at least one dose of the study drug, defined in the current study as the modified ITT population. An overview of the studies and analysis populations included in the base-case, subgroup, and sensitivity analyses of each endpoint is provided in Supplementary Table S4. Findings from each outcome analysis are presented visually using forest plots.
Results
Overview of studies identified from the systematic literature review
Fifteen relevant RCTs evaluating the efficacy and safety of botulinum toxin type A for refractory NDO were identified in the SLR (). After removing comparators not relevant for aboBoNT-A (see Feasibility assessment for details), six unique RCTs that evaluated aboBoNT-A or onaBoNT-A were eligible for inclusion in the base-case ITCs: the CONTENT1 and CONTENT2 trials (N = 226 and 257, respectively), which compared aboBoNT-A 600 U and 800 U with placeboCitation14,15, and four clinical trials comparing onaBoNT-A 200 U with placebo, namely DIGNITY-1 (N = 416); DIGNITY-2 (N = 275); Apostolidis et al. (N = 73); and Schurch et al. (N = 59)Citation24–27. Overall, there were 1,306 patients across the six trials. Patient characteristics for each study are shown in , including demographics, disease factors (duration, etiology [multiple sclerosis or spinal cord injury]), and current treatment use. Depending on the outcome, time point, and/or patient group of interest, all analyses (including subgroup and sensitivity analyses) were informed by three to six studies (). An overview of the studies included in the base-case, subgroup, and sensitivity analyses by efficacy and safety outcomes is provided in Supplementary Table S4.
Figure 1. SLR Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram. aBibliographies were searched for relevant citations. Note, no additional studies were identified during targeted searches performed in September 2021. Abbreviations. CDSR, Cochrane Database of Systematic Reviews; CENTRAL, Cochrane Central Register of Controlled Trials; DARE, Database of Abstracts of Reviews of Effectiveness; ITC, indirect treatment comparison; MA, meta-analysis; NMA, network meta-analysis; SLR, systematic literature review.
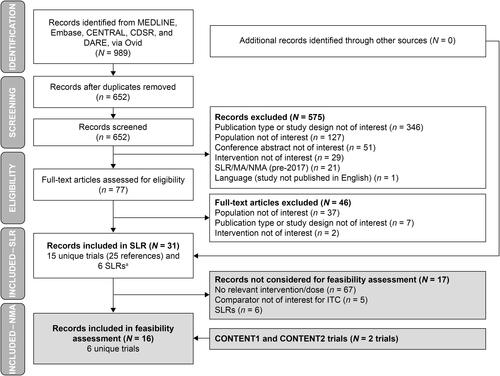
Figure 2. Network diagram for base-case analysis and CIC-experienced subgroup analysis. Note, base-case analysis includes all studies; CIC-experienced subgroup analysis excludes the Apostolidis et al.Citation24 trial and the two DIGNITY trials; sensitivity analysis excludes the Apostolidis et al.Citation24 trial. Abbreviations. CIC, clean intermittent catheterization; U, units.

Five of the six RCTs were phase 3 clinical trials; Apostolidis et al.Citation24 was a phase 2 clinical trial. The six RCTs generally had similar enrollment criteria, including patients aged 18 years or older, with an average of two UI episodes per day, whose disease was not adequately managed by anticholinergics. Patients with a history of hematuria, pelvic or urological abnormalities, bladder or prostate cancer, or prior surgery affecting bladder function were excluded. Patient populations across the six clinical trials were generally similar regarding age and sex, apart from the Apostolidis et al.Citation24 trial population, which was younger and included more male patients than other trial populations (). The two CONTENT trialsCitation14,15 and the Schurch et al.Citation27 study were limited to CIC-experienced patients, whereas the other clinical trials included some patients without prior CIC experience at baseline.
Overall, risk of bias was low across all six RCTs included in the ITCs and across all individual Cochrane Risk of Bias tool domains (Supplementary Table S5). There were some concerns regarding the randomization process for DIGNITY-1Citation26; however, these concerns did not warrant downgrading of the overall bias rating.
Outcome data for each individual study included in the ITCs are presented in Supplementary Table S6.
Change from baseline in weekly urinary incontinence frequency
In the broad base-case population, results for all time points were comparable between aboBoNT-A 600 U and onaBoNT-A 200 U (), with a minor numerical trend in favor of aboBoNT-A. The mean difference in change from baseline was more than three UI episodes per week in favor of aboBoNT-A 800 U compared with onaBoNT-A 200 U, at 12 and 24 weeks of follow-up; these differences exceeded the clinically meaningful threshold estimated in the literature, although they were not statistically significant. The results were not materially different in the sensitivity analysis, from which the Apostolidis et al.Citation24 trial was excluded (Supplementary Table S7).
Figure 3. Mean difference in change from baseline of number of weekly UI episodes – ABO 600 U and 800 U versus onabotulinumtoxinA 200 U. For base-case analyses, ITT populations were used where available; otherwise, modified ITT populations were used. Fixed-effects results were only used when random-effects meta-analyses could not be conducted because of lack of data. Abbreviations. ABO, abobotulinumtoxinA; CI, confidence interval; CIC, clean intermittent catheterization; ITT, intention-to-treat; U, units; UI, urinary incontinence.
In the CIC-experienced subgroup, there was a consistent numerical trend in favor of aboBoNT-A for both doses and at all time points (). In almost all cases, the size of the differences was clinically meaningful according to a threshold of three UI episodes per week and, in one case (aboBoNT-A 800 U vs onaBoNT-A 200 U at 12 weeks), the difference was statistically significant (mean difference = −9.42; 95% CI = −18.46, −0.39; p < 0.05) ().
Proportion of patients with a 100% reduction in number of weekly urinary incontinence episodes
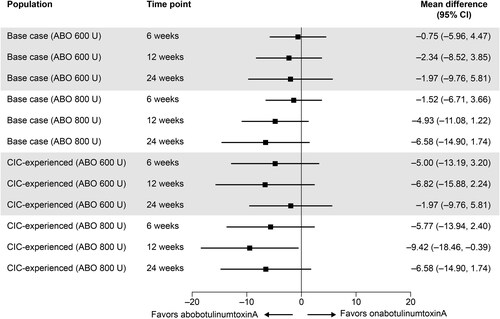
In the base-case population, aboBoNT-A 600 U and 800 U were associated with numerically greater odds of a 100% reduction in number of weekly UI episodes than onaBoNT-A 200 U (ORs [95% CI], 3.03 [0.60, 15.34] and 2.14 [0.66, 7.00], respectively) at the 6-week time point (). This trend was reflected in the sensitivity analysis. However, no CIC-experienced subgroup data on this outcome were available for onaBoNT-A 200 U; therefore, no indirect comparison with aboBoNT-A 600 U or 800 U could be made in this patient group of interest.
Figure 4. Proportion of patients with a 100% reduction in number of weekly UI episodes at week 6 – ABO 600 U and 800 U versus onabotulinumtoxinA 200 U. For base-case analyses, ITT populations were used where available; otherwise, modified ITT populations were used. Abbreviations. ABO, abobotulinumtoxinA; CI, confidence interval; ITT, intention-to-treat; U, units; UI, urinary incontinence.
Proportion of patients with treatment-emergent urinary tract infections
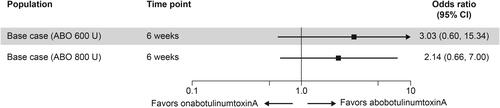
Data on TE-UTIs were reported in all six RCTs identified. Results suggested that patients treated with aboBoNT-A 600 U and 800 U were less likely to experience TE-UTIs than patients treated with onaBoNT-A 200 U, although the ORs (ranging from 0.41 to 0.90) were not statistically significant (p > 0.05). This trend was observed in both the base-case analysis and the CIC-experienced subgroup analysis ().
Figure 5. Odds ratio comparing TE-UTIs in patients receiving ABO 600 U and 800 U with those in patients receiving onabotulinumtoxinA 200 U. Modified ITT is the base-case analysis. Abbreviations. ABO, abobotulinumtoxinA; CI, confidence interval; CIC, clean intermittent catheterization; ITT, intention-to-treat; TE-UTI, treatment-emergent urinary tract infection; U, units.
Discussion
We have reported findings from the first ITC to compare the efficacy and safety of two botulinum toxin type A treatments (onaBoNT-A and aboBoNT-A) in the NDO indication. For the treatment of NDO, and particularly for CIC-experienced patients with NDO, there was a favorable trend for aboBoNT-A 600 U and 800 U compared with onaBoNT-A 200 U for the key efficacy outcomes: change from baseline in weekly UI frequency and proportion of patients with a 100% reduction in number of weekly UI episodes. Although results were not statistically significant, the favorable trend was consistent and, in several analyses, the magnitude of the difference in UI frequency between treatments was clinically meaningful. In terms of safety, there was a numerical trend for fewer UTIs with aboBoNT-A 600 U and 800 U than with onaBoNT-A 200 U, but no significant differences were found.
These ITCs included data from RCTs that were identified from a PRISMA-compliant SLR and used well-established methodology for comparing treatments indirectlyCitation17,18. Although searches were performed in September 2020, more recent targeted searches (September 2021) did not reveal any additional RCTs that would have been eligible for inclusion. The findings of this ITC address an unmet need in terms of providing comparative evidence for botulinum toxin type A treatments for NDO. The study populations identified were of direct relevance to the indicated population for aboBoNT-A (i.e. NDO related to multiple sclerosis or spinal cord injury). The eligibility criteria permitted NDO related to any other neurological conditions; however, no other conditions were identified among the study populations.
The analyses are associated with limitations, owing to small study size and heterogeneity in study design. This resulted in wide CIs for all outcomes. In particular, sparse reporting and a rarity of events with placebo led to wide 95% CIs for indirect comparisons between aboBoNT-A and onaBoNT-A for the proportion of patients with a 100% reduction in number of weekly UI episodes. Thus, the power of the statistical analysis was low to detect significant differences. In this context, it is important to interpret the findings from the standpoint of clinical meaningfulness, together with the statistical significance of differencesCitation28,29. In addition, given that the impact of unmanaged NDO on patients’ quality of life due to UI can be considerableCitation1, even small improvements could have a large, positive impact on the patient. We considered a difference of three UI episodes per week to be clinically important, based on a published minimal clinically important change estimated in patients with OAB and incontinenceCitation21. Although this estimate was not derived from an NDO population, we believe that it is an appropriate threshold to use for NDO because the baseline number of weekly UI episodes in the OAB cohortCitation21 was similar to that observed in the NDO study populations used in the current study.
The current standard of care for patients unable to urinate spontaneously or to empty their bladder, CIC, is associated with several limitations; patient inexperience and/or impaired cognitive functioning can make self-catheterization difficult, and leaving the catheter in place (i.e. indwelling catheterization) can increase risks of UTIs and other AEsCitation2,5,6. CIC is relevant in the current ITC because a key source of heterogeneity among studies was CIC experience in the study populations. The possibility of effect modification due to CIC experience was explored through subgroup analyses. Analysis of the change from baseline in weekly UI frequency in CIC-experienced patients was conducted on the most relevant studies available: CONTENT1 and CONTENT2Citation14,15 for aboBoNT-A 600 U and 800 U, and Schurch et al.Citation27 for onaBoNT-A 200 U. The study by Schurch et al.Citation27 was the only placebo-controlled onaBoNT-A 200 U trial available to inform this CIC-experienced subgroup analysis; it included only 19–21 patients per treatment arm. No onaBoNT-A trials were available to inform an ITC with aboBoNT-A in CIC-experienced patients on the second key efficacy outcome: the proportion of patients with a 100% reduction in number of weekly UI episodes. Even with sparse data, it was deemed important to obtain comparative evidence in this patient subgroup, given that patients experienced with CIC may have a different profile than non-experienced patients or those just starting CIC (e.g. the population in the DIGNITY trials)Citation26; therefore, the efficacy and safety of botulinum toxins type A in CIC-experienced patients may differ from those in other patients. Overall, our subgroup analyses in CIC-experienced patients yielded consistent clinically meaningful trends in favor of aboBoNT-A 600 U and 800 U over onaBoNT-A; owing to small sample sizes, caution is advised regarding interpretation of the analyses, and further comparative studies are needed.
Conclusions
For the treatment of NDO, and particularly for CIC-experienced patients with NDO, this analysis showed a favorable, clinically meaningful trend for aboBoNT-A 600 U and 800 U to provide better key efficacy outcomes, and a numerical trend for a lower risk of developing UTIs, compared with onaBoNT-A 200 U.
Transparency
Declaration of financial/other interests
FC has been consultant and/or investigator for AbbVie (Allergan), Astellas, Bayer, Ipsen, and Recordati. ND and JW were employed by Ipsen at the time of the study. KF, AF, and JT are employees of Evidera, which received funding from Ipsen for performing the SLR and statistical analyses that informed this study.
Author contributions
FC contributed to the interpretation of the data. ND and JW contributed to the conception and design of the study, and the interpretation of the data. KF, AF, and JT performed the statistical analyses and contributed to the interpretation of the data. All authors contributed to the drafting of the paper or revising it critically for intellectual content. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Acknowledgements
Lisa Law (ORCID: https://orcid.org/0000-0002-9837-6609) of Oxford PharmaGenesis, Oxford, UK, provided medical writing support, which was funded by Ipsen, in accordance with Good Publication Practice 3 (GPP3).
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (64.3 KB)Additional information
Funding
Notes
i Proprietary name: Dysport (Ipsen).
ii Proprietary name: BOTOX (Allergan).
References
- Cardarelli WJ. Managed care aspects of managing neurogenic bladder/neurogenic detrusor overactivity. Am J Manag Care. 2013;19(10 Suppl):S205–S208.
- Alsulihem A, Corcos J. Evaluation, treatment, and surveillance of neurogenic detrusor overactivity in spinal cord injury patients. Neuroimmunol Neuroinflammation. 2019;12(6):404–412.
- Yonnet GJ, Fjeldstad AS, Carlson NG, et al. Advances in the management of neurogenic detrusor overactivity in multiple sclerosis. Int J MS Care. 2013;15(2):66–72.
- Madersbacher H, Murtz G, Stöhrer M. Neurogenic detrusor overactivity in adults: a review on efficacy, tolerability and safety of oral antimuscarinics. Spinal Cord. 2013;51(6):432–441.
- Ellsworth PI, Coyle PK, Esquenazi A, et al. Consensus statement on neurogenic detrusor overactivity: multiple sclerosis and spinal cord injury. 2012. Corpus ID: 59115883.
- Taweel WA, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85–99.
- National Institute for Health and Care Excellence (NICE). Urinary incontinence and pelvic organ prolapse in women: management. NICE guideline, published April 2, 2019. https://www.nice.org.uk/guidance/ng123/resources/urinary-incontinence-and-pelvic-organ-prolapse-in-women-management-pdf-66141657205189.
- National Institute for Health and Care Excellence (NICE). Lower urinary tract symptoms in men: management. NICE guideline, published May 23, 2010. https://www.nice.org.uk/guidance/cg97/resources/lower-urinary-tract-symptoms-in-men-management-pdf-975754394053
- Cruz F. Targets for botulinum toxin in the lower urinary tract. Neurourol Urodyn. 2014;33(1):31–38.
- Thakker MM, Rubin PA. Pharmacology and clinical applications of botulinum toxins a and B. Int Ophthalmol Clin. 2004;44:147–163.
- Nitti VW. Botulinum toxin for the treatment of idiopathic and neurogenic overactive bladder: state of the art. Rev Urol. 2006;8(4):198–208.
- U.S. Food and Drug Administration [Internet]. Prescribing information, BOTOX (onabotulinumtoxinA). 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf
- Ipsen [Internet]. Ipsen receives positive opinion in Europe for Dysport® in the management of urinary incontinence in adults with neurogenic detrusor overactivity due to multiple sclerosis or spinal cord injury. 2022. https://www.ipsen.com/press-releases/ipsen-receives-positive-opinion-in-europe-for-dysport-in-the-management-of-urinary-incontinence-in-adults-with-neurogenic-detrusor-overactivity-due-to-multiple-sclerosis-or-spinal-cord-injury/
- ClinicalTrials.gov [Internet]. Dysport® treatment of urinary incontinence in adults subjects with neurogenic detrusor overactivity (NDO) due to spinal cord injury or multiple sclerosis – study 1 (CONTENT1). 2021. https://clinicaltrials.gov/ct2/show/NCT02660138
- ClinicalTrials.gov [Internet]. Dysport® treatment of urinary incontinence in adults subjects with neurogenic detrusor overactivity (NDO) due to spinal cord injury or multiple sclerosis – study 2 (CONTENT2). 2021. https://clinicaltrials.gov/ct2/show/NCT02660359
- Denys P, Giannantoni AG, Thompson C, et al. Significant reduction of urinary incontinence episodes in patients with neurogenic detrusor overactivity treated with abobotulinumtoxinA (aboBoNT-a): pooled results of the phase III CONTENT program. Eur Urol. 2021;79(Suppl 1):S32–S33.
- Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14(4):429–437.
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417–428.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2021. Accessed November 29, 2021.
- Homma Y, Koyama N. Minimal clinically important change in urinary incontinence detected by a quality of life assessment tool in overactive bladder syndrome with urge incontinence. Neurourol Urodyn. 2006;25(3):228–235.
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691.
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
- Apostolidis A, Thompson C, Yan X, et al. An exploratory, placebo-controlled, dose-response study of the efficacy and safety of onabotulinumtoxinA in spinal cord injury patients with urinary incontinence due to neurogenic detrusor overactivity. World J Urol. 2013;31(6):1469–1474.
- Cruz F, Herschorn S, Aliotta P, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol. 2011;60(4):742–750.
- Ginsberg D, Gousse A, Keppenne V, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012;187(6):2131–2139.
- Schurch B, de Seze M, Denys P, Botox Detrusor Hyperreflexia Study Team, et al. Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174(1):196–200.
- Dahlberg SE, Korn EL, Le-Rademacher J, et al. Clinical versus statistical significance in studies of thoracic malignancies. J Thorac Oncol. 2020;15(9):1406–1408.
- Schober P, Bossers SM, Schwarte LA. Statistical significance versus clinical importance of observed effect sizes: what do P values and confidence intervals really represent? Anesth Analg. 2018;126(3):1068–1072.
