Abstract
Background
Extraintestinal Pathogenic Escherichia coli (ExPEC) is a leading cause of invasive disease, including bacteremia and sepsis. Invasive ExPEC disease (IED) has the potential to complicate the clinical treatment of other conditions and is associated with an increased mortality, hospitalization, and worse outcomes. Older adults and individuals with comorbid conditions are at higher risk of IED. ExPEC is of particular concern in the Asia Pacific region due to aging populations and rising antimicrobial resistance.
Objectives
This study aimed to synthesize most recent data on the epidemiology, clinical and economic burden of IED in the elderly/high risk populations in China, Japan, South Korea, Taiwan, and Australia.
Methods
A targeted literature review was conducted using Embase, Medline, as well as local scientific databases. We included studies published in English and local languages published from January 1, 2010 to October 7, 2020 that were relevant to the research objectives. Studies were narratively synthesized.
Results
A total of 1,047 studies were identified and 34 of them were included in this review. ExPEC accounted for 46.0% (1,238/2,692) of bacteria-related invasive diseases in patients aged above 60 years in South Korea, followed by China (44.4% (284/640)), Taiwan (39.0% (1,244/3,194)), and Japan (18.1% (581/3,206)), while Australia reported ExPEC out of all pathogens (54.7% (4,006/7,330)) in general adults. Comorbidities such as diabetes or cancer were common in these patients. Studies reported increases in length-of-stay, and in-hospital 30-day all-cause mortality related to ExPEC associated bacteremia was between 9% to 12%. From a cost perspective, a 3-fold increase in sepsis-associated cost was reported in South Korea between 2005 and 2012. In Australia, antimicrobial resistance contributed to an additional cost of AUD $5.8 million per year (95% uncertainty interval [UI], $2.2–$11.2 million) in the treatment of bloodstream infections (BSIs).
Conclusion
ExPEC was a major cause of blood stream infection across China, Japan, South Korea, Taiwan, and Australia. Both the clinical and economic burden associated to ExPEC infections as well as the antimicrobial resistance observed in the elderly call for preventive and curative actions in these regions.
PLAIN LANGUAGE SUMMARY
Extraintestinal Pathogenic Escherichia coli (ExPEC) is a leading cause of invasive disease, including bacteremia and sepsis.
A targeted literature review included the most recent data from 34 published studies on the epidemiology and clinical and economic burden of IED in the elderly/high risk populations in China, Japan, South Korea, Taiwan, and Australia.
ExPEC accounted for 46.0% (1,238/2,692) of bacteria-related invasive diseases in patients aged above 60 years in South Korea, followed by China (44.4% (284/640)), Taiwan (39.0% (1,244/3,194)), and Japan (18.1% (581/3,206)), while Australia reported ExPEC out of all pathogens (54.7% (4,006/7,330)) in general adults. Studies reported increases in length-of-stay and in-hospital 30-day all-cause between 9% to 12%. These factors, along with antimicrobial resistance observed in the elderly, call for preventive and curative actions in these regions.
Data for costs associated with ExPEC induced BSI or sepsis in this region are limited, but evidence shows increasing expenditures.
Introduction
Escherichia coli (E. coli) is the most common gram-negative species causing extraintestinal infection in the community, as well as in the ambulatory, long-term care, and hospital settings. E. coli strains of biological significance to humans are broadly categorized as commensal, intestinal pathogenic (InPEC), and extraintestinal pathogenic E. coli (ExPEC)Citation1.
ExPEC strains are E. coli that can cause urinary tract, bloodstream, prostate, and other type of infections at non-intestinal sites and are responsible for the majority of human extraintestinal infections globallyCitation2. ExPEC is the most common cause of bacteremia, which primarily affects the elderly, and is a frequent cause of meningitis in neonates. Global morbidity and mortality rates due to diseases induced by ExPEC infections are significant and increasing over timeCitation3.
The clinical management of E. coli infections is challenging due to the emergence of multi-drug resistance, which frequently leads to treatment failure, increased rates of hospitalization, length-of-stay, morbidity, mortality, and healthcare and societal costsCitation3. In a recent review of the global burden of bacterial antimicrobial resistance (AMR), E. coli was found to be the leading pathogen for deaths associated with AMRCitation4. Other studies found that antimicrobial-resistant ExPEC infections led to higher hospitalization rates and infectious complications after prostate biopsy, and ExPEC was identified as a risk factor for poor outcome in patients with bacteremiaCitation5,6. Consequently, there is an urgent need for effective preventive measures, such as vaccination, to reduce the global burden of invasive disease caused by ExPEC.
This targeted literature review aims to synthesize evidence on the burden and epidemiology of ExPEC infections in the elderly population across five countries in the Asia Pacific region, namely Taiwan, China, Japan, South Korea, and Australia.
Methods
Study question
The study aimed to determine the epidemiological and medical burden of ExPEC infection in older adults (≥60 years) in China, Japan, South Korea, Taiwan, and Australia. Given that ExPEC infection is pervasive across all age groups and studies normally include patients with a mixture of ages, this review will also include those studies in which the mean/median age of the included patients is around 60 years old. The research was framed according to the PICOS framework (Population, Intervention, Comparator, Outcomes and Study type) outlined in .
Table 1. PICOS Framework for ExPEC infectionsa.
Study selection and analysis
A structured search was conducted for English language articles published from January 1, 2010 to October 7, 2020 in Medline and Embase databases (via EMBASE.com). Moreover, the following databases were also searched: Chinese language databases Wanfang and CNKI; the Korean databases Koreamed and kmbase; and the Japanese database Ichushi-Web. Furthermore, supplementary hand searching in Google Scholar was conducted, and review articles were thoroughly cross-referenced to identify additional relevant articles. Finally, databases and reports linked to sentinel surveillance systems for ExPEC and nosocomial infection in each of the regions were searched for data on the incidence of ExPEC infection in the target population.
ExPEC related search strings included but were not limited to “Escherichia coli”, “E. coli”, “extraintestinal pathogenic E.coli”, “bacteremia”, “bacteraemia”, “sepsis”, and “urosepsis”. The outcomes of interest included “incidence”, “mortality”, “prevalence”, “hospitalization”, “survival”, “incidence rate”, “death”, “mechanical ventilation”, “high risk patient”, and “transmission”. The full version of search terms used for Embase is presented in . Study selection was done based on predefined selection criteria following the PICOS framework. Titles and abstracts of citations were screened and quality checked by two reviewers independently. Those included at the title and abstract screening stage were full text reviewed and further assessed for eligibility.
Table 2. Search strategy and preliminary number of studies in Embase.
The geographical focus of the review was on Taiwan, China, South Korea, Japan, and Australia. Observational studies and surveillance reports conducted in elderly patients (age ≥60) or patients with underlying conditions that reported the outcomes of interest were included. Case reports, narrative reviews, commentaries, modelling, and review articles were excluded. Included studies were narratively summarized and results descriptively analyzed. No formal quantitative statistical assessment was done as part of this research. Our study did not involve research on human subjects and/or any patient or cohort level data. We utilized only evidence from already published studies. As such, it was deemed unnecessary to seek approval from an institutional review board.
Results
Study selection
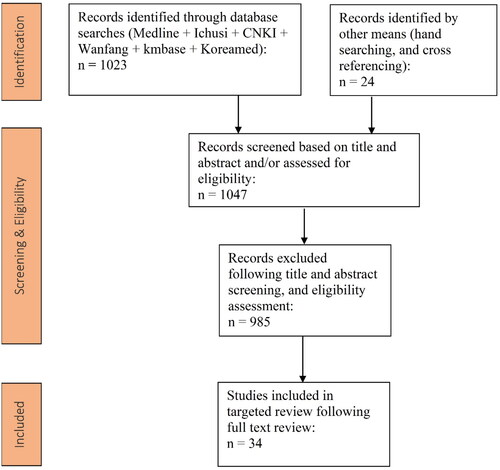
The results of the search are summarized in the simplified PRISMA diagram shown in . The initial database search identified a total of 1,047 records, while an additional 24 articles were identified via supplementary manual searching. Following screening activities, a total of 34 studies were selected for data extractionCitation7–40, including two studies focused on economic burden due to ExPEC-associated BSI39,40.
Characteristics of included studies
An overview of the studies reporting the prevalence of invasive diseases caused by ExPEC in the regions of interest is shown in and . BSI/bacteremia and sepsis are the most reported invasive diseases caused by E. coli, i.e. in 30/32 studiesCitation7–32,Citation35–38, while the remaining two studies focused on acute pyelonephritisCitation33,34. Studies were predominantly hospital-based, and prevalence rate was commonly reported as a proportion of the number of hospitalized patients with ExPEC-associated BSI over the number of patients with confirmed gram-negative pathogens.
Table 3. Prevalence of extra-intestinal pathogenic E. coli in China, Japan, South Korea, Taiwan, and Australia.
Table 4. Hospital stay, ICU frequency/stay, and all-cause mortality rate among patients with BSI in China, Japan, Korea, and Australia.
Epidemiology of invasive diseases caused by ExPEC
The highest proportion of elderly patients with invasive diseases (BSI/bacteremia or sepsis) caused by E. coli were reported in studies from South Korea (up to 46.0% (1,238/2,692))Citation27, followed by China (44.4% (284/640))Citation7, Taiwan (39.0% (1,244/3,194))Citation35, and Japan (18.1% (581/3,206))19. In Australia, such data was only available for the general adult population, being 54.7% (4,006/7,330)Citation38.
South Korea
Four studies focusing on South Korea reported sepsis/BSIs due to E. coli, including two derived from the Korean Global AMR Surveillance System (Kor-GLASS)Citation27,28. Both studies, Lee et al.Citation28 and Liu et al.Citation27, examined blood samples collected from patients in sentinel hospitals and identified E. coli as the leading cause of infection, with a prevalence of 43.6% (1,536/3,523) and 42.9% (1,772/4,129), respectively. In Liu et al.Citation27, where the number of patients with bacteremia was stratified by age and specific pathogens, E. coli infection peaked among patients over 85 years old (51% (229/447)).
In the other two Korean studies, the prevalence of E. coli among cases of invasive disease differed due to differences in the underlying populationCitation25,26. Park et al.Citation25 included 1,192 adult patients admitted into the intensive care unit (ICU) due to sepsis from 2005 to 2009, from which E. coli was detected in 22.1% (mean age = 65 years). Among the 422 patients who developed bacteremia, E. coli was present in 37.7% of them. A separate study by Son et al.Citation26 analyzed 380 community-acquired (mean age = 57.0 years), 558 hospital-acquired (mean age = 47.3 years), and 206 healthcare-associated cases of bacteremia (mean age = 53.7 years), and identified E. coli as the leading cause in the first (47.1%) and third (27.2%) group.
Taiwan
The incidence rate of ExPEC infections at a regional level in Taiwan was available from a study by Hsieh et al.Citation35. The authors examined 104,641 blood cultures from adult patients with BSIs from a regional hospital in northern Taiwan during 2008–2013. E. coli was the most commonly identified organism (39.0% (1,244/3,194)) in bacteremia cases in patients aged over 61 yearsCitation35. In another study by Lee et al.Citation36, the authors included adult patients (n = 1,141, mean age >68) with bacteremia caused by E. coli, Klebsiella species or Proteus mirabilis, from which 72.4% were found to be caused by E. coli. This high prevalence could be biased due to the exclusion of patients infected with other pathogens different than the above-mentioned.
China
In China, a multicenter study by Quan et al.Citation7 examined a total of 919 consecutive episodes of community-onset bloodstream infections (COBSIs) from 28 tertiary hospitals across the country. E. coli was identified in 69.64% (640/919) of cases, and 44.4% (284/640) were from patients over 65 years old. It should be noted that this was the rate of elderly patients among all age groups with bloodstream infections, specifically caused by E. coli and K. pneumoniae. Additionally, studies reporting regional level data showed that the prevalence of E.coli among cases of BSI varied from 20% to 53% across different provinces or cities, including Hunan, Beijing, Lanzhou, and WuhanCitation8–12.
Japan
The proportion of hospitalized patients with E. coli BSI in Japan was similar to other regions in three multicenter studiesCitation18–20. Takeshita et al.Citation19 reported that 18.1% (581/3,206) of hospitalized elderly patients with BSI had E. coli as the causative agent. The prevalence of E. coli was slightly higher (20.23%) in NagaoCitation18, which included 2,941 hospitalized patients with BSI. Similarly, a single center study of 2,105 hospital patients with BSIs by Hattori et al.Citation20, reported that 20.6% of the infections were due to E. coli. In contrast, higher prevalence of E. coli was found in three studies with smaller sample size (n = 179–438). In these studies, E. coli was identified in 44–85% of patients with gram-negative bacteremia or extended-spectrum β-lactamase (ESBL) producing bacteremiaCitation15–17.
Australia
In Australia, epidemiological data came from the Sepsis Outcome Programs 2015 Report by the Australian Commission on Safety and Quality in Health Care. The document included common prevalence of E. coli in BSI patients across 32 institutions, and in each state and territory of Australia. E. coli was the most common organism causing bacteremia in the country, accounting for 54.7% (4,006/7,330) of all BSI episodes reportedCitation38.
Clinical burden of ExPEC in Asia Pacific
Patient comorbidities
Comorbidities including cancer, diabetes, and kidney diseases were frequently found in patients with BSI from all regions of study except for Australia. Cancer was present in 15–40% (median across studies: 33.74%) of the patients across all studies, between 5% and 26% (median across studies: 15%) had kidney diseases, while 3% to 42% (median across studies: 20.51%) were found to have diabetes.
When studies from individual regions were assessed, in the Quan et al.Citation7 study from China, diabetes was the most common underlying disease in 27.8% (178/640) of patients with BSI, followed by urinary system disease (e.g. obstructive urinary tract disease, renal failure) in around 13% (82/640), chronic kidney disease (7.97%), and autoimmune disease (5.78%).
In Japan, the presence of a solid tumor was found in 31–40% of patients with BSI according to three large sample studies included in this review, Takeshita et al. (n = 3,206), Nagao (n = 2,941), and Hattori et al. (n = 2,105)Citation18–20.
With regards to South Korea, 71.1% of the patients with BSI in the study by Park et al.Citation25 had at least one type of comorbidity (i.e. cardiovascular disease = 37.85%, metabolic disorder = 28.1%, cancer = 14.68%). On the other hand, solid tumor (33.74%) and diabetes (18%) were the most common underlying disease in Son et al.Citation26.
Finally, in the Lee et al.Citation36 study from Taiwan, hypertension (49.4%, 564/1,141), diabetes mellitus (38.9%, 444/1,141), and malignancy (26.9%, 307/1,141) were the most common comorbidities in patients with bacteremia. Similar to E. coli prevalence, the high rate of comorbidities in this study could be biased due to the selection of patients.
Hospitalizations and ICU frequency
Apart from reporting the burden of E. coli BSI, several studies included hospitalization rates, ICU care, and in-hospital mortality associated with this disease ().
The longest mean hospital stay (mean = 46.3 days, SD = 56.0) was seen in the Japanese study by Hattori et al.Citation20, although this number was based on analysis of a mixture of patients infected with a variety of gram-negative bacteria and not exclusively with E. coli. In comparison, Chinese and Korean studies showed shorter hospital stays (means <31 days across studies). But the shortest hospitalization time was observed in Taiwan, with a mean length of 15 days in patients with BSI due to E. coli. Interestingly, Kang et al.Citation33 found that patients aged ≥65 years had significantly longer hospital stays than those under this age (median 7 days vs. 5 days, p = 0.009), and diabetes mellitus was significantly associated with a longer length-of-stay (OR = 5.34, 95% CI = 1.76–16.2, p = 0.003).
Information on length-of-stay in Australian patients with E. coli induced BSI was limited. In this regard, Wozniak et al.Citation40 conducted a population-based modelling study to investigate the health and economic burden of antimicrobial-resistant infections in Australian hospitals. They found that BSI caused by ceftriaxone-resistant E. coli led to an additional 4.89 days (95% UI = 1.1–8.7) of hospital stay compared to patients susceptible to E. coli infections.
With regards to ICU admission in patients with BSI, several factors such as severity of infection, underlying diseases, and older age could influence its rate. For example, severe urosepsis was associated with a significantly higher rate of ICU admission compared to non-urosepsis hospitalizations (41.07% vs. 18.42, p = 0.0208) in the study by Jiang et al.Citation41, although this condition did not impact the length of stay as the days of ICU stay were not significantly different (p = 0.8051).
30-Day mortality
E. coli associated BSI has been shown to lead to considerable mortality, with some evidence suggesting that it has increased in recent years ()Citation22. The highest 30-day mortality in patients suffering from E. coli BSI was reported in Taiwan (19.2–33.3%)Citation36, followed by China (12.2–25.5%)Citation13, South Korea (23.6%)Citation25, Australia (8.4–21.3%)Citation38, and Japan (6.45%)Citation20. However, this number in Japan is on the rise according to Tsuzuki et al.Citation22, in which a 40% increase in BSI attributable deaths caused by E. coli was found during 2011–2017, from 7.1 per 100,000 population to 11.1 per 100,000 population (p < 0.001). Finally, the case-fatality rate further increased when only patients with E. coli BSIs at the ICU were considered, reaching 30% according to another Japanese study by NagaoCitation18.
When 30-day mortality was compared between hospital-onset and community-onset BSI, the first one was associated with a higher mortality rate than the second one. These results were similar across different regions analyzed, including Australia, Korea, Japan, and TaiwanCitation19,26,37,38.
When variant-specific results were analyzed, the Chinese study Zhang et al.Citation13 found that bacteremia due to AmpC β-lactamase-producing E. coli was associated with a higher 30-day mortality compared with that induced by non-AmpC β-lactamase-producing E. coli (25.5% vs. 12.2%, p = 0.018) in cancer patients. Another Chinese study, Xiao et al.Citation14, analyzed the risk factors associated with ESBL-E. coli induced BSI, and no significant difference was found in 30-day mortality between the ESBL group and the non-ESBL group (11.1% vs. 9.2%, p = 0.642). In this case, a significant higher risk of mortality was associated to a respiratory tract originated E. coli infection (OR = 2.050; p = 0.031) and prior use of carbapenems (OR = 3.491; p = 0.003). Similar results were found in the Japanese study by Namikawa et al.Citation24, where 30-day mortality did not differ between the two groups (9.7% vs 9.2%). However, two other Japanese studies found that ESBL-E. coli BSI led to higher mortality than non-ESBL-E. coli BSI, but they all suggested that this finding should be further investigated as differences in baseline characteristics between groups could have impacted survivalCitation21,23,24. For example, in Haruki et al.Citation23, the ESBL group had a significantly higher proportion of patients with chronic renal failure than the non-ESBL group, and further sub-analyses suggested that antibiotic use could have impacted the outcomes. Finally, in South Korea, Ha et al.Citation32 and Cho et al.Citation29 focused specifically on elderly patients with ESBL-E. coli induced BSI and found similar 30-day mortality rates (14.9%, 12.6%, and 10.9%). In contrast, Ku et al.Citation31 reported an all-cause 28-day mortality of 20% for the same type of patients.
Additional Korean studies also assessed risk factors associated with mortality. In Yoon et al.Citation30, the leading risk factor for mortality in patients with E. coli BSI (30-day mortality of 9.5%, n = 1,492) was an ICU admission, accounting for 31.9% of these early deaths. Nosocomial origin of the infection was also reported in 36.9% of these patients. In this same line, Park et al.Citation25 reported higher in-hospital mortality (28.0%, 334/1192) and sepsis-related mortality (23.6%, 281/1192) among ICU patients, although this study was not restricted to patients with E. coli. Finally, a higher mortality rate was found in older patients (age >65) with acute pyelonephritis due to E. coli. Kang et al.Citation33 reported a 14-day mortality of 4.2% vs. 2.3% when comparing older to younger patients. This number increased considerably in a different study, by Lee et al.Citation34, where 25.9% of 54 bacteremic acute pyelonephritis patients with septic shock due to E. coli died within 7 days of admission, although results in this study were not stratified by age and the sample size was limited.
Antimicrobial resistance
Antimicrobial resistance (AMR) in ExPEC infections was found to be a common issue across China, Japan, South Korea, and Taiwan, with a resistance rate across studies of 30% to >90% to most antibiotics such as ampicillin (>65%), piperacillin (>55%), cefazolin (>42%), and cefotaxime (>35%). In contrast, the AMR rate is relatively lower in Australia, with the highest AMR rate to ampicillin being approximately 60% and under 16% to the others.
Resistance rates of E. coli to different antibiotics were considerably high. Resistance to ampicillin and piperacillin was reported in China (90% to both)Citation11, South Korea (65% and 55%, obtained from the Korean governmental antimicrobial resistance AMR surveillance system), and Taiwan (70% for ampicillin)Citation42, while Australia showed slightly lower resistance to ampicillin (50.9% for community onset and 61.6% for hospital onset sepsis) and piperacillin–tazobactam (2.1% and 6.7%, for community and hospital onset sepsis, respectively)Citation38. A continuous decrease in sensitivity of E. coli to other antibiotics was also observed in the same regions, as reported in the Chinese study by Tian et al.Citation11, the Korean governmental antimicrobial resistance AMR surveillance system (42% to cefazolin, 40% to ciprofloxacin, 35% to cefotaxime), and the study by Chen et al.Citation42 from Taiwan (51% to trimethoprim/sulfamethoxazole, 56% to ampicillin/sulbactam). In contrast, Australia showed lower resistance rates for several antibiotics including ceftriaxone (9.1–15.8%), ciprofloxacin (11.3–15.1%), amoxicillin–clavulanate (7.7–13%), mainly attributable to the conservative use of antibioticsCitation38. This is in line with a Korean analysis that found a positive correlation between consumption of antimicrobials such as fluoroquinolone, cefoxitin, and cefotaxime with the resistance rates of E. coli to these agentsCitation43.
When AMR in specific E. coli strains were assessed, these numbers increased significantly in all regions and for most antimicrobials tested. ESBL-E. coli isolates were highly resistant to different antimicrobials (e.g. cefazolin, ceftriaxone, ceftazidime, cefotaxime, cefepime, levofloxacin, ciprofloxacin, trimethoprim/sulfamethoxazole) in China and Japan, with AMR rates of over 63% for the most effective agents and over 90% for the less effective onesCitation12. In addition, prevalence of ESBLs was very high (80.95%) in the Chinese study by Jiang et al.Citation12.
Economic burden of ExPEC in the Asia Pacific
Costs associated with ExPEC induced BSI or sepsis were not comprehensively documented across the five regions, with limited data only available for South Korea and Australia. Kim et al.Citation39 estimated that the costs of sepsis in Korea have tripled between 2005 and 2012, growing 311.8% from 4,252 × 100,000,000 KRW to 13,226.5 × 100,000,000 KRW. In Australia, the population-based modeling study conducted by Wozniak et al.Citation40 found that an additional AUD $5.8 million (95% UI: $2.2–$11.2 million) per year was spent to treat ceftriaxone-resistant E. coli BSIs.
Discussion
To our knowledge this is the first review of the overall burden of ExPEC infections in the five Asia Pacific countries studied. Findings on the epidemiology of BSIs caused by ExPEC in China, Japan, South Korea, Taiwan, and Australia indicated higher incidence among older patients, which may further increase in the future with aging and growing trends in pathogen resistance. Importantly, our review identified E. coli as the most common organism causing bacteremia among the elderly across several studies in the regions of interest.
These findings are in line with evidence from outside the Asia Pacific region. Older populations were also shown to be more susceptible to ExPEC infections according to surveillance data in the UK in 2018 (150/100,000 in age = 65–74 years and 430/100,000 in age >75 years)Citation44. A recent reviewCitation45 on the epidemiology of ExPEC induced BSIs which covered western countries found that, overall, E. coli accounted for 27% of documented bacteremia episodes. Furthermore, the review suggested that ExPEC infection is a major health issue in older adults and at-risk groups, with approximately three quarters of ExPEC BSIs being contracted in the community.
Our review also highlighted the complications and comorbidities accompanied to ExPEC infections, which is reflected in the long hospital and ICU stays as well as the considerable in-hospital and ICU mortality rates. Although not all the studies assessed trends for mortality, evidence from Japan shows a significant increase in E. coli BSI-related mortality which may be under-documented in the other regions. This is expected to some extent as elderly patients suffer in general from higher prevalence of cancer, diabetes, renal, and other issues.
Some of the studies we identified reported issues with antimicrobial resistance (AMR) and the associated consequences. This deserves particular attention as the emergence of AMR in the Enterobacter species has been reported to be associated with significantly (p < 0.001) longer hospitalization and higher treatment cost, as well as a higher risk of death (relative risk = 5.02; p = 0.01) compared with susceptible strainsCitation46. Furthermore, recent work by Tsuzuki et al. studying the population-level burden of disease associated with AMR BSIs in Japan found that 137.9 (95% CI = 130.7–145.2) disability-adjusted life-years (DALYs) per 100,000 population were attributable to BSIs caused by nine antimicrobial-resistant bacteria in 2018. Of those, methicillin-resistant Staphylococcus aureus (MRSA), fluoroquinolone-resistant E. coli (FQREC), and third-generation cephalosporin-resistant E. coli (3GREC) accounted for 87.2% overall burden, which was particularly high in those aged 65 or olderCitation47. AMR also places additional burden on the clinical practice by contributing to more infections, undermining the outcome of the treatment, inducing extra expenses, and interrupting the capacity of healthcare infrastructuresCitation48. A recent study of the economic burden associated with AMR in gram-negative bacteria estimated that the additional costs associated with hospitalizations in Japan were USD $1.1 billionCitation49. For all these reasons, managing AMR has become an important component to the achievement of sustainable development as highlighted by the United Nation (UN). The UN introduced a new sustainable development goal (SDG) indicator specifically related to E. coli induced BSIs in March 2020, along with several recommended approaches to cope with AMR such as infection prevention and control programsCitation50.
It has been argued that appropriate prevention strategies could help reduce the pressure which ExPEC causes on healthcare systems and individual patient sufferingCitation45. Such prevention strategies, including vaccinations, have been effective in reducing the burden of other infections in the elderly, such as influenza, pneumococcal infections, and herpes zoster. In addition, vaccination also reduces usage of antibiotics, therefore contributes to preventing expansion of drug-resistant pathogens. A recent Japanese study estimated that reducing AMR levels of gram-negative pathogens including E. coli had the potential of increasing clinical and economic gainsCitation49. With aging populations in many of the Asia Pacific regions, especially in Japan, where 28% of the population is over 65 years old and the aging trend is still increasingCitation51, preventing ExPEC infections is likely to have significant implications in public health and the associated economy in the long-run.
Finally, this review has several limitations. Most notably, data were overall limited despite the number of studies identified. Secondly, the considerable heterogeneity in study design and regional medical practice limits comparability across studies. The different selection criteria of patients in the studies deserve special attention when interpreting the findings to avoid overestimation or underestimation of disease burden. Thirdly, E. coli has been classified into three serotypes (O:H:K), with the O-groups being the most common ones (N = 186)Citation52. However, none of the included studies reported the serotyping of the E. coli identified.
Conclusion
This review found that ExPEC was a major cause of blood stream infections across China, Japan, South Korea, Taiwan, and Australia. Our findings provide evidence on the significant clinical and economic consequences of such infections which are largely linked to the acute care for patients as well as AMR. With length of hospital stay and ICU stay exceeding 20 days in many of the studies, the average cost per patient would be considerable. In the Asia Pacific region, with an aging population and increasing difficulty to treat ExPEC infections, the burden to the healthcare system is expected only to increase. Considering the diverse characteristics of different serotypes, an in-depth exploration would facilitate the development of vaccination strategies. Further work into the evidence gaps identified in this review in and the implications of ExPEC prevention are likely to provide key information for policy makers.
Table 5. Evidence gap analysis for ExPEC in patients aged 60 years and over in China, Japan, South Korea, Taiwan, and Australia.
Transparency
Declaration of financial/other relationships
LHP, KL, MCN: Employees of Janssen. PA, XJ are employees of Amaris Consulting, which received funding from Janssen Asia Pacific during the conduct of the study and has no other funding, financial relationships, or conflict of interest to disclose.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
| Abbreviations | ||
| ExPEC | = | Extraintestinal pathogenic Escherichia coli |
| IED | = | Invasive ExPEC disease |
| AUD | = | Australian dollar |
| UI | = | Uncertainty interval |
| BSIs | = | Bloodstream infections |
| E. coli | = | Escherichia coli |
| InPEC | = | Intestinal pathogenic Escherichia coli |
| AMR | = | Antimicrobial resistance |
| PICOS | = | Population, Intervention, Comparator, Outcomes and Study type |
| ICU | = | Intensive care unit |
| COBSIs | = | Community-onset bloodstream infections |
| ESBL | = | Extended-spectrum β-lactamase |
| OR | = | Odds ratio |
| KRW | = | Korean Won |
| DALYs | = | Disability-adjusted life-years |
| MRSA | = | Methicillin-resistant Staphylococcus aureus |
| FQREC | = | Fluoroquinolone-resistant E. coli |
| 3GREC | = | Third-generation cephalosporin-resistant E. coli |
| USD | = | US dollar |
| UN | = | United Nation |
| SDG | = | Sustainable development goal |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SD | = | Standard deviation |
| CI | = | Confidence interval |
| UK | = | United Kingdom |
Acknowledgements
Jun Feng and David Wu, both employees of Janssen, provided valuable review and feedback. Xue Chen of Amaris provided additional support.
Additional information
Funding
References
- Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5(5):449–456.
- Manges AR, Geum HM, Guo A, et al. Global Extraintestinal pathogenic Escherichia coli (ExPEC) lineages. Clin Microbiol Rev. 2019;32(3):e00135-18.
- Poolman JT, Wacker M. Extraintestinal Pathogenic Escherichia coli, a common human pathogen: challenges for vaccine development and progress in the field. J Infect Dis. 2016;213(1):6–13.
- Murray CJ, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399(10325):629–655.
- Leibovici L, Shraga I, Drucker M, et al. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Int Med. 1998;244(5):379–386.
- Williamson D, Barrett LK, Rogers B, et al. Infectious complications following transrectal ultrasound-guided prostate biopsy: new challenges in the era of multidrug-resistant Escherichia coli. Clin Infect Dis. 2013;57(2):267–274.
- Quan J, Zhao D, Liu L, et al. High prevalence of ESBL-producing Escherichia coli and Klebsiella pneumoniae in community-onset bloodstream infections in China. J Antimicrob Chemother. 2017;72(1):273–280.
- Liu B. 2016-2017年成人血流感染临床特点和病原学分析2016-2017 Clinical characteristics and etiological analysis of adult bloodstream infections-Chongqing municipal health information center. Digital J System. 检验医学与临床. 2020;17(4):269. http://paper.cqshic.com/paper.aspx?id=194063
- Bai Y, Zheng Z, Du M, et al. Bloodstream Infection and its clinical characteristics and relevant factors associated with interventional therapy in a large tertiary hospital: a six years surveillance study. BioMed Res Int. 2019;2019:1–7.
- Feng Q, Yuejuan S, Xiaoqin H. Epidemiology of pathogenic microorganisms, basic diseases and clinical biomarker analysis in patients with bloodstream infections from a 3A general hospital in Lanzhou of China. Afr J Microbiol Res. 14(7):332–339.
- Tian L, Zhang Z, Sun Z. Antimicrobial resistance trends in bloodstream infections at a large teaching hospital in China: a 20-year surveillance study (1998-2017). Antimicrob Resist Infect Control. 2019;8(1):86.
- Jiang Y, Li J, Zhang Y, et al. Clinical Situations of bacteriology and prognosis in patients with urosepsis. BioMed Res Int. 2019;2019:1–9.
- Zhang Q, Zhang W, Li Z, et al. Bacteraemia due to AmpC β-lactamase-producing Escherichia coli in hospitalized cancer patients: risk factors, antibiotic therapy, and outcomes. Diagn Microbiol Infect Dis. 2017;88(3):247–251.
- Xiao T, Wu Z, Shi Q, et al. A retrospective analysis of risk factors and outcomes in patients with extended-spectrum beta-lactamase-producing Escherichia coli bloodstream infections. J Glob Antimicrob Resist. 2019;17:147–156.
- Kosai K, Yamagishi Y, Hashinaga K, et al. Multicenter surveillance of the epidemiology of gram-negative bacteremia in Japan. J Infect Chemother. 2020;26(3):193–198.
- Mitsuboshi S, Tsuruma N, Watanabe K, et al. Advanced age is not a risk factor for mortality in patients with bacteremia caused by extended-spectrum β-lactamase-producing organisms: a multicenter cohort study. Jpn J Infect Dis. 2020;73(4):288–292.
- Miyazato K, Natomi H, Shiroma Y, et al. Bacteremia in cholangiolithic cholangitis. Gastroenterol Endoscopy. 2018;60:2377–2386.
- Nagao M. A multicentre analysis of epidemiology of the nosocomial bloodstream infections in Japanese university hospitals. Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis. 2013;19(9):852–858.
- Takeshita N, Kawamura I, Kurai H, et al. Unique characteristics of community-onset healthcare- associated bloodstream infections: a multi-Centre prospective surveillance study of bloodstream infections in Japan. J Hosp Infect. 2017;96(1):29–34.
- Hattori H, Maeda M, Nagatomo Y, et al. Epidemiology and risk factors for mortality in bloodstream infections: a single-center retrospective study in Japan. Am J Infect Control. 2018;46(12):e75–9–e79.
- Komatsu Y, Kasahara K, Inoue T, et al. Molecular epidemiology and clinical features of extended-spectrum beta-lactamase- or carbapenemase-producing Escherichia coli bacteremia in Japan. PLoS ONE. 2018;13(8):e0202276.
- Tsuzuki S, Matsunaga N, Yahara K, et al. National trend of blood-stream infection attributable deaths caused by Staphylococcus aureus and Escherichia coli in Japan. J Infect Chemother. 2020;26(4):367–371.
- Haruki Y, Hagiya H, Haruki M, et al. Clinical characteristics and outcome of critically ill patients with bacteremia caused by extended-spectrum β-lactamase-producing and non-producing Escherichia coli. J Infect Chemother. 2018;24(11):944–947.
- Namikawa H, Yamada K, Fujimoto H, et al. Clinical characteristics of bacteremia caused by extended-spectrum beta-lactamase-producing Escherichia coli at a tertiary hospital. Intern Med. 2017;56(14):1807–1815.
- Park DW, Chun BC, Kim JM, et al. Epidemiological and clinical characteristics of Community-Acquired severe sepsis and septic shock: a prospective observational study in 12 university hospitals in korea. J Korean Med Sci. 2012;27(11):1308–1314.
- Son JS, Song JH, Ko KS, et al. Bloodstream infections and clinical significance of healthcare-associated bacteremia: a multicenter surveillance study in korean hospitals. J Korean Med Sci. 2010;25(7):992–998.
- Liu C, Yoon EJ, Kim D, et al. Antimicrobial resistance in South Korea: a report from the korean global antimicrobial resistance surveillance system (Kor-GLASS) for 2017. J Infect Chemother. 2019;25(11):845–859. 1
- Lee H, Yoon EJ, Kim D, et al. Antimicrobial resistance of major clinical pathogens in South Korea, may 2016 to april 2017: first one-year report from Kor-GLASS. Eurosurveillance. 2018;23(42):1800047.
- Cho SY, Kang CI, Cha MK, Korean Network for Study on Infectious Diseases, et al. Clinical features and treatment outcomes of bloodstream infections caused by extended-spectrum β-lactamase-producing escherichia coli sequence type 131. Microb Drug Resist. 2015;21(4):463–469.
- Yoon EJ, Choi MH, Park YS, et al. Impact of host-pathogen-treatment tripartite components on early mortality of patients with Escherichia coli bloodstream infection: prospective observational study. EBioMedicine. 2018;35:76–86.
- Ku NS, Kim YC, Kim MH, et al. Risk factors for 28-day mortality in elderly patients with extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae bacteremia. Arch Gerontol Geriatr. 2014;58(1):105–109.
- Ha YE, Kang CI, Cha MK, et al. Epidemiology and clinical outcomes of bloodstream infections caused by extended-spectrum β-lactamase-producing Escherichia coli in patients with cancer. Int J Antimicrob Agents. 2013;42(5):403–409.
- Kang SJ, Jung SI, Wi YM, et al. Clinical characteristics and outcomes of community-onset acute pyelonephritis caused by Escherichia coli in elderly patients. Eur Geriatr Med. 2014;5(2):78–81.
- Lee JH, Lee YM, Cho JH. Risk factors of septic shock in bacteremic acute pyelonephritis patients admitted to an ER. J Infect Chemother. 2012;18(1):130–133.
- Hsieh WS, Tsai YT, Chi WM, et al. Epidemiology and prevalence of bloodstream infections in a regional hospital in Northern Taiwan during 2008-2013. J Exp Clin Med Taiwan. 2014;6(6):187–189.
- Lee CC, Lee CH, Hong MY, et al. Propensity-matched analysis of the impact of extended-spectrum β-lactamase production on adults with community-onset Escherichia coli, Klebsiella species, and Proteus mirabilis bacteremia. J Microbiol Immunol Infect. 2018;51(4):519–526.
- Lee JC, Lee NY, Lee HC, et al. Clinical characteristics of urosepsis caused by extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella pneumonia and their emergence in the community. J Microbiol Immunol Infect. 2012;45(2):127–133.
- Australian Commission on Safety and Quality in Health Care. Australian Group on Antimicrobial Resistance. Sepsis outcome programs 2015 report. 2016.
- Kim J, Kim K, Lee H, et al. Epidemiology of sepsis in korea: a population-based study of incidence, mortality, cost and risk factors for death in sepsis. Clin Exp Emerg Med. 2019;6(1):49–63.
- Wozniak TM, Bailey EJ, Graves N. Health and economic burden of antimicrobial-resistant infections in Australian hospitals: a population-based model. Infect Control Hosp Epidemiol. 2019;40(3):320–327.
- Jiang W, Wu M, Zhou J, et al. Etiologic spectrum and occurrence of coinfections in children hospitalized with community-acquired pneumonia. BMC Infect Dis. 2017;17(1):787.
- Chen LF, Chiu CT, Lo JY, et al. Clinical characteristics and antimicrobial susceptibility pattern of hospitalised patients with community-acquired urinary tract infections at a regional hospital in Taiwan. Healthc Infect. 2014;19(1):20–25.
- Kim YA, Park YS, Youk T, et al. Trends in South Korean antimicrobial use and association with changes in Escherichia coli resistance rates: 12-year ecological study using a nationwide surveillance and antimicrobial prescription database. Karunasagar I, editor. PLoS ONE. 2018;13(12):e0209580.
- Health protection: Infectious diseases - detailed information - GOV.UK. 2022. https://www.gov.uk/topic/health-protection/infectious-diseases
- Bonten M, Johnson JR, van den Biggelaar AHJ, et al. Epidemiology of Escherichia coli bacteremia: a systematic literature review. Clin Infect Dis. 2021;72(7):1211–1219.
- Cosgrove SE, Kaye KS, Eliopoulous GM, et al. Health and economic outcomes of the emergence of Third-Generation cephalosporin resistance in Enterobacter species. Arch Intern Med. 2002;162(2):185–190.
- Tsuzuki S, Matsunaga N, Yahara K, et al. Disease burden of bloodstream infections caused by antimicrobial-resistant bacteria: a population-level study, Japan, 2015–2018. Int J Infect Dis. 2021;108:119–124.
- Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect. 2016;22(5):416–422.
- Matsumoto T, Darlington O, Miller R, et al. Estimating the economic and clinical value of reducing antimicrobial resistance to three gram-negative pathogens in Japan. J Health Econ Outcomes Res. 2021;8(2):64–75.
- World Health Organization. Regional Office for Europe. The fight against antimicrobial resistance is closely linked to the Sustainable Development Goals [Internet]. World Health Organization. Regional Office for Europe; 2020. Report No.: WHO/EURO:2020-1634-41385-56394. https://apps.who.int/iris/handle/10665/337519
- Akiyama H. Aging well: an update. Nutr Rev. 2020;78(12 Suppl 2):3–9.
- Fratamico PM, DebRoy C, Liu Y, et al. Advances in molecular serotyping and subtyping of Escherichia coli†. Front Microbiol. 2016;7:644.