Abstract
Aims
The National Health Service (NHS) in England is facing extreme capacity pressures. The backbone of prostate cancer care is gonadotropin-releasing hormone agonist (GnRHa) therapy, commonly administered every month or 3 months. We estimated the cost and capacity savings associated with increased use of 6-monthly GnRHa therapy in England.
Methods
A capacity and cost-minimization model including a societal perspective was developed (in Microsoft Excel) to generate cost and capacity estimates for GnRHa drug acquisition and administration for “Current practice” and for a “Base case” scenario. In the “Base case” scenario, 50% of patients who were receiving monthly or 3-monthly GnRHa therapy in “Current practice” switched/transitioned to a 6-monthly formulation. Cost/capacity estimates were calculated per patient per administration and scaled to annualized population levels. Sensitivity analyses were conducted to assess the impact of individual model assumptions: 1 tested the impact of drug acquisition costs; 2 and 3 tested the level of nurse grade and the time associated with treatment administration, respectively; 4 tested the rate of switch/transition to 6-monthly GnRHa therapy; and 5 tested differing diagnostic patterns following the coronavirus disease 2019 pandemic.
Results
Compared with “Current practice”, the “Base case” scenario was associated with annual cost savings of £5,164,296 (148,478 fewer appointments/year and 37,119 fewer appointment-hours/year). The largest savings were in drug administration (£2.2 million) and acquisition (£1.6 million) costs. Annual societal cost savings totaled £1.4 million, mainly in reduced appointment-related travel, productivity and leisure time opportunity losses. Increased use of 6-monthly versus monthly or 3-monthly GnRHa therapy consistently achieved system-wide annual cost and capacity savings across all sensitivity analysis scenarios.
Conclusions
Our holistic model suggests that switching/transitioning men from monthly or 3- monthly GnRHa therapy to a 6-monthly formulation can reduce NHS cost and capacity pressures and the societal and environmental costs associated with prostate cancer care.
PLAIN LANGUAGE SUMMARY
Men with prostate cancer often receive hormone injections to slow their cancer progression and relieve their symptoms. In England, most men who are prescribed hormone injections receive them once every month or 3 months; however, a 6-monthly option would reduce the number of injection appointments required each year. If some men who are receiving hormone injections every month or every 3 months switched to treatment once every 6 months, it could reduce the impact of prostate cancer treatment on their lives. It might also reduce the demands of prostate cancer treatment on the National Health Service (NHS). We developed a computer-based model to assess how NHS costs and nursing would be affected if half of the men in England who are receiving hormone injections every month or 3 months switched to injections every 6 months. According to our model, this change could save the NHS about £5.2 million each year. The main cost savings would be in reduced nursing costs. The change would also benefit the NHS because nurses would have almost 150,000 fewer injections to give, meaning that they could spend their time providing care elsewhere. Given that men would have to attend fewer appointments, they would also benefit from reduced time traveling, which would benefit the environment as well. Overall, these benefits to society would contribute about £1.4 million of savings per year. Given how stretched the NHS is in England, particularly after the COVID-19 pandemic, opportunities to reduce time and staffing pressures are very important.
Introduction
The National Health Service (NHS) in England is facing unprecedented capacity challenges, with record levels of staff vacancies and growing service demandCitation1. The coronavirus disease 2019 (COVID-19) pandemic added to existing pressures created by an aging population in an era of increasingly sophisticated and costly therapies. In the aftermath of the pandemic, a fatigued and depleted workforce is faced with the pressures of meeting ongoing COVID-19 care requirements, as well as meeting usual care requirements and reducing backlogs in elective care servicesCitation1–4. In prostate cancer alone, an estimated 14,000 men in England delayed accessing care during the pandemicCitation5.
Hormonal treatment with androgen deprivation therapy (ADT) is a cornerstone of the therapeutic management of prostate cancerCitation6. Androgens promote the development and growth of cancerous prostate cells via binding and activation of androgen receptorsCitation7,Citation8. ADT using a gonadotropin-releasing hormone agonist (GnRHa) can reduce androgen levelsCitation9, thereby helping to slow disease progression and relieve symptomsCitation10. Several GnRHa therapies are approved for use in England; most are formulated for administration once every month or once every 3 months. Approved options include goserelin acetate (3.6 mg monthly or 10.8 mg 3-monthly)Citation11,Citation12, leuprorelin acetate (3.75 mg monthly or 11.25 mg 3-monthly)Citation13,Citation14, and triptorelin acetate (3 mg monthly or 11.25 mg 3-monthly)Citation15,Citation16. Triptorelin is also formulated for use once every 24 weeks (i.e. approximately once every 6 months; triptorelin pamoate 22.5 mg)Citation17.
In terms of care delivery, implementation of a 6-monthly GnRHa regimen requires fewer patient–healthcare interactions than monthly or 3-monthly regimens. Reducing the number of such interactions potentially releases capacity within the system and broadens the range of care pathways available to patients. Increasing patient choice aligns with the key principles of the NHS Choice FrameworkCitation18 and the NHS Long Term PlanCitation19, which outline commitments to deliver efficient and effective care and to offer people more control over their health and more choice about how their healthcare is provided. In addition to service capacity and patients’ choice considerations, prostate cancer management guidelines published since the start of the COVID-19 pandemic endorse the use of 6-monthly GnRHa therapy, from a patient safeguarding perspective, because reducing healthcare touch points decreases the risk of viral infection and transmissionCitation20,Citation21. The interim COVID-19 management strategy for prostate cancer published by the British Association of Urological Surgeons (BAUS) explicitly called for expedited access to 6-monthly GnRHA preparationsCitation22. Although the initial phase of the COVID-19 pandemic has past, this principle of care remains relevant for future periods of high COVID-19 and influenza infection/re-infection and related system pressures.
The impact of transitioning patients to 6-monthly GnRHa therapy after an initial 12 months of monthly or 3-monthly treatment was evaluated by Cornford and colleagues in adults (aged 53–99 years) with prostate cancer treated in metastatic, neoadjuvant, or adjuvant settings at one of three UK hospital trusts (n = 41)Citation23. The transition to 6-monthly treatment led to a significant reduction in total patient–healthcare interactions (41.5% reduction; p<.0001) and associated per-patient costs. Median serum prostate-specific antigen (PSA) levels were stabilized in patients who transitioned from monthly or 3-monthly regimens to 6-monthly therapy and remained low post transition (n = 36; 0.35 ng/mL at baseline versus 0.18 ng/mL at 6 months and 0.24 ng/mL at 12 months post switch/transition). Among patients at the same study centres who were newly initiated on 6-monthly GnRHa therapy (n = 47), treatment was associated with a pronounced reduction in median serum PSA levels (n = 45; 23.5 ng/mL at baseline versus 0.84 ng/mL and 1.30 ng/mL at 6- and 12-months, respectively). Adverse events for 6-monthly GnRHa therapy were consistent with those for more frequent GnRHa formulationsCitation23. Thus, at a national level, increased use of 6-monthly GnRHa therapy may offer the potential for considerable system-wide cost and capacity savings without an anticipated diminution in treatment outcomes.
In addition to considering the impact of prostate cancer care decisions on the healthcare service, it is also relevant to consider the implications of management approaches from the wider societal perspective. More holistic health economic analyses help to support optimal decision-making by factoring in societal welfare, as well as the welfare of the individuals and/or organizations directly involvedCitation24,Citation25. Within this context, a reduction in required healthcare appointments for GnRHa therapy could reduce related patient travel, thereby decreasing not only time lost to travel, but also travel costs, CO2 emissions, and (for those still of working age) workplace absenteeism. Potential reductions in unnecessary patient travel and CO2 emissions align with the commitment of the NHS to decrease its carbon footprint, as outlined in its “net zero” goalsCitation26. The relevance of taking a holistic approach to economic modeling was highlighted by an economic evaluation of 6-monthly versus 3-monthly GnRHa therapy with leuprorelin acetate for men with prostate cancer in Japan. The study found that one less GnRHa injection (over a 6-month period) not only reduced direct medical costs and indirect lost productivity costs, but also offered value by reducing intangible costs through fewer injection site reactions and medical consultations, and by virtue of reduced waiting time and treatment-related pain. Overall, the authors concluded that the intangible value of a GnRHa regimen requiring fewer injections might not be negligible, and was likely to influence patient-centered healthcare decision-makingCitation27.
We used publicly available data to develop a holistic model estimating the cost and capacity savings associated with increased use of 6-monthly GnRHa therapy in men receiving prostate cancer treatment in England. This may be particularly timely given that integrated care boards (ICBs) have recently been established to optimize (and potentially reconfigure) NHS service deliveryCitation28.
Methods
Model overview
The model was designed to assess potential savings for the NHS and society from the transition of eligible patients from monthly or 3-monthly GnRHa injections to 6-monthly injections (once every 24 weeks) of triptorelin pamoate 22.5 mg. The model was developed in accordance with best practice guidelines for conducting budget impact analysesCitation29.
A time horizon of 12 months was used (2021–2022), and the population considered was men in England with prostate cancer for whom a GnRHa was indicated and prescribed within its licensed indication. The model included quantifiable costs only, specifically those associated with drug acquisition and administration, and the societal costs from primary care visits for treatment administration. The capacity, financial, and societal impacts were evaluated by comparing estimated GnRHa-related treatment expenditure and societal costs under a “Current practice” scenario compared with a simulated “Base case” scenario, assuming a 50% switch/transition rate from monthly or 3-monthly GnRHa (any formulation) to 6-monthly triptorelin. Following on from previous work demonstrating that switching/transitioning patients to 6-monthly GnRHa therapy from more frequent dosing regimens is a rational management approachCitation23, the model was not designed to compare the safety or efficacy of any included treatments. Compliance with GnRHa therapy (i.e. treatment implementation and persistence) was assumed to be consistent across all scenarios.
Model inputs
As far as possible, model inputs were estimated conservatively based on published evidence. If no published evidence was available, inputs were informed by data on file, expert insights, and/or conservative assumptions. Details of the assumptions underlying the model are summarized below and detailed in and .
Table 1. Population estimate funnel for prevalent prostate cancer population in England eligible for GnRHa therapy.
Table 2. Assumptions within the model: “Current practice” and “Base case” scenarios.
Patient population assumptions
The number of men eligible for GnRHa therapy for prostate cancer in England was estimated using national population projections for England for 2022, the prevalence of prostate cancer, and the stage of disease at diagnosis ().Citation30–33,Citation35 The estimated prevalence of prostate cancer was 347,878 patients. In the absence of published data on GnRHa treatment use, or on disease stage distribution data, for the prevalent prostate cancer population in England, the number of patients who were eligible for GnRHa therapy was calculated based on the distribution of disease stage at diagnosis: stage 1, 35.5% (n = 123,497); stage 2, 13.6% (n = 47,311); stage 3, 23.9% (n = 83,143); stage 4, 17.0% (n = 59,139); and stage unknown, 10.0% (n = 34,788)Citation33. The proportions of men in each disease stage category who were assumed to initiate GnRHa treatment were: stage 2, 21%; stage 3, 54%; and stage 4, 75%Citation35. Men with a diagnosis of stage-1 prostate cancerCitation34 or with unknown disease stage were excluded from the analysis on the basis that GnRHa therapy is not indicated for use in these patientsCitation15. Thus, the estimated size of the population of patients initiated on GnRHa was calculated as 99,187, and this figure was used for the “Current practice” scenario and the “Base case” simulated scenarios.
Drug acquisition cost assumptions
Total annual GnRHa acquisition costs per patient were estimated by multiplying the unit cost per GnRHa injection by the number of injections required per year (). Unit drug costs were taken from the NHS Business Services Authority website listings in March 2022Citation36, and the number of annual injections was based on the posology of each GnRHa therapy, as per their summary of product characteristicsCitation11–17.
To achieve a population-level cost estimate, the per-patient drug acquisition cost was then multiplied based on size of the overall prevalent population () and the estimated market share of each GnRHa product. In the “Current practice” scenario, the distribution of use of each GnRHa product was informed by unpublished market analysis data collected by Ipsen Ltd (Slough, UK). To quantify the theoretical impact of widescale implementation of switching/transitioning eligible patients to 6-monthly GnRHa therapy, the “Base case” scenario assumed that 50% of patients with prostate cancer who were being treated with monthly or 3-monthly GnRHa therapy (the “Current practice” scenario) were switched/transitioned to treatment with 6-monthly triptorelin. Based on this assumption, the overall proportion of GnRHa-treated patients using 6-monthly triptorelin increased from 6.5% in the “Current practice” scenario to 53.2% in the “Base case” scenario.
Administration time and cost assumptions
The model assumed that GnRHa injections were administered by a band-5 nurse during an appointment lasting 15 min. This estimate for appointment duration was informed by a noninterventional evaluation of the effects of switching/transitioning men to 6-monthly GnRHa therapy in the UKCitation23. The costs associated with administration were based on rates listed in the unit costs from the Personal Social Services Research Unit ()Citation37. The total administration cost per year was calculated by multiplying the number of patients receiving each product by the number of administrations per year and by the £11.00 per-administration cost for a band-5 nurse.
In both the “Current practice” and the “Base case” scenarios, it was assumed that patients would not need to see a general practitioner during these appointments. In recognition of the fact that GnRHa administration appointments can be used opportunistically to conduct PSA tests, the cost of PSA testing was factored into the model; this was based on UK cost (inflated to 2022 prices) and a 26.6% reduction in test frequency following a switch/transition to 6-monthly therapy (from monthly or 3-monthly), as reported previouslyCitation23.
Societal cost assumptions
The societal cost of treatment was calculated for each scenario as the sum of four separate cost components: travel; environmental; lost productivity; and lost leisure time opportunity costs.
The assumptions underlying these cost estimates are summarized in . All societal costs were estimated on a “per administration appointment” basis and were then converted to annual population-level costs by multiplying by the number of patients receiving each product and the number of administrations per year.
The cost of travel was calculated based on an estimated fuel cost of £1.84 per journey (assuming a return journey time of 16 minCitation38, an average speed of 30 miles per hour, an average price of petrol of £1.88 per liter [on 21 July 2022]Citation43, and mileage of 6.6 miles per liter of fuelCitation44).
The environmental cost of treatment was estimated per journey and based on greenhouse gas emissions of 1,793 g (0.001793 tons) of CO2 per journey (221.4 g per mileCitation39 at an average speed of 30 miles per hour for 16 min) and a cost to the environment of £248.00 per ton of CO2 (UK government estimateCitation40).
The cost of lost productivity was calculated based on an estimated 51 min of work lost for each appointment (15-minute appointment plus 16 min of travel time and an additional 20 min spent parking and waiting), using a conservative estimate that 10% of patients with prostate cancer are active in the workforce, and a gross value added of £35.70 of labor productivity per hour of work in EnglandCitation41.
Overall cost of lost leisure time opportunity was estimated by multiplying the total lost time (51 min per appointment) by a per-hour cost of £6.75 (estimated based on an assessment of “Willingness to Pay” for leisure timeCitation42).
Sensitivity analyses
To assess the sensitivity of the budget impact estimates to different model assumptions, several sensitivity analyses were conducted. Each varied one input from the “Base case” scenario (Supplementary Table S1). Sensitivity analysis 1 evaluated the impact of a 20% overestimation or underestimation of GnRHa drug acquisition costs. Sensitivity analysis 2 assessed the impact of the level of expertise of the nurse administering the injection (i.e. the effect of administration by a band-7 rather than a band-5 nurse, thus increasing the cost to £16.50 per administrationCitation37). Sensitivity analysis 3 evaluated the impact of the administrative set-up time required for GnRHa treatment administration, factoring in 30 min of additional time for patient coordination, treatment sourcing and procurement, and set-up. Sensitivity analysis 4 evaluated the impact of different levels of switching/transitioning from monthly or 3-monthly to 6-monthly formulations of GnRHa therapy, assuming a 20% overestimation or underestimation within the “Base case” scenario. Sensitivity analysis 5 evaluated the impact of a higher proportion of patients receiving a diagnosis of later-stage disease, anticipated based on changes in the pattern of healthcare utilization at the height of the COVID-19 pandemic. A decrease of 20 percentage points in patients presenting with stage-1 disease was used as a proxy for the reduction in patients entering the healthcare system and being referred for prostate cancer diagnosis because of elective care backlogs and increasing challenges accessing careCitation20,Citation21.
Software
The model was developed in Microsoft Excel, version 2101 (Build 13628.20274).
Results
“Base case” scenario
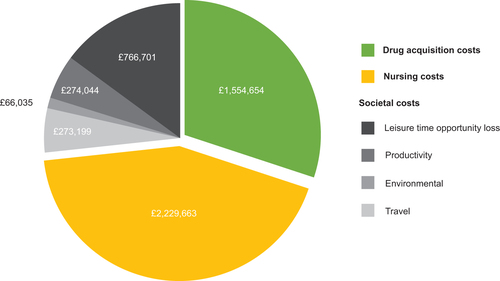
According to model estimates, a 50% switch/transition from monthly or 3-monthly to 6-monthly GnRHa therapy in men receiving these treatments for prostate cancer in England would reduce the estimated overall annual NHS budget and societal cost from £105,389,365 to £100,225,069 – a total saving of £5,164,296 ( and Supplementary Table S2). The largest driver of cost savings in the model was nurse administration costs, totaling £2,229,663 per annum ( and Supplementary Table S2). Estimated annual drug acquisition costs were reduced by £1,554,654, and savings were also predicted in all four societal cost domains (travel, environmental, productivity loss, and leisure time opportunity loss), totaling £1,379,978.
Figure 1. Estimated cost savings in the “Base case” scenario compared with “Current practice”. Cost savings are given in Great British Pounds.
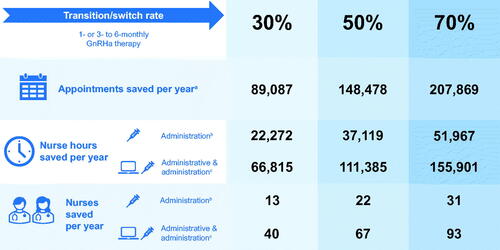
Reductions in administration costs were driven by a decreased number of appointments (and appointment-hours) required for administration of the 6-monthly GnRHa formulation. In total, an estimated 148,478 appointments could be avoided, saving 37,119 h of nursing time each year (equivalent to an NHS capacity saving of approximately 22 full-time equivalent [FTE] nurses, assuming an FTE nurse works 1672.5 h per yearCitation45) ().
Figure 2. Estimated NHS capacity savings with different switch/transition rates and resourcing estimates compared with “Current practice”. a“Nurses saved per year” data are based on full-time equivalent nurses working 37.5 h per week with 37 days of annual leave per year, equating to 1,672.5 working hours per yearCitation45. bAdministration time is based on a 15-minute appointment to administer the GnRHa injectionCitation23. cAdministrative time includes appointment set-up time, including booking patients for repeat visits and ensuring that GnRHa treatment is ordered and available. Abbreviations. GnRHa, gonadotropin-releasing hormone agonist; NHS, National Health Service.
Sensitivity analysis scenarios
Sensitivity analysis 1 evaluated the impact of a 20% overestimation or underestimation of GnRHa drug acquisition costs in the model assumptions. Increasing drug acquisition costs by 20% increased the costs of the “Current practice” and “Base case” scenarios (from £105,389,365 to £123,832,751 and from £100,225,069 to £118,357,523, respectively). If drug acquisition costs were 20% higher, a 50% switch/transition to 6-monthly GnRHa therapy (as per the “Base case” scenario) would still save the NHS £5,475,227 each year compared with “Current practice” (). Conversely, in the scenario with acquisition costs reduced by 20%, a 50% switch/transition from monthly or 3-monthly to 6-monthly therapy would achieve a more modest annual saving (£4,853,365) compared with “Current practice” ().
Figure 3. Estimated cost savings in the sensitivity analyses compared with the “Current practice” scenario. Abbreviation. GBP, Great British Pounds.
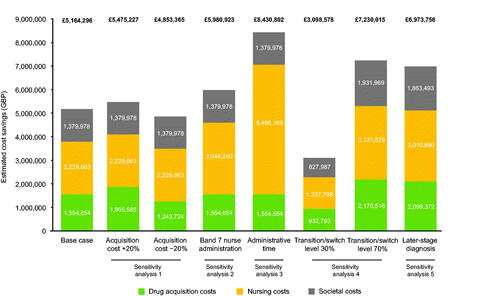
Sensitivity analysis 2 assessed the effect of a band-7 rather than a band-5 nurse administering GnRHa injections in both the “Current practice” and “Base case” scenarios. Compared with “Current practice”, the “Base case” scenario required 37,119 fewer nursing hours, lessening the system-wide costs of a higher-banded nurse administering therapy. Thus, if GnRHa treatment was routinely administered by a band-7 nurse, a transition to 6-monthly therapy by 50% of patients receiving monthly or 3-monthly injections would save the NHS £3,046,290 in nursing costs (and £5,980,923 overall) each year compared with “Current practice” ().
Sensitivity analysis 3 estimated the broader costs of GnRHa treatment implementation, taking into account not only 15 min for nurse administration of treatment, but also an additional 30 min of nurse time per patient per injection for set-up/administrative tasks (e.g. appointment booking and care coordination, treatment ordering and preparation, checking the PSA value and discussing it with the patient, and dealing with patient concerns). Thus, the total required nurse time in this analysis was 45 min. The addition of nurse set-up time increased annual nursing costs and time estimates for the “Current practice” scenario (from £8,412,040 to £19,680,239 and from 128,048 h to 384,143 h, respectively) and for the “Base case” scenario (from £6,182,376 to £14,184,070 and from 90,928 h to 272,785 h, respectively). Overall, with appointment set-up time being factored into GnRHa total cost and time estimates, a 50% switch/transition rate to 6-monthly GnRHa therapy (as per the “Base case” scenario) could achieve an estimated annual saving of £8,430,802 (£5,496,169 in nursing costs) and a saving of 111,358 appointment-hours compared with “Current practice” ( and ).
Sensitivity analysis 4 assessed the effect on the model estimates of different levels of switching/transitioning from monthly or 3-monthly to 6-monthly GnRHa therapy, assuming a 20% overestimation or underestimation in the “Base case” scenario. In the scenario in which the switch/transition rate was 70% (i.e. 20% higher than in the “Base case”), the annual cost and nurse hours saved compared with “Current practice” were £7,230,015 and 51,967 h, respectively (compared with £5,164,296 and 37,119 h, respectively, for the “Base case” scenario). In the scenario in which the switch/transition rate was 30%, the annual cost and capacity savings compared with “Current practice” were more modest: £3,098,578 and 22,272 h, respectively ( and ).
Finally, sensitivity analysis 5 assessed the impact of a higher proportion of patients receiving a diagnosis of later-stage disease as a result of the COVID-19 pandemic. The hypothesized shift to later-stage diagnosis was quantified as a decrease of 20 percentage points in men presenting with stage-1 disease, redistributed equally among stages 2–4. In the later-stage disease presentation scenario, a 50% switch/transition rate to 6-monthly GnRHa therapy was estimated to save the NHS £6,973,756 and 50,125 nurse hours per annum compared with “Current practice” ().
Discussion
At a time when the NHS is facing unprecedented staffing challengesCitation1–4, opportunities to increase capacity without compromising patient care are not only highly desirable but also much needed. In the context of this challenging care landscape, our model assessed the impact of different GnRHa treatment scenarios on NHS costs and nursing capacity.
The model builds on earlier work by Cornford and colleagues (the DESERVE study), which evaluated the impact of a switch/transition to 6-monthly GnRHa therapy at three UK hospitalsCitation23. Several of our model assumptions were informed by their results but extrapolated to consider system-wide cost and capacity effects on a per annum basis. We also modeled the possible effect of the COVID-19 pandemic on diagnostic patterns and factored in a societal perspective to offer a holistic evaluation of the societal economic impact of a change in prostate cancer careCitation24,Citation25. Overall, we estimated that switching/transitioning 50% of men who are receiving monthly or 3-monthly GnRHa therapy to a 6-monthly formulation could reduce GnRHa appointments by almost 150,000 each year, free up over 37,000 appointment-hours (approximately 22 FTE nurses) and save over £5 million in NHS and societal costs (NHS: £3.8 million; society: £1.3 million). The 50% switch/transition rate used for the “Base case” scenario was a hypothetical rate chosen to allow demonstration of the scale of capacity and cost benefits potentially realizable through widescale implementation of guideline recommendations to switch/transition eligible patients to a 6-montlhy GnRHa treatment regimenCitation20–22; the rate was not intended to quantify the impact of current trends in prescribing practice. Further, a 50% switch/transition rate is reasonable in the context of the new integrated care system and provider collaborations in England, which enable best care practice changes to be implemented at scaleCitation28.
Greater use of 6-monthly GnRHa therapy was predicted to yield cost and capacity savings (vs “Current practice”) across all sensitivity analysis scenarios, including those in which: drug acquisition costs or switch/transition rates were 20% higher or lower than in the “Base case” scenario; treatment was administered by a higher-band nurse (7 vs. 5); nursing time extended beyond the duration of the appointment to include associated administrative tasks; and COVID-19 increased the proportion of men receiving a diagnosis of later-stage prostate cancer because of delays in accessing care during the pandemicCitation5. The greatest cost and capacity savings were predicted in sensitivity analysis 3, which evaluated the impact of a 50% switch/transition rate to 6-monthly therapy, taking into consideration the time that nurses spend coordinating and preparing for injection appointments, as well as the time spent with patients, administering the injection. In this scenario, total annual cost and capacity savings were estimated to be approximately £8.4 million, 148,478 appointments, and 111,358 appointment-hours. This is equivalent to approximately 67 FTE nurses.
There is some ambiguity in the size of the population input used in the model owing to the assumption that prevalent disease stage distribution is consistent with incident disease stage. This assumption was necessary because of the lack of disease distribution data published for the prevalent prostate cancer population and the challenges of estimating true GnRHa therapy use. Eligibility for GnRHa therapy is dependent on disease stage, and the population maintained on GnRHa therapy at any point in time comprises not only newly diagnosed eligible cases, but also newly eligible cases resulting from disease progression and disease recurrence. Despite the use of incident disease stage distribution as a proxy for prevalent disease stage distribution, the resultant 99,187 men estimated to be receiving GnRHa therapy in England in 2022 is broadly consistent with estimates published by Bourke and colleagues for the UK: that 125,000 men in were maintained on ADT therapy in 2018, and that this number would likely increase to 200,000 men by 2020, and to 300,000 men by 2030, because of the aging population in the UK and increasing rates of prostate cancerCitation46. It should be noted that the model focused on quantifiable costs within the primary care setting where most GnRHa therapy is administered; some cost and capacity considerations associated with GnRHa therapy are unquantifiable and were beyond the scope of the present work. For instance, pragmatic and logistical challenges associated with care delivery and implementation – particularly for a condition like prostate cancer, for which care is often split between specialties (e.g. urology and oncology) and settings (primary, secondary, and pharmacy care) – were beyond the primary care scope of the model. The model was also limited by its lack of inclusion of pricing adjustments for drug rebates and discounts. Rebate and discount assumptions were not included because of a lack of transparency surrounding these issues and because of likely variation between primary care practices. Accurate cost savings would need to take into account local pricing arrangements. Nevertheless, the internal consistency of the model means that the model accurately estimates the relative drug cost savings across the different scenarios considered. Regardless of the drug cost savings, the societal benefits and capacity savings, which contribute the greatest cost and time savings in each switch/transition scenario, would persist because they are underpinned by reductions in appointment number and are independent of drug costs. Also beyond the scope of the model was the impact of increased use of 6-monthly GnRHa therapy on health-related quality of life. However, it is plausible that increased use of a less onerous GnRHa treatment regimen (and in some instances introducing it to the range of existing treatment options) may have a beneficial impact on quality of life by virtue of increasing patient choice and/or reducing the impact of treatment administration on patients’ daily living.
It is relevant to reiterate that the model assumed equivalence in the effectiveness and tolerability of the different GnRHa formulations available for use in England at the time of writing. Direct comparative evidence is limited for the different GnRHa therapies available in England. A systematic review of direct comparative data for goserelin, triptorelin, and leuprorelin noted that the published studies are restricted by small sample sizes and lack power to confirm equivalence, but the review authors noted that there was no evidence of major differences in the ability of these GnRHa therapies to reduce testosterone or PSA levelsCitation47. The assumption of equivalence used in the present analysis is in keeping with this assertion, and with the unqualified call for greater use of 6-monthly GnRHa therapy published by the BAUS in their interim management guidance for prostate cancer during the COVID-19 pandemicCitation22. The assumption of equivalence is also supported by evidence from the UK DESERVE study, which suggested that switching from shorter-acting GnRHa therapy to a 6-monthly formulation is a pragmatic and rationale treatment approach in real-world UK clinical practiceCitation23. In addition, in the absence of robust comparative data for the available GnRHa alternatives, the present analysis assumed that a switch/transition to 6-monthly therapy would only be implemented to optimize patient care, based on patient preference and clinical eligibility. The hypothesized 50% switch/transition rate used in the “Base case” scenario assumes that a switch/transition to 6-monthly GnRHa therapy is neither feasible, nor appropriate in all men currently receiving monthly or 3-monthly treatment. GnRHa formulations requiring more frequent dosing may enable closer management and monitoring of potential treatment-related adverse event management (e.g. of hot flushes, erectile dysfunction, cognitive impairments, and risk of cardiometabolic conditions), particularly in the months immediately following new treatment initiationCitation48,Citation49. Finally, if appointments for GnRHa treatment administration serve as an opportunity and prompt for regular PSA testing in routine care, it is also plausible that a reduction in the number of required GnRHa injections may reduce the opportunity for earlier detection of disease progression. However, in the absence of a clinical guideline specifying a gold standard for frequency of PSA testing, coupled with the challenges of quantifying the potential capacity benefit of detecting disease progression 3–5 months earlier (i.e. at a 3-monthly or monthly vs 6-monthly GnRHa administration appointment), such considerations were not factored into the design of the model.
The cost and capacity savings estimated by the model for different patterns of GnRHa prescribing should be considered, not only at a national level, but also in the context of regional services and the potential for optimizing local prostate cancer care. Care model optimization is particularly important within the new paradigms being proposed under ICBs in EnglandCitation50, which are seeking opportunities to reconfigure services and establish more efficient and effective ways of working.
In this context, another important consideration is the potential impact of increased use of 6-monthly GnRHa therapy on the frequency of PSA testing. The present model assumes that PSA testing would be reduced by 26.6% in patients who switch/transition from monthly or 3-monthly to 6-monthly GnRHa therapy. This assumption was based on observations reported by Cornford and colleagues in their real-world GnRHa switch studyCitation23. Although a reduction in coincident PSA testing may be welcomed from a financial and capacity costs perspective, there is currently no formal guidance on the optimal PSA testing regimen for individuals with confirmed prostate cancer to determine whether reductions associated with less frequent GnRHa administration would be advantageous or whether additional PSA test touch points might be required to exclude castration-resistant disease.
Sensitivity analysis 3 included consideration of the administrative time involved with GnRHa treatments (e.g. appointment booking and care coordination, and treatment ordering and preparation). In this scenario, the nurse time required per appointment was three times greater than that required for treatment administration only. The model did not include any estimation of the upfront time investment required to review patient eligibility for switching/transitioning to 6-monthly GnRHa therapy; such time is not trivial but was considered to be a one-off investment with the potential to release costs and capacity back into the system in subsequent years. In addition, conducting treatment reviews and engaging with patients about their care pathway and preferences aligns with the NHS Choice FrameworkCitation18. The recent establishment of ICBs in England makes this an opportune moment to consider the role that specialist clinics (e.g. within hospital settings or designated service hubs in a primary care setting) may play in conducting such reviews. This could help to optimize system-wide care by streamlining the patient pathway and to reduce inequalities, as well as realizing potential capacity and cost benefits.
Conclusions
At a time when the NHS in England was facing significant system-wide cost and capacity pressures, our holistic model estimated substantial cost and capacity savings if half of the men currently receiving monthly or 3- monthly GnRHa therapy in England switched/transitioned to a 6-monthly formulation. The greatest opportunity was in reducing the number of GnRHa injections required each year and, in so doing, reducing the associated demands on nursing time and related costs. In addition to the reduced impact on nursing time and capacity, large-scale switching/transitioning from monthly and 3-monthly GnRHa therapy to a 6-monthly formulation was predicted to reduce drug acquisition costs and, from a societal and patient perspective, to reduce the time and costs associated with appointment-related travel, lost leisure and productivity time, and environmental costs. These benefits were predicted for all sensitivity scenarios modeled and should be realizable when seeking to optimize prostate cancer care services at a system, as well as national, level.
Transparency
Author contributions
PC: visualization, review and editing. CH: conceptualization, methodology, visualization, review and editing. JS: visualization, review and editing. IF: conceptualization, methodology, visualization, review and editing. SB: visualization, review and editing.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have received an honoraria for advice and speaking for Ipsen on a different product. Another reviewer carried out paid consultancy for Ipsen (2015–16) in connection with an HTA submission for triptorelin. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Previous presentations
Not applicable.
Supplemental Material
Download MS Word (30.2 KB)Acknowledgements
The authors thank John Whalen, MBA, (formerly of Ipsen) for his contributions to the conception and methodology of the model. The authors also thank Alison Chisholm, MPH, of Oxford PharmaGenesis, Oxford, UK, who provided medical writing and editorial support, which was sponsored by Ipsen in accordance with Good Publication Practice guidelines.
Declaration of interest
This study was sponsored by Ipsen.
Declaration of funding/other interests
PC reports honoraria from AstraZeneca, Bayer, Ferring Pharmaceuticals, Ipsen and Janssen; scientific advisory board meetings for Accord, AstraZeneca, Bayer, Bristol Myers Squibb, Ferring Pharmaceuticals and Janssen; and travel grants from Janssen and Bayer.
CH, JS and IF are employees and shareholders of Ipsen.
SB reports consulting or advisory role honoraria from Ipsen.
References
- Health and Social Care Committee. Workforce: recruitment, training and retention in health and social care: third report of session 2022–23; 2022 July 15 [Internet] [cited 2022 October]. Available from: https://committees.parliament.uk/committee/81/health-and-social-care-committee/news/172310/persistent-understaffing-of-nhs-a-serious-risk-to-patient-safety-warn-mps/.
- National Health Service Confederation. Real risk that thousands of NHS staff will leave unless they are allowed to recover; 2021 March 24 [Internet] [cited 2022 October]. Available from https://www.nhsconfed.org/news/real-risk-thousands-nhs-staff-will-leave-unless-they-are-allowed-recover.
- National Health Service England. Delivery plan for tackling the COVID-19 backlog of elective care; 2022 February [Internet] [cited 2022 October]. Available from: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2022/02/C1466-delivery-plan-for-tackling-the-covid-19-backlog-of-elective-care.pdf.
- Royal College of Nursing. Radical action needed to boost nurse workforce; 2022 May 18 [Internet] [cited 2022 October]. Available from: https://www.rcn.org.uk/news-and-events/news/uk-radical-action-needed-to-boost-nursing-workforce-180522.
- Prostate Cancer UK. Find the 14,000 men: we join forces with the NHS to find men who haven’t started prostate cancer treatment due to the pandemic; 2022 February 16 [Internet] [cited 2022 October]. Available from: https://prostatecanceruk.org/about-us/news-and-views/2022/02/find-the-14-000-men-nhs-campaign.
- National Institute for Health and Care Excellence. Prostate cancer: diagnosis and management. NICE guideline [NG131]; 2019 May 9. [Internet] [cited 2022 October]. Available from: https://www.nice.org.uk/guidance/ng131/chapter/Recommendations.
- Massie CE, Lynch A, Ramos-Montoya A, et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. Embo J. 2011;30(13):2719–2733.
- Hååg P, Bektic J, Bartsch G, et al. Androgen receptor down regulation by small interference RNA induces cell growth inhibition in androgen sensitive as well as in androgen independent prostate cancer cells. J Steroid Biochem Mol Biol. 2005;96(3–4):251–258.
- Perlmutter MA, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol. 2007;9(Suppl 1):S3–S8.
- National Health Service. Prostate cancer: treatment; 2021 October 18 [Internet] [cited 2022 October]. Available from: https://www.nhs.uk/conditions/prostate-cancer/treatment/.
- AstraZeneca UK Ltd. Summary of product characteristics: goserelin acetate (Zoladex Implant), 3.6 mg [Internet] [first authorized 2001 May 1; updated 2017 January 24; cited 2022 October]. Available from: https://www.medicines.org.uk/emc/product/1543/smpc.
- AstraZeneca UK Ltd. Summary of product characteristics: goserelin acetate (Zoladex LA), 10.8 mg [Internet] [first authorized 2001 May 1; updated 2017 January 24; cited 2022 October]. Available from: https://www.medicines.org.uk/emc/product/1567.
- Takeda UK Ltd. Summary of product characteristics: leuprorelin acetate (PROSTAP SR DCS), 3.75 mg [Internet] [first authorized 2011 April 28; updated 2020 August 24; cited 2022 October]. Available from: https://www.medicines.org.uk/emc/product/4650/smpc.
- Takeda UK Ltd. Summary of product characteristics: leuprorelin acetate (PROSTAP 3 DCS), 11.25 mg [Internet] [first authorized 2011 April 28; updated 2020 August 24; cited 2022 October]. Available from: https://www.medicines.org.uk/emc/product/4651/smpc.
- Ipsen Ltd. Summary of product characteristics: triptorelin acetate (Decapeptyl SR), 3 mg [Internet] [first authorized 1994 December 29; updated 2017 September 13; cited 2022 October]. Available from: https://www.medicines.org.uk/emc/product/963/smpc.
- Ipsen Ltd. Summary of product characteristics triptorelin pamoate (Decapeptyl SR), 11.25 mg [Internet] [first authorized 2002 October 16; updated 2009 March 16; cited 2022 October]. Available from: https://www.medicines.org.uk/emc/product/780/smpc.
- Ipsen Ltd. Summary of product characteristics: triptorelin pamoate (Decapeptyl SR), 22.5 mg. [Internet] [first authorized 2010 September 14; updated 2016 December 17; cited 2022 October]. Available from: https://www.medicines.org.uk/emc/product/5906/smpc.
- Department of Health and Social Care. The NHS choice framework [Internet] [cited 2022 October]. Available from: https://www.gov.uk/government/publications/the-nhs-choice-framework.
- National Health Service UK. The NHS long term plan; 2019 January 7 [Internet] [updated 2019 August 21; cited 2022 October]. Available from: https://www.longtermplan.nhs.uk/publication/nhs-long-term-plan/.
- Wallis CJD, Catto JWF, Finelli A, et al. The impact of the COVID-19 pandemic on genitourinary cancer care: re-envisioning the future. Eur Urol. 2020;78(5):731–742.
- Obek C, Doganca T, Argun OB, et al. Management of prostate cancer patients during COVID-19 pandemic. Prostate Cancer Prostatic Dis. 2020;23(3):398–406.
- British Association of Urological Surgeons (Section of Oncology). COVID-19 strategy for the interim management of prostate cancer [Internet] [cited 2022 October]. Available from: https://wmcanceralliance.nhs.uk/images/Documents/Urology_including_Prostate/COVID-19_BAUS_Oncology_Prostate_final.pdf.
- Cornford P, Jefferson K, Cole O, et al. Effects of initiating or switching to a six-monthly triptorelin formulation on prostate cancer patient-healthcare interactions and hospital resource use: a real-world, retrospective, non-interventional study. Oncol Ther. 2018;6(2):173–187.
- Jönsson B. Ten arguments for a societal perspective in the economic evaluation of medical innovations. Eur J Health Econ. 2009;10(4):357–359.
- Byford S, Raftery J. Perspectives in economic evaluation. BMJ. 1998;316(7143):1529–1530.
- National Health Service England. Delivering a ‘net zero’ national health service 2020 October [Internet] [cited 2022 October]. Available from: www.england.nhs.uk/greenernhs/wp-content/uploads/sites/51/2020/10/delivering-a-net-zero-national-health-service.pdf.
- Goto R, Uda A, Hiroi S, et al. Cost analysis of leuprorelin acetate in Japanese prostate cancer patients: comparison between 6-month and 3-month depot formulations. J Med Econ. 2017;20(11):1155–1162.
- National Health Service England. Integrated care systems: guidance [Internet] [cited 2022 October]. Available from: https://www.england.nhs.uk/publication/integrated-care-systems-guidance/.
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR task force on good research practices–budget impact analysis. Value Health. 2007;10(5):336–347.
- Office for National Statistics. National population projections: 2020-based interim. Projections for ENGLAND only; 2022 [Internet] [cited 2022 October]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/bulletins/nationalpopulationprojections/2020basedinterim.
- Prostate Cancer UK. About prostate cancer. Facts and figures. 2018 [Internet] [cited 2022 October]. Available from: https://prostatecanceruk.org/prostate-information/about-prostate-cancer.
- Office for National Statistics. Population estimates for the UK, England and Wales, Scotland and Northern Ireland: mid 2017 [Internet] [cited 2022 October]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2017.
- Cancer Research UK. Prostate cancer incidence statistics [Internet] [cited 2022 October]. Available from: www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence.
- Cancer Research UK. Survival; 2020 [Internet] [cited 2022 October]. Available from: www.cancerresearchuk.org/about-cancer/prostate-cancer/survival.
- Kantar Health. Market research report: cancerMPact treatment architecture. Prostate Cancer, EU5. 2020 (Ipsen data on file).
- National Health Service (NHS) Business Services Authority NHS Prescription Services. NHS Business Services Authority NHS prescription services -dm + d [Internet] [cited 2022 October]. Available from: https://services.nhsbsa.nhs.uk/dmd-browser/.
- Personal Social Services Research Unit. Community based healthcare staff; 2021. [Internet] [cited 2022 October]. Available from: https://www.pssru.ac.uk/pub/uc/uc2021/communitybasedhcstaff.pdf.
- UK Government. Department for Transport. Official statistics. Journey time statistics, England; 2019 [Internet] [cited 2022 October]. Available from: www.gov.uk/government/statistics/journey-time-statistics-england-2019/journey-time-statistics-england-2019.
- NimbleFins. Average CO2 emissions per car in the UK [Internet] [cited 2022 October]. Available from: https://www.nimblefins.co.uk/average-co2-emissions-car-uk.
- UK Government. Department for Business, Energy & Industrial Strategy. Valuation of greenhouse gas emissions: for policy appraisal and evaluation; 2021 [Internet] [cited 2022 October]. Available from: www.gov.uk/government/publications/valuing-greenhouse-gas-emissions-in-policy-appraisal/valuation-of-greenhouse-gas-emissions-for-policy-appraisal-and-evaluation.
- Office for National Statistics. Subregional productivity: labour productivity indices by UK ITL2 and ITL3 subregions [Internet] [cited 2022 October]. Available from: www.ons.gov.uk/employmentandlabourmarket/peopleinwork/labourproductivity/datasets/subregionalproductivitylabourproductivitygvaperhourworkedandgvaperfilledjobindicesbyuknuts2andnuts3subregions.
- Verbooy K, Hoefman R, van Exel J, et al. Time is money: investigating the value of leisure time and unpaid work. Value Health. 2018;21(12):1428–1436.
- Allstar Business Solutions. UK fuel prices near me [Internet] [cited 2022 October]. Available from: https://fleetcor-prod-ukw-allstar.azurewebsites.net/tools/uk-fuel-prices/?_ga=2.20420667.2054603868.1675102289-1061929705.1675102289.
- NimbleFins. Average MPG for Cars UK; 2022 [Internet] [cited 2022 October]. Available from: https://www.nimblefins.co.uk/cheap-car-insurance/average-mpg.
- Royal College of Nursing. NHS conditions of employment. Standard working hours, overtime, annual leave and on-call working arrangements [Internet] [cited 2022 October]. Available from: https://www.rcn.org.uk/employment-and-pay/NHS-conditions-of-employment.
- Bourke L, Turner R, Greasley R, STAMINA investigators, et al. A multi-centre investigation of delivering national guidelines on exercise training for men with advanced prostate cancer undergoing androgen deprivation therapy in the UK NHS. PLOS One. 2018;13(7):e0197606.
- Bolton EM, Lynch T. Are all gonadotrophin-releasing hormone agonists equivalent for the treatment of prostate cancer? A systematic review. BJU Int. 2018;122(3):371–383.
- Edmunds K, Tuffaha H, Galvão DA, et al. Incidence of the adverse effects of androgen deprivation therapy for prostate cancer: a systematic literature review. Support Care Cancer. 2020;28(5):2079–2093.
- Meani D, Solarić M, Visapää H, et al. Practical differences between luteinizing hormone-releasing hormone agonists in prostate cancer: perspectives across the spectrum of care. Ther Adv Urol. 2018;10(2):51–63.
- National Health Service England. What are integrated care systems? [Internet] [cited 2022 October]. Available from: https://www.england.nhs.uk/integratedcare/what-is-integrated-care/.
